Abstract
Artificial intelligence (AI) is rapidly advancing, aiming to mimic human cognitive abilities, and is addressing complex medical challenges in the field of biological science. Over the past decade, AI has experienced exponential growth and proven its effectiveness in processing massive datasets and optimizing decision-making. The main content of this review paper emphasizes the active utilization of AI in the field of stem cells. Stem cell therapies use diverse stem cells for drug development, disease modeling, and medical treatment research. However, cultivating and differentiating stem cells, along with demonstrating cell efficacy, require significant time and labor. In this review paper, convolutional neural networks (CNNs) are widely used to overcome these limitations by analyzing stem cell images, predicting cell types and differentiation efficiency, and enhancing therapeutic outcomes. In the biomedical sciences field, AI algorithms are used to automatically screen large compound databases, identify potential molecular structures and characteristics, and evaluate the efficacy and safety of candidate drugs for specific diseases. Also, AI aids in predicting disease occurrence by analyzing patients’ genetic data, medical images, and physiological signals, facilitating early diagnosis. The stem cell field also actively utilizes AI. Artificial intelligence has the potential to make significant advances in disease risk prediction, diagnosis, prognosis, and treatment and to reshape the future of healthcare. This review summarizes the applications and advancements of AI technology in fields such as drug development, regenerative medicine, and stem cell research.
1. Introduction
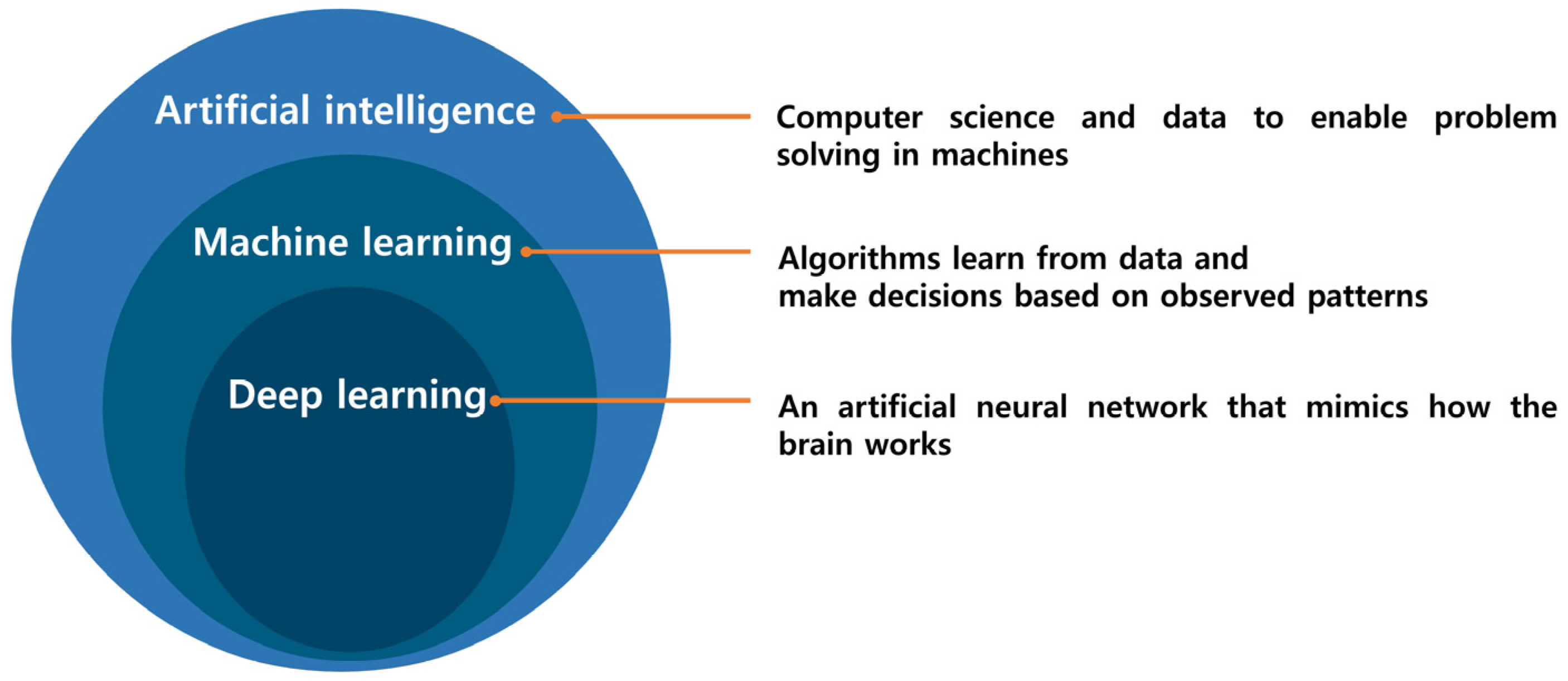
In the fields of bioscience and healthcare, numerous companies and researchers are dedicated to developing novel treatments, including new medicines through drug discovery, new gene therapies, and new stem cell therapies, with the aim of accurately diagnosing and treating diseases. However, these endeavors demand diverse technologies and specialized knowledge, and each area is constrained by significant time and cost limitations. To overcome these challenges, approaches such as virtual screening (VS) and molecular docking have been employed in drug development. Yet, these computational methods exhibit inaccuracies and inefficiencies, contributing to an average cost of $2.8 billion and a 15-year timeline for drug discovery [1,2]. Additionally, the complexities of aspects such as chemical structures and drug–protein interactions pose challenges, making it difficult to handle big data manually [3]. Consequently, the need to develop new methods for handling these time- and cost-intensive tasks has been recognized. Figure 1 illustrates the relationship between artificial intelligence (AI), deep learning (DL), and machine learning (ML). Artificial intelligence is widely utilized across various industries. In bioscience, there is a significant emphasis on overcoming the challenges of drug design and discovery [4], where AI is employed in processes such as drug target prediction, bioavailability prediction, and de novo drug design [5,6]. Major pharmaceutical companies, including Bayer, Roche, and Pfizer, have initiated collaborations with information technology (IT) companies to develop AI-based methodologies for drug design [1].
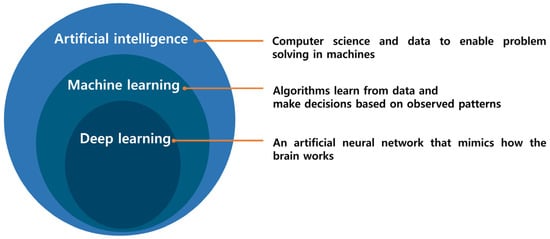
Figure 1.
Artificial Intelligence encompasses a broad spectrum, with Machine Learning being one of its subsets. Within Machine Learning, the category of Deep Learning is a subset specifically within the domain of Artificial Neural Networks.
A linkage between stem cell biology and AI research has firmly established itself as a revolutionizing approach in biological studies, offering tremendous potential for elucidating the characteristics of stem cells and their applications in the field of therapeutics. Stem cells, with their remarkable abilities for self-renewal and differentiation into specific cell types, play a central role in regenerative medicine and developmental biology [7,8]. Their extraordinary potential to regenerate damaged tissues and organs has positioned them as key elements in exploring novel therapeutic interventions, ranging from cancer treatments to those addressing neurodegenerative diseases [9,10,11].
While numerous researchers strive to develop successful and safe stem cell therapies, experiments involving the stable cultivation of stem cells and induced differentiation into desired cell types are extremely time-consuming and labor-intensive [12]. To overcome these limitations, various global companies and research institutes are employing AI technology to investigate the safe cultivation of stem cells and the efficient development of stem cell therapies. Artificial intelligence possesses exceptional analytical and pattern recognition capabilities for processing vast and complex datasets, enabling unprecedented efficiency in exploring the intricacies of stem cell biology by analyzing and predicting high-dimensional cellular imaging data [13].
Among the various algorithms contributing to stem cell research, convolutional neural networks (CNNs) are seen as essential deep learning models for analyzing and identifying image patterns. Recent advancements in CNNs allow researchers to not only discern the types of stem and cancer cells from unlabeled images but also predict the efficiency of differentiation into desired cell types and the level of genetic safety. The advancements in artificial intelligence and deep learning present excellent opportunities for efficient drug development and early disease diagnosis and will affect diverse research areas involving stem cells. Ultimately, these technologies are poised to have a significant impact on humanity.
This review paper will explore how AI technology is utilized in the field of stem cells; introduce current cases of its application; and suggest future directions for AI development. Additionally, it will briefly present ways AI is being applied in regenerative medicine and drug development.
2. AI Technology in Regenerative Medicine
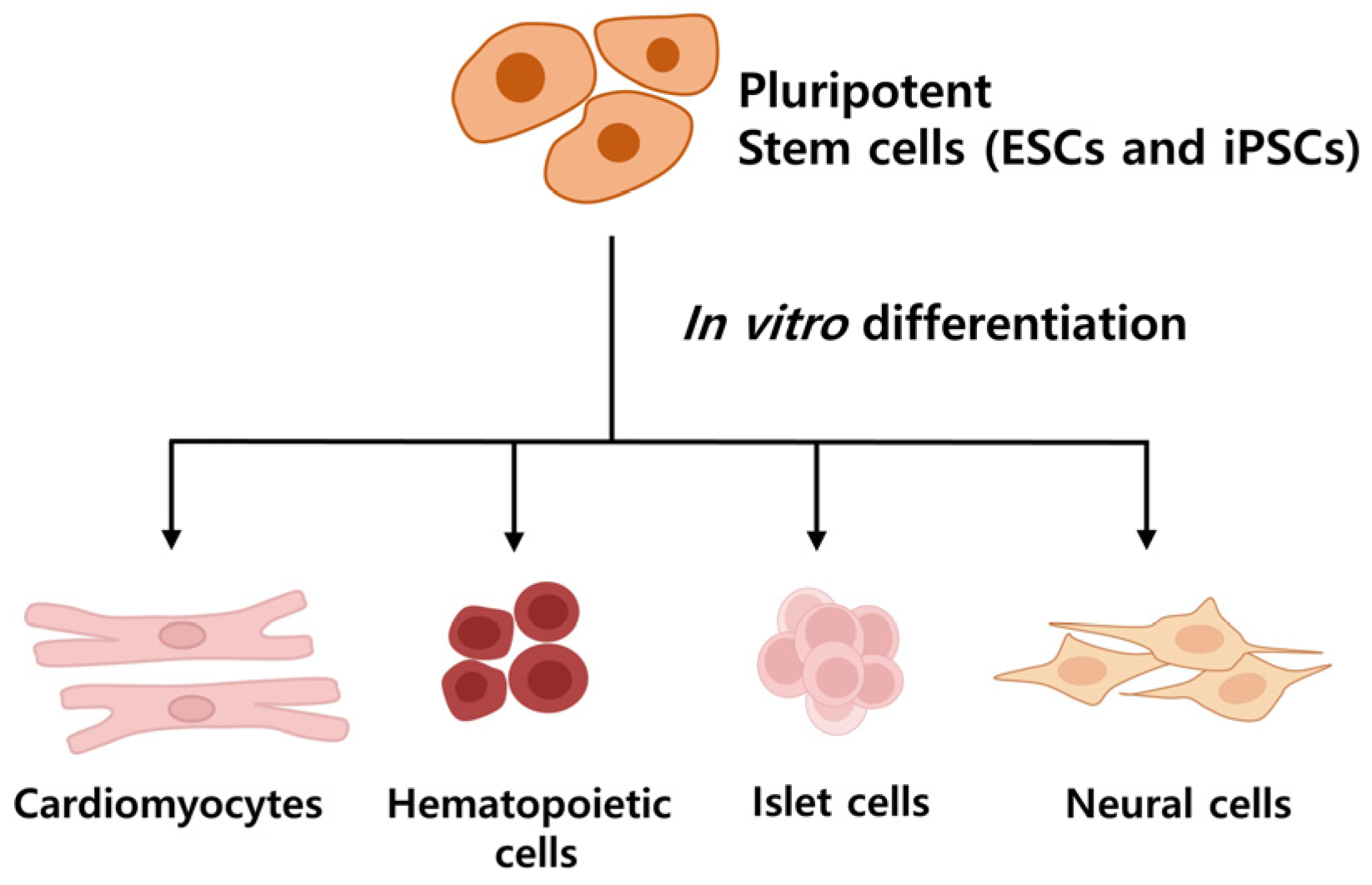
Stem cells are characterized as undifferentiated cells possessing the capability for both self-renewal division and differentiation into various cell types within an organism [14,15]. Pluripotent stem cells (Figure 2), such as embryonic stem cells (ESCs), originate from the inner cell mass of the blastocyst and have the potential to differentiate into all cell types of the embryo and adult, excluding the embryo itself. However, their study has raised ethical concerns due to embryo destruction [16]. Induced pluripotent stem cells (iPSCs), generated from artificially reprogrammed adult somatic cells, share similar functional properties with pluripotent stem cells [17], providing a valuable alternative to ESCs for drug development, disease modeling, and regenerative medicine without significant ethical concern [15,18].
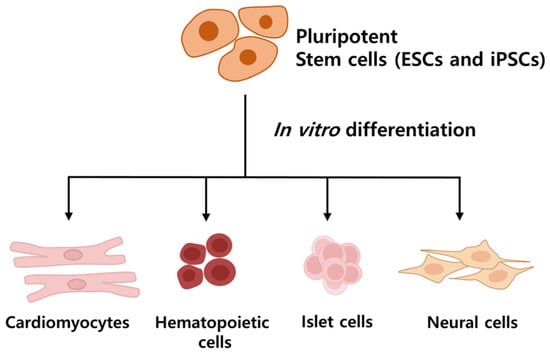
Figure 2.
Differentiation of Pluripotent Stem Cells. Pluripotent stem cells can be used to obtain desired cells through differentiation. They have come to represent a crucial turning point in regenerative medicine, with potential applications in disease treatment.
Regenerative medicine, involving advanced technologies such as stem-cell-based therapies, gene therapy, and tissue engineering, aims to restore or replace damaged tissues and organs [19,20]. Therefore, regenerative medicine holds significant promise for patients afflicted with challenging diseases like heart conditions, diabetes, and neurological disorders [21,22]. However, the development of regenerative therapies necessitates the analysis of complex and extensive data, a task where the capabilities of AI technology can be applied. The remainder of this section aims to explore the utilization of AI in the field of regenerative medicine, discussing outcomes and proposing new research directions, while specific examples of AI’s use in regenerative medicine are discussed in the next section. This comprehensive perspective seeks to underscore the potential growth of AI in the field.
Cell-based therapy is a promising field in regenerative medicine that utilizes stem cells to treat damaged tissues and organs. Stem cells are considered ideal candidates for repairing damaged tissues and organs [23]. Cell therapy also holds the potential to address chronic diseases without current treatment options [24]. However, there are drawbacks, such as the time- and cost-intensive cultivation of stem cells in a stable manner and the challenges of inducing specific cell differentiation. Moreover, despite ongoing clinical trials, a fully developed cure through stem cell therapy is yet to be realized [25]. Many scientists are turning to AI to analyze extensive datasets and assist in identifying optimal cells for specific patients. One of the key advantages of employing AI in stem cell therapy is its capability to predict the most effective cell types by analyzing patients’ genetic information and medical records. This not only aids in identifying conditions for the differentiation of specific cells and optimizing cell cultures but also allows the prediction of cell types based solely on cell morphology. In this context, various algorithms are utilized, and we aim to explore the algorithms predominantly used and provide illustrative examples.
In DL, CNN is the most famous and commonly used algorithm. Its advantage is that it automatically identifies the characteristics of the data without human intervention. The CNN algorithm is widely applied in various fields such as computer vision, voice processing, and facial recognition [26].
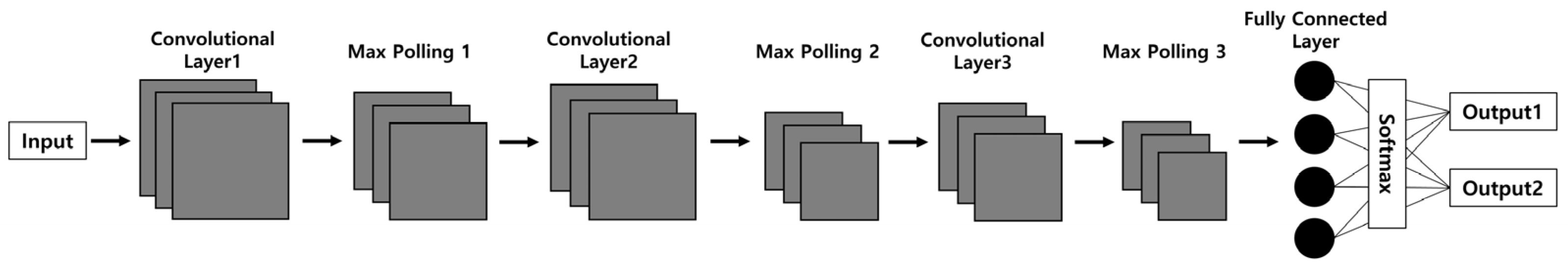
The CNN model structure commonly used in the field of biosciences is illustrated in Figure 3. The main framework of the CNN structure consists of convolution layers, max-pooling layers, and softmax layers [27]. Initially, the input data passes through a series of convolution layers paired with max-pooling layers to extract features from the data. Subsequently, the classification task is carried out through fully connected layers.
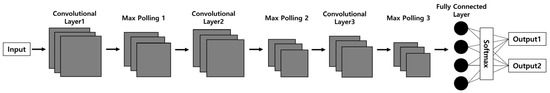
Figure 3.
The common convolutional neural network model used in biosciences. The CNN model consists of three convolutional layers extracting features from input images and three max-pooling layers reducing the feature map size followed by a fully connected layer for image classification. The number of convolutional layers and max pooling layers can be customized based on the model’s application.
The convolution layers play a crucial role in generating feature maps of various sizes, which are then reduced through pooling layers before proceeding to the next layer. The fully connected layers and softmax function map the extracted features into a final output, such as classification. The initial layers serve to recognize the basic structures of the images, while neurons in deeper layers are specialized in identifying more complex structures [28,29].
2.1. Potential of CNNs in Cell-Image-Based Classification
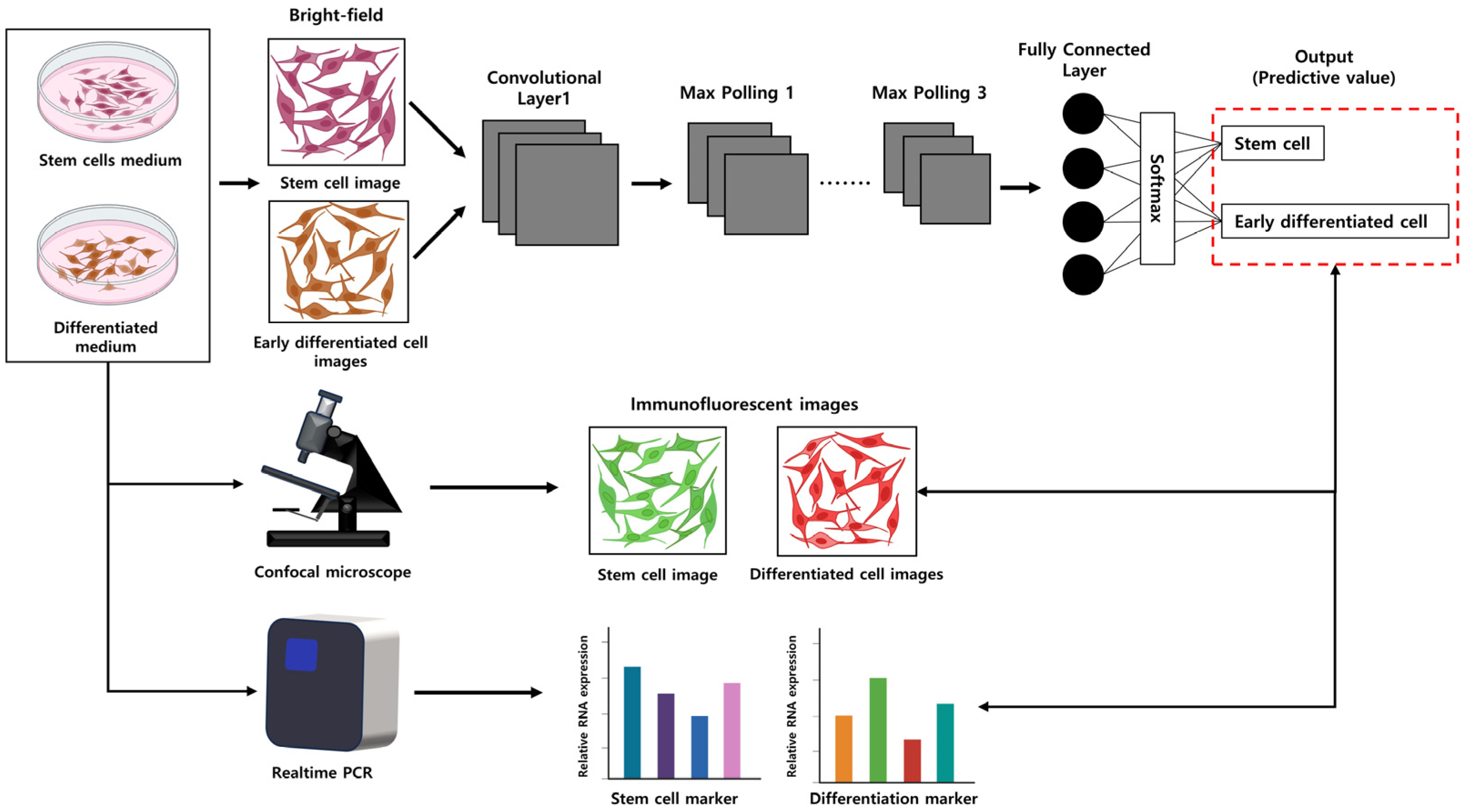
Microscopes are the most important tools in the field of medicine, allowing the close observation of cell shapes and the detection of abnormal cells. It is important in biology for researchers to maintain cell cultures safely and detect the desired cell morphologies; however, this is time-consuming and prone to errors. Deep learning can overcome these limitations by efficiently analyzing vast amounts of data. Molecular biology is one significant field where deep learning technology can be effectively applied as it deals with the unique shapes of each cell, proven through various experiments [30]. Figure 4 illustrates the capability of CNNs to discern subtle changes in cell morphology induced by a cell culture medium that are imperceptible to the human eye. Worth exploring is whether it is possible to classify the desired neuronal cells solely based on cell images by comparing them with the morphology of existing stem cells. Such cell-image-based classification aims to ascertain whether early cell differentiation can be recognized or if cell types can be identified in early differentiation. If it is successful, utilizing only the images of early differentiating cells could significantly reduce the overall time and cost involved in differentiation experiments. The results predicted by CNNs can be validated by staining stem cells and the desired differentiated cells and observing them through a confocal microscope while also assessing stem cell and differentiation-related gene expression through real-time PCR experiments.
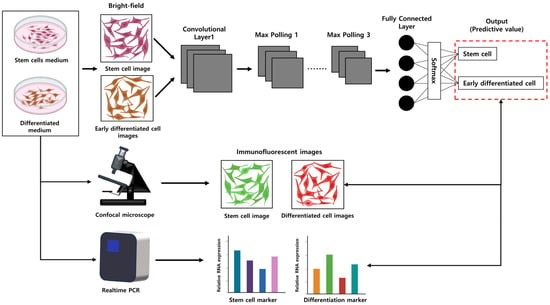
Figure 4.
The overall experimental process using CNNs for assessing cell differentiation. The illustration demonstrates the CNN model’s ability to distinguish subtle differences in morphology between stem cells and early differentiating cells. The accuracy of CNN predictions can be confirmed by staining both stem cells and differentiated cells, observing them with a confocal microscope, and simultaneously evaluating gene expression related to stem cell differentiation using real-time PCR.
2.2. Applications of CNNs in Stem Cell Culture and Differentiation
Pluripotent stem cells play a significant role in regenerative medicine, disease modeling, and drug testing due to their capacity to differentiate into various cell types within an organism [31,32]. Among the pluripotent stem cells, ESCs and iPSCs are two distinct forms: ESCs are derived during the early phases of embryo development, while iPSCs are generated by reprogramming genes, a process that reverses terminally differentiated somatic cells into a pluripotent state [14,20]. iPSC-derived cells offer a targeted examination of cellular physiology, rendering them valuable for activities such as drug screening, disease analysis, and regenerative medicine. Furthermore, the utilization of mature endothelial cells, derived from iPSCs via differentiation, holds potential for disease modeling and organ development [33]. However, even with well-trained researchers adept at consistently cultivating iPSCs and conducting differentiation experiments into desired cell types, significant time and budget allocation are required for this research. Therefore, trained AI is essential to effectively assist experimenters during the iPSC production phase. Table 1 presents examples of CNN applications in the field of biology and identifies the various cell types and/or disease categories tested in each application. The rest of this section details some of these studies and the major advancement in this field.

Table 1.
Applications of CNNs in the stem cell field.
The DNN model introduced by Christiansen et al. [61] can predict cell types and the location of cell nuclei from microscopic images without conducting cell immunology experiments. Segmentation based on CNNs can classify images pixel by pixel, assigning each pixel to a particular object category. Additionally, the CNN model enables the detection of object boundaries and categorization within boundary-delineated areas. Consequently, semantic segmentation finds extensive application in fields like cell biology and medicine, not only for identifying the cell’s position but also for determining its categories [30].
Kim et al. [31] investigated whether a CNN algorithm, Resnet50, could distinguish subtle changes in the shapes of stem cells, including ESCs and iPSCs, under different culture conditions. These conditions included a medium containing leukemia inhibitory factor (LIF) to maintain pluripotency, a medium without LIF, and a medium with insulin/transferrin/selenium (ITS) to induce differentiation. Data obtained from transmitted light microscopy capturing changes in cell morphologies over a 24-h period were utilized, and the algorithm demonstrated an accuracy of over 95% in identifying the culture conditions and cell types solely based on cell morphology.
Edlund et al. [62] developed the LIVECell system, which can accurately classify eight different types of cells using phase-contrast microscopy images. Even without molecular labeling, this enables the visualization of not only cell types but also intracellular components, along with their localization and types [63,64]. The process of stem cell differentiation was also analyzed using AI by securing image data with a microscope. The differentiation of C2C12 cells and hematopoietic stem cells was conducted with high accuracy. Furthermore, the utilization of an RNN designed to analyze time-series data enabled accurate predictions of hematopoietic stem cell differentiation from microscopic images [65,66].
The CNN model can be employed in unlabeled cell classification systems, as demonstrated by Ugawa et al. [67], who created a ghost cell measurement system capable of identifying undifferentiated human iPSCs, iPSC-derived differentiated cells, neuroendothelial cells (NECs), and hepatic endothelial cells (HECs), and categorizing surrounding white blood cell types. Additionally, CNNs are capable of categorizing cardiac tissue contractility, molecular images, and cell morphologies [68,69,70].
Though theoretically straightforward, stem cell therapy becomes highly challenging if the cells are not stable or homogeneous. Moreover, conventional testing methods may incur more errors than accurate predictions [71]. Scientists believe that various artificial intelligence techniques, such as ML-SVM and DL-CNN, can assist in addressing these complexities and limitations, potentially serving as the key to perfecting the formula for stem cell therapy [71]. Fan et al. [69] derived iPSCs from human urine cells and utilized a CNN for colony recognition and a semi-supervised segmentation method, two essential aspects of machine learning, to understand visual information from limited labeled data, enabling the detection of colony positions and boundaries.
A vector-based CNN (V-CNN) model was utilized to identify iPSC colonies using phase-contrast images [72]. The input data were healthy and unhealthy iPSC colonies, and the training results of an SVM classifier model and the V-CNN model were compared. The results showed that the V-CNN model could detect the quality of iPSC colonies with an accuracy of 95.5%, while the SVM classifier exhibited an accuracy of 75.2%. Thus, the V-CNN model greatly outperformed the SVM classifier.
A CNN model was also employed to precisely distinguish between stable PSCs and those undergoing early differentiation [32]. Images were acquired at multiple time points within the 24-h period following the onset of differentiation and used to train the CNN model. The training results showed that the CNN model could identify differentiating PSCs with high accuracy just 20 min after the onset of differentiation, distinguishing between undifferentiated and differentiating cells with over 99% accuracy within 24 h.
Recently, an interesting paper focused on discerning morphological distinctions between cells derived from Parkinson’s disease patients and healthy individuals [73]. Parkinson’s disease has a variety of causes and progression patterns, with significant differences among patients. The team divided patients’ cells into five classes, comprising a group of healthy control neurons and four different disease subtype neuron groups. Then, the group trained the model using microscopy image data from all groups obtained after multidimensional fluorescent labeling. This model achieved an accuracy of 82% using tabular data based on the fluorescent cell imagery, while utilizing the microscopy images themselves as the input data resulted in an accuracy of 95%. The model demonstrated consistent accuracy across all Parkinson’s disease subtypes used in the experiment, suggesting that beyond diagnosis and drug discovery, AI technology could be used to directly identify the pathological mechanisms of Parkinson’s disease.
3. AI Technology in Medical Image Analysis and New Drug Development
3.1. Image Analysis
Medical imaging, crucial for clinical diagnosis and disease treatment, plays a pivotal role in healthcare by generating visual data of the human body. Artificial intelligence has emerged as a leading technology for the analysis of medical imaging and big data [74].
In the realm of medical imaging analysis for disease diagnosis, CNN stands out as the most successful and commonly used deep learning model. This model’s widespread adoption in medical image research is primarily attributed to its remarkable performance, made possible by its utilization of graphics processing units (GPUs). Numerous studies have actively employed CNNs to predict and diagnose various diseases, including research projects utilizing MRI images to predict Alzheimer’s disease, analyzing CT images to identify pancreatic cancer, and focusing on the early diagnosis of breast cancer [75,76,77]. The application of CNNs plays a crucial role in predicting and treating a diverse range of diseases. They facilitate efficient diagnosis and the prescription of treatments by analyzing image data through segmentation and classification tasks, enabling clinicians to make effective diagnostic and treatment decisions based on a detailed analysis of visual information [28]. Table 2 elucidates instances of CNN utilization within the biomedical domain, serving as exemplary cases for research and clinical applications.

Table 2.
Applications of CNNs in the biomedical field.
3.2. New Drug Development
In the field of drug discovery, AI utilizes both supervised and unsupervised learning [94]. Supervised learning involves training data using labeled information, where there are explicitly defined answers. Conversely, unsupervised learning clusters unlabeled data based on similar features to predict outcomes for new data [95]. Supervised learning techniques can be further categorized into classification and regression algorithms, while unsupervised learning techniques are divided into clustering and dimensionality reduction algorithms. Each AI technique is detailed extensively in Chen et al. [1], and Table 3 categorizes the use of AI algorithms according to different types of drug development introduced later.

Table 3.
Comparison of major AI techniques used in drug development.
3.2.1. Drug Screening
The traditional drug development process involves synthesizing and testing a large number of compounds to distinguish potential drug candidates, often taking over 10 years and costing an average of around $28 billion. Moreover, despite substantial investments, nine out of ten drug candidates fail in Phase II clinical trials and the regulatory approval process [109]. In response, powerful AI-based tools have emerged that can analyze large compound datasets to predict which treatments will work best for specific diseases. Databases such as ChEMBL, ChemDB, the Collection of Open Natural Products (COCONUT), the Drug–Gene Interaction Database (DGIdb), DrugBank, the Drug Target Commons (DTC), and the Intelligent Network Pharmacology Platform Unique for Traditional Chinese Medicine (INPUT) freely provide diverse information, including molecule names, molecular structures, structural characteristics, bioactive molecules, chemical compounds, bioactivity, and genetic data [110,111,112,113,114,115,116].
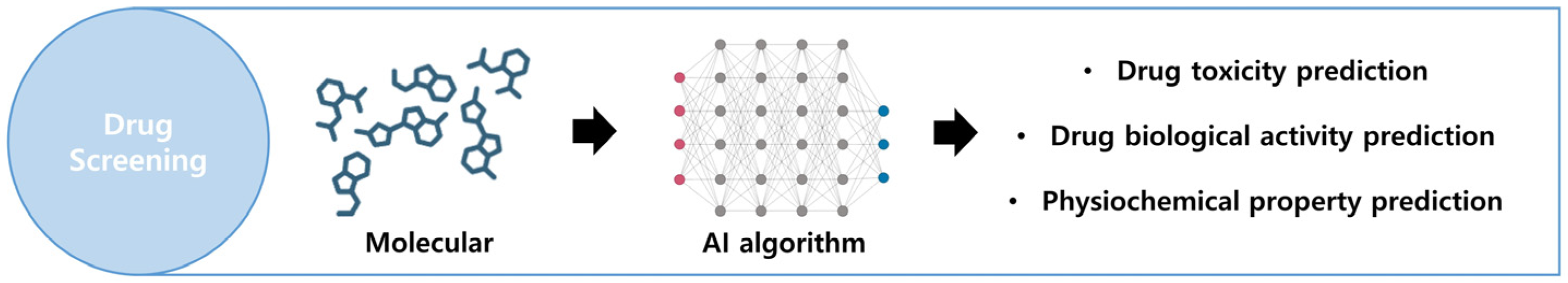
In drug screening, AI is employed to predict the toxicity, biological activity, and physicochemical properties of prospective novel drugs (Figure 5). The utilized algorithms encompass nearest-neighbor classifiers, random forest (RF), extreme learning machines, support vector machines (SVMs), and deep neural networks (DNNs). These computational methods are applied in VS with a focus on synthetic feasibility, offering predictions of in vivo activity and toxicity [117,118]. While these predictions demonstrate high accuracy, the cost of screening potential drug candidates from a vast array of natural compounds remains expensive. Several pharmaceutical companies, including Bayer, Roche, and Pfizer collaborate with IT companies to advance the development of diverse therapeutics solutions. The following subsections delve into the facets of integrating AI into VS [119].
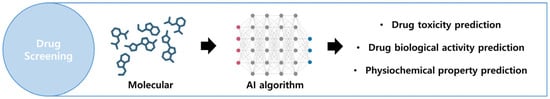
Figure 5.
Overview of the drug screening process. By using AI technology to analyze the molecular structure of specific drugs, it is possible to predict drug usage. Created with BioRender.com.
Drug Toxicity Prediction
It is essential to predict the toxicity of drug molecules. Various advanced artificial intelligence techniques are used to identify substances that may have harmful effects on humans. Multiple computational methods were utilized to assess the toxicity of 12,707 environmental compounds and drugs in the Tox21 Challenge [120]. An ML algorithm named “DeepTox” is an ensemble model designed for predicting compound toxicity that combines multiple DNNs [121]. It encodes molecular shapes using 0D to 3D molecular structure models as the inputs for the DNNs. Comparative results based on the Tox21 dataset indicate that DeepTox, with 2500 toxicophore features, outperforms its competitors in toxicity prediction [121,122].
Drug Biological Activity Prediction
Predictions of the biological activity of prospective drugs are widely utilized in areas such as anticancer, antiviral, and antimicrobial drug development, playing a significant role in pharmaceutical discovery [123,124]. The efficacy of drug molecules is determined by their affinity for a target protein or receptor. However, novel drug molecules can also exhibit toxicity due to unintended interactions with target and nontarget proteins or receptors. Therefore, predicting drug–target binding affinity (DTBA) is crucial. Initially, artificial intelligence can be used to measure the binding affinity of a drug by considering the features or similarities between the drug and its target. Subsequently, recognizing the chemical components of the drug and its target is essential for determining feature vectors [125]. Various strategies, including ML and DL approaches such as KronRLS, SimBoost, DeepDTA, and PADME, have been employed to determine DTBA.
For example, Shen et al. [126] employed AutoMolDesigner to automatically design new antibiotics by considering compounds’ structures and properties, addressing challenges such as antibiotic scarcity, inhibitory effects, and antibiotic resistance. This open-source tool has proven valuable for researchers seeking to develop novel antibiotics [126].
Physiochemical Property Prediction
The diverse nature of drug physicochemical properties, which includes solubility, partition coefficient (logP), degree of ionization, and intrinsic permeability, necessitates an indirect yet essential understanding of drug action [127]. In particular, the solubility of a drug has a significant impact on its pharmaceutical efficacy, influencing both the pharmacokinetic properties and the formulation of the drug [128]. Considerable investment has been made in developing AI-based solubility prediction models that leverage large datasets of physicochemical properties generated during compound optimization during training with DL models such as DNN or CNN [120].
Panapitiya et al. [129] compared various deep learning architectures, including fully connected neural networks, recurrent neural networks (RNNs), graph neural networks, and SchNet, presenting the strengths and weaknesses of each model. Fully connected neural networks, leveraging molecular descriptors, demonstrated the best performance in solubility prediction.
3.2.2. Key Areas for Drug Discovery
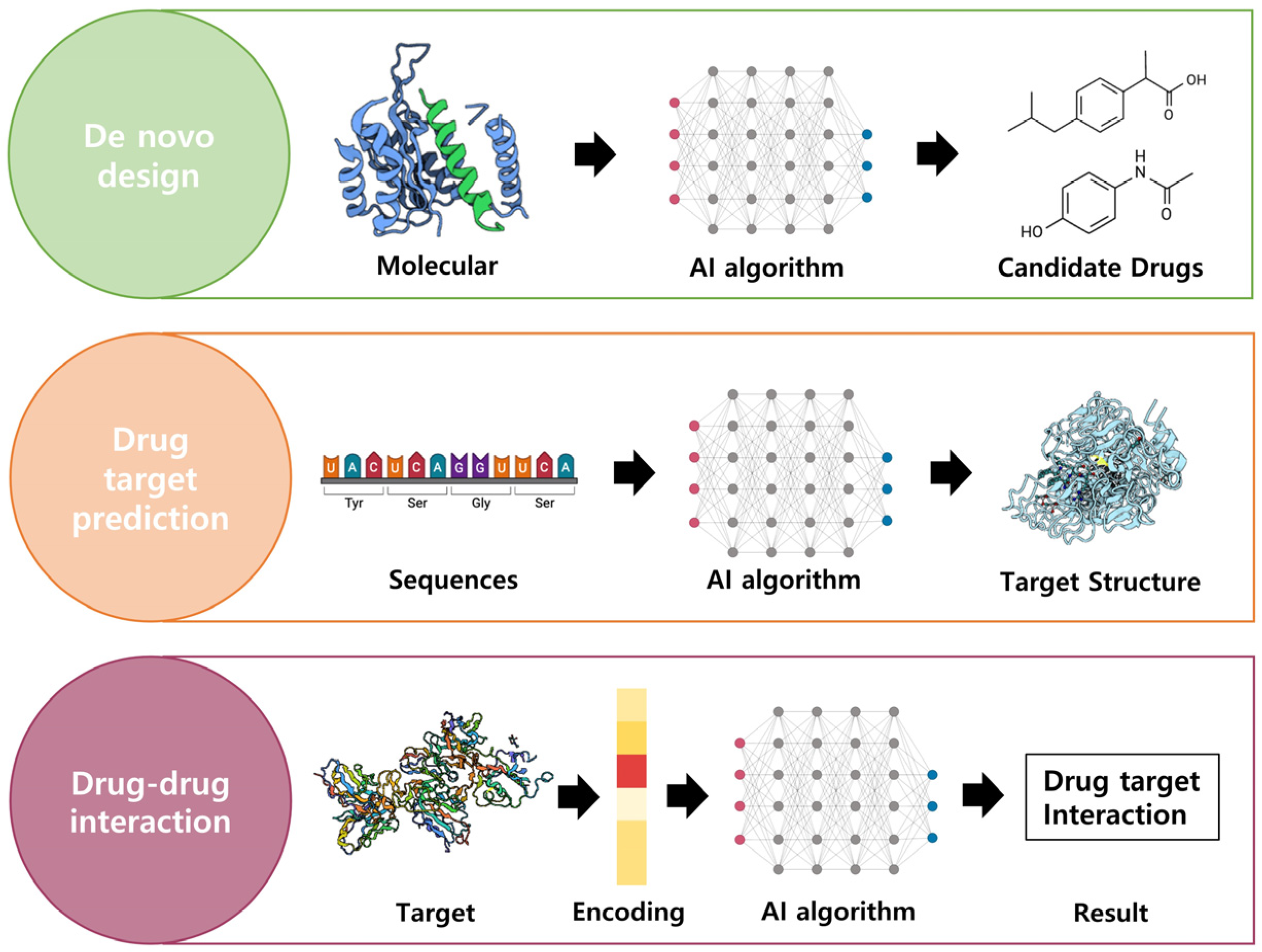
The pursuit of drug discovery involves the identification of active compounds that exhibit therapeutic effects for specific diseases. This subsection aims to elucidate the fundamental elements necessary for drug discovery, highlighting the utilization of AI in key areas such as de novo drug design, target structure prediction, and drug–target interaction (DTI) prediction. Figure 6 illustrates the application of AI technology in drug discovery, as previously discussed.
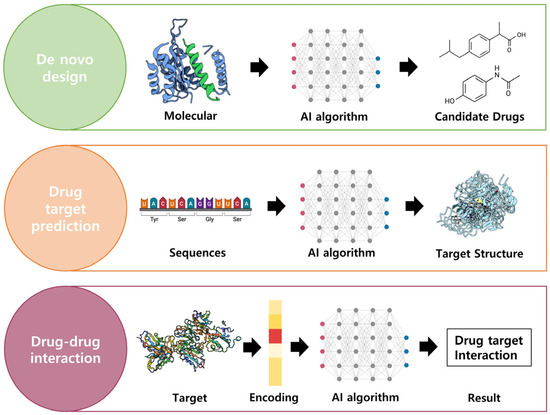
Figure 6.
AI technologies to discover drugs. There are de novo design, drug target prediction, and drug–drug interaction elements to drug discovery. By utilizing AI technologies, it is possible to predict the structure of the desired drug target and determine the potential interactions. Created with BioRender.com.
De Novo Drug Design
Artificial intelligence can aid in designing new molecules optimized for specific therapeutic applications, significantly improving the drug discovery process. Computer-aided drug design methods (CADD) that utilize computers to develop drugs have recently become prominent [130]. De novo drug design, a technique for generating novel molecular structures, has garnered significant attention, and various artificial neural network models, such as the reinforcement-learning-based ReLeaSE, the encoder–decoder-based ChemVAE, the GAN-based GraphINVENT, and the RNN-based MolRNN, have been applied in de novo drug design [131,132,133,134]. Molecular representation plays a pivotal role in de novo drug design, with inputs for deep learning algorithms derived from simplified molecular-input line-entry system (SMILES), fingerprint, molecular-graph, and 3D geometry data [107,135]. Furthermore, when the structure of a receptor is known, molecular docking information is used, and in cases where the receptor’s structure is unknown, quantitative structure–activity relationship (QSAR) and pharmacophore modeling can be employed to predict the 3D structure of the receptor. More recently, deep learning technology has also been applied to various aspects of drug discovery and development [136,137].
Drug Target Prediction
Drug target prediction has, to date, deciphered the structures of approximately 100,000 proteins, but this accounts for only a small fraction of the known protein universe [138]. However, succeeding in drug development with AI still requires addressing challenges such as difficulties in understanding protein tertiary structures. Predicting the 3D structure of proteins, which may consist of thousands of amino acids, demands significant time and resources [95]. To more efficiently predict protein structures, DeepMind has developed the neural-network-based tool AlphaFold, which can predict the 3D structure of proteins from amino acid sequences [138,139].
Predicting Drug–Drug Interactions
Drug target interaction (DTI) prediction, assessing the interactions between compounds and protein targets in an organism, is widely utilized through deep learning and is an essential process in drug development [140]. Prediction methods for DTI using biological data can be categorized into five approaches: ligand-based methods, docking simulations, genetic-algorithm-based methods, text-mining-based methods, and network-based methods [141].
Initially, the encoding of compounds and proteins is carried out using their respective features. Subsequently, the input for the deep learning methods involves the use of the feature embeddings of both compounds and proteins. Models based on deep confidence neural networks, CNNs, and multi-layer perceptrons are commonly employed for DTI prediction [125,142,143].
In in silico drug development, accurately predicting drug–protein interactions is a crucial step. This is essential for understanding the success of treatments and the efficacy and effects of drugs [128]. However, large-scale predictions for countless unknown interactions can involve complex processes. Therefore, semi-supervised learning techniques, primarily utilizing technologies that integrate compound structures, drug–protein interaction network data, and genome sequence data are commonly used [144].
Dhakal et al. [145] discussed that protein–ligand binding sites, ligand binding affinity, and binding structures can be predicted using various machine learning and deep learning techniques. Particularly noteworthy is the discussion on addressing data imbalance issues, where methods like multiple random undersampling and classifier ensembles are introduced to balance sample distributions and reduce information loss. Furthermore, the prediction accuracy is improved by leveraging successful prior research that used convolutional and recurrent neural network architectures to predict and interpret protein structures. The introduction of techniques such as the RF method for enhancing the prediction of ligand binding affinities and the utilization of various algorithms (RF, SVM, neural networks) is also highlighted.
Yaseen et al. [146] focused on predicting drug–target interactions based on text, utilizing data from drug databases and the drug–drug interaction corpus (DDI corpus). Both the CNN model and SVM used in this study demonstrated high performance, achieving excellent accuracy even when using an ensemble model. The study employed CNN models and machine-learning-based classifier SVMs. Both the single CNN and SVM models demonstrated high performances, achieving excellent accuracy even when using an ensemble model. Specifically, the single CNN model showed an F1-score of 0.82, and the ensemble model achieved an impressive 96.72% approved accuracy. The SVM model, in the machine-learning-based implementation, faced challenges due to the availability of negative DTI data but yielded good results in terms of area under the ROC curve (AUC) values. The paper also discusses techniques for addressing class imbalance issues and introduces threshold moving in ensemble models. Suggested future directions include integrating pre-trained embedding layers and position embeddings for improved performance.
4. Discussion
Over the past few years, the integration of AI technology into stem cell therapy, regenerative medicine, and drug development has made significant progress. Artificial intelligence has played a crucial role in recognizing and analyzing vast amounts of data that would be extremely challenging for humans, contributing greatly to the advancement of these fields [147]. However, there are many technological challenges to address before fully realizing the potential of AI in this field.
One of the limitations of AI technology in the fields of stem cell therapy, regenerative medicine, and drug development is the need for large-scale and high-quality data [124]. However, obtaining vast amounts of data is difficult for small patient populations, such as those with rare diseases [148].
In the field of stem cell research, experiments involving the stable culture of stem cells and their differentiation into desired cell types are time-consuming and costly, with inconsistent outcomes. These limitations can be addressed by incorporating AI technology. AI can analyze cell images to predict cell states and improve cell quality. These advantages can overcome the existing challenges of stem cell therapies, making them more effective [8]. In addition, the use of AI technology could ultimately bring about a paradigm shift in tests for development of the therapeutic stem cells, which require accurate and systematic technology in both preclinical and clinical trials.
The stem cells developed for patient treatment currently require substantial time and support. Continuous collaboration among researchers, healthcare providers, and AI developers is essential to continually advance AI technology in the field and achieve more effective personalized treatment solutions [149]. With the increasing availability of high-quality data, the opportunity to fine-tune and customize AI algorithms specifically for regenerative purposes will expand. The machine learning methods used on cells derived from patients with specific diseases have demonstrated high accuracy in predicting disease states, suggesting that the accurate classification capabilities of AI technology could revolutionize disease diagnosis, drug discovery, and the identification of pathological mechanisms in the future [73].
Furthermore, while many AI-based models are being developed, most of them lack freely available web servers or source codes. Even if some smart tools are developed, they are often only available commercially, restricting their application. Therefore, there is a need to develop open tools or packages that can serve as essential resources for applying these models in drug discovery and development [1].
In the field of drug development, it will be possible to predict drug side effects and develop personalized medications for individual diseases using vast amounts of data. Furthermore, the drug development process can be accelerated if collaboration among industry, academia, and regulatory agencies is established to collect, analyze, and validate large datasets.
However, AI algorithms must understand biological complexity to predict drug–target interactions, and the ability to interpret AI models is essential. In drug development, there are considerations for drug safety, efficacy, and ethical issues, requiring substantial investment in technology, infrastructure, and expertise. Despite these challenges, AI continues to advance and offers tremendous potential. As this field progresses and these obstacles are overcome, AI is expected to revolutionize the drug discovery process, leading to faster, more cost-effective, and personalized treatments, ultimately improving patient outcomes [148]. Therefore, the realization of AI’s potential may pave the way for a new era in tackling intractable diseases, ultimately contributing to the advancement of medicine and societal well-being.
Author Contributions
Conceptualization, M.K. and S.H.; methodology, M.K.; writing—original draft preparation, M.K.; writing—review and editing, S.H.; supervision, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Research Foundation of Korea, grant NRF-2020R1A2C1101294, and by Ministry of Health and Welfare of the government of the Republic of Korea, grant RS-2022-00060247.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, W.; Liu, X.; Zhang, S.; Chen, S. Artificial intelligence for drug discovery: Resources, methods, and applications. Mol. Ther.-Nucleic Acids 2023, 31, 691–702. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. The significance of artificial intelligence in drug delivery system design. Adv. Drug Deliv. Rev. 2019, 151, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef] [PubMed]
- Duch, W.; Swaminathan, K.; Meller, J. Artificial intelligence approaches for rational drug design and discovery. Curr. Pharm. Des. 2007, 13, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, X.; Pan, X.; Wang, B.; Ji, C.; Qi, Y.; Zhang, J.Z. HobPre: Accurate prediction of human oral bioavailability for small molecules. J. Cheminform. 2022, 14, 1. [Google Scholar] [CrossRef]
- Agrawal, M.; Alexander, A.; Khan, J.; Giri, T.K.; Siddique, S.; Dubey, S.K.; Patel, R.J.; Gupta, U.; Saraf, S.; Saraf, S. Recent biomedical applications on stem cell therapy: A brief overview. Curr. Stem Cell Res. Ther. 2019, 14, 127–136. [Google Scholar] [CrossRef]
- Mukherjee, S.; Yadav, G.; Kumar, R. Recent trends in stem cell-based therapies and applications of artificial intelligence in regenerative medicine. World J. Stem Cells 2021, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Ntege, E.H.; Sunami, H.; Shimizu, Y. Advances in regenerative therapy: A review of the literature and future directions. Regen. Ther. 2020, 14, 136–153. [Google Scholar] [CrossRef]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Pardal, R.; Morrison, S.J. Diverse mechanisms regulate stem cell self-renewal. Curr. Opin. Cell Biol. 2004, 16, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.-Y.; Chen, T.-T.; Tsai, E.-T.; Hsiao, Y.-J.; Lee, N.; Gao, C.-E.; Yang, Y.-P.; Chen, S.-J.; Yarmishyn, A.A.; Hwang, D.-K. Recognizing the Differentiation Degree of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cells Using Machine Learning and Deep Learning-Based Approaches. Cells 2023, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Nosrati, M. Artificial intelligence in regenerative medicine: Applications and implications. Biomimetics 2023, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2012, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Romito, A.; Cobellis, G. Pluripotent stem cells: Current understanding and future directions. Stem Cells Int. 2016, 2016, 9451492. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Altyar, A.E.; El-Sayed, A.; Abdeen, A.; Piscopo, M.; Mousa, S.A.; Najda, A.; Abdel-Daim, M.M. Future regenerative medicine developments and their therapeutic applications. Biomed. Pharmacother. 2023, 158, 114131. [Google Scholar] [CrossRef]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Rajabzadeh, N.; Fathi, E.; Farahzadi, R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Jiang, Y. Endogenous pancreatic β cell regeneration: A potential strategy for the recovery of β cell deficiency in diabetes. Front. Endocrinol. 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.I.; Costa-Almeida, R.; Gershovich, P.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Cell-based approaches for tendon regeneration. In Tendon Regeneration; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–203. [Google Scholar]
- Farini, A.; Sitzia, C.; Erratico, S.; Meregalli, M.; Torrente, Y. Clinical applications of mesenchymal stem cells in chronic diseases. Stem Cells Int. 2014, 2014, 306573. [Google Scholar] [CrossRef] [PubMed]
- Munir, H.; McGettrick, H.M. Mesenchymal stem cell therapy for autoimmune disease: Risks and rewards. Stem Cells Dev. 2015, 24, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, R.R.; Abd Hamid, Z.; Zaki, W.M.D.W.; Huddin, A.B.; Mathialagan, R. Stem cell imaging through convolutional neural networks: Current issues and future directions in artificial intelligence technology. PeerJ 2020, 8, e10346. [Google Scholar] [CrossRef] [PubMed]
- Rawat, W.; Wang, Z. Deep convolutional neural networks for image classification: A comprehensive review. Neural Comput. 2017, 29, 2352–2449. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, D.; Yuasa, S. The application of convolutional neural network to stem cell biology. Inflamm. Regen. 2019, 39, 14. [Google Scholar] [CrossRef]
- Kim, M.; Namkung, Y.; Hyun, D.; Hong, S. Prediction of Stem Cell State Using Cell Image-Based Deep Learning. Adv. Intell. Syst. 2023, 5, 2300017. [Google Scholar] [CrossRef]
- Waisman, A.; La Greca, A.; Möbbs, A.M.; Scarafía, M.A.; Velazque, N.L.S.; Neiman, G.; Moro, L.N.; Luzzani, C.; Sevlever, G.E.; Guberman, A.S. Deep learning neural networks highly predict very early onset of pluripotent stem cell differentiation. Stem Cell Rep. 2019, 12, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chu, L.-F.; Hou, Z.; Schwartz, M.P.; Hacker, T.; Vickerman, V.; Swanson, S.; Leng, N.; Nguyen, B.K.; Elwell, A. Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proc. Natl. Acad. Sci. USA 2017, 114, E6072–E6078. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, D.; Lachmann, M.; Kunihiro, T.; Yuasa, S.; Kishino, Y.; Kimura, M.; Katsuki, T.; Itoh, S.; Seki, T.; Fukuda, K. Automated deep learning-based system to identify endothelial cells derived from induced pluripotent stem cells. Stem Cell Rep. 2018, 10, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Theagarajan, R.; Guan, B.X.; Bhanu, B. DeephESC: An automated system for generating and classification of human embryonic stem cells. In Proceedings of the 2018 24th International Conference on Pattern Recognition (ICPR), Beijing, China, 20–24 August 2018; pp. 3826–3831. [Google Scholar]
- Orita, K.; Sawada, K.; Koyama, R.; Ikegaya, Y. Deep learning-based quality control of cultured human-induced pluripotent stem cell-derived cardiomyocytes. J. Pharmacol. Sci. 2019, 140, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Abe, K.; Yokota, H.; Sudo, K.; Nakamura, Y.; Tsai, M.-D. Human induced pluripotent stem cell region detection in bright-field microscopy images using convolutional neural networks. Biomed. Eng. Appl. Basis Commun. 2019, 31, 1950009. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Abe, K.; Yokota, H.; Sudo, K.; Nakamura, Y.; Chu, S.-L.; Hsu, C.-Y.; Tsai, M.-D. Human induced pluripotent stem cell reprogramming prediction in microscopy images using LSTM based RNN. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 2416–2419. [Google Scholar]
- Chen, Y.-C.; Zhang, Z.; Yoon, E. Early prediction of single-cell derived sphere formation rate using convolutional neural network image analysis. Anal. Chem. 2020, 92, 7717–7724. [Google Scholar] [CrossRef]
- Kegeles, E.; Naumov, A.; Karpulevich, E.A.; Volchkov, P.; Baranov, P. Convolutional neural networks can predict retinal differentiation in retinal organoids. Front. Cell. Neurosci. 2020, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.F.; Lu, Y.; Oh, S.; Conduit, G.J. Machine learning to predict mesenchymal stem cell efficacy for cartilage repair. PLoS Comput. Biol. 2020, 16, e1008275. [Google Scholar] [CrossRef]
- Ahmadzadeh, E.; Jaferzadeh, K.; Shin, S.; Moon, I. Automated single cardiomyocyte characterization by nucleus extraction from dynamic holographic images using a fully convolutional neural network. Biomed. Opt. Express 2020, 11, 1501–1516. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Chen, Z.; He, Y.; Liu, Q.; Huang, D.-S. Locating transcription factor binding sites by fully convolutional neural network. Brief. Bioinform. 2021, 22, bbaa435. [Google Scholar]
- Hirose, T.; Kotoku, J.i.; Toki, F.; Nishimura, E.K.; Nanba, D. Label-free quality control and identification of human keratinocyte stem cells by deep learning-based automated cell tracking. Stem Cells 2021, 39, 1091–1100. [Google Scholar] [CrossRef]
- Dursun, G.; Tandale, S.B.; Gulakala, R.; Eschweiler, J.; Tohidnezhad, M.; Markert, B.; Stoffel, M. Development of convolutional neural networks for recognition of tenogenic differentiation based on cellular morphology. Comput. Methods Programs Biomed. 2021, 208, 106279. [Google Scholar] [CrossRef]
- Yan, R.; Fan, C.; Yin, Z.; Wang, T.; Chen, X. Potential applications of deep learning in single-cell RNA sequencing analysis for cell therapy and regenerative medicine. Stem Cells 2021, 39, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Au Yeung, W.K.; Maruyama, O.; Sasaki, H. A convolutional neural network-based regression model to infer the epigenetic crosstalk responsible for CG methylation patterns. BMC Bioinform. 2021, 22, 341. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Nakatsuka, R.; Fujioka, T. Automatic discrimination of human hematopoietic tumor cell lines using a combination of imaging flow cytometry and convolutional neural network. Hum. Cell 2021, 34, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, R.; Wu, Z.; Song, S.; Cheng, L.; Zhu, R. Deep learning-based predictive identification of neural stem cell differentiation. Nat. Commun. 2021, 12, 2614. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Jeon, J.H.; Park, K.; Kim, S.W.; Kim, D.H.; Lee, S. High throughput screening of mesenchymal stem cell lines using deep learning. Sci. Rep. 2022, 12, 17507. [Google Scholar] [CrossRef]
- Mai, M.; Luo, S.; Fasciano, S.; Oluwole, T.E.; Ortiz, J.; Pang, Y.; Wang, S. Morphology-based deep learning approach for predicting adipogenic and osteogenic differentiation of human mesenchymal stem cells (hMSCs). Front. Cell Dev. Biol. 2023, 11, 1329840. [Google Scholar] [CrossRef]
- Chu, S.-L.; Sudo, K.; Yokota, H.; Abe, K.; Nakamura, Y.; Tsai, M.-D. Human induced pluripotent stem cell formation and morphology prediction during reprogramming with time-lapse bright-field microscopy images using deep learning methods. Comput. Methods Programs Biomed. 2023, 229, 107264. [Google Scholar] [CrossRef]
- Lan, Y.; Huang, N.; Fu, Y.; Liu, K.; Zhang, H.; Li, Y.; Yang, S. Morphology-based deep learning approach for predicting osteogenic differentiation. Front. Bioeng. Biotechnol. 2022, 9, 802794. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.; Yon, J.-M.; Kim, S.W.; Lee, S.Y.; Jeong, I.; Jang, J.; Lee, S.; Cho, D.-W. Predicting multipotency of human adult stem cells derived from various donors through deep learning. Sci. Rep. 2022, 12, 21614. [Google Scholar] [CrossRef] [PubMed]
- Hanai, Y.; Ishihata, H.; Zhang, Z.; Maruyama, R.; Kasai, T.; Kameda, H.; Sugiyama, T. Temporal and Locational Values of Images Affecting the Deep Learning of Cancer Stem Cell Morphology. Biomedicines 2022, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Marzec-Schmidt, K.; Ghosheh, N.; Stahlschmidt, S.R.; Küppers-Munther, B.; Synnergren, J.; Ulfenborg, B. Artificial intelligence supports automated characterization of differentiated human pluripotent stem cells. Stem Cells 2023, 41, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, A.; Krasnova, O.; Khvorova, I.; Kozlov, K.; Gursky, V.; Samsonova, M.; Tikhonova, O.; Neganova, I. Quality Control of Human Pluripotent Stem Cell Colonies by Computational Image Analysis Using Convolutional Neural Networks. Int. J. Mol. Sci. 2022, 24, 140. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.; Arai, Y.; Kanda, J.; Kondo, T.; Ikegame, K.; Uchida, N.; Doki, N.; Fukuda, T.; Ozawa, Y.; Tanaka, M. A convolutional neural network-based model that predicts acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Commun. Med. 2023, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Witmer, A.; Theagarajan, R.; Bhanu, B. Triplet-net Classification of Contiguous Stem Cell Microscopy Images. IEEE/ACM Trans. Comput. Biol. Bioinform. 2023, 20, 2314–2327. [Google Scholar] [CrossRef]
- He, L.; Li, M.; Wang, X.; Wu, X.; Yue, G.; Wang, T.; Zhou, Y.; Lei, B.; Zhou, G. Morphology-based deep learning enables accurate detection of senescence in mesenchymal stem cell cultures. BMC Biol. 2024, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, E.M.; Yang, S.J.; Ando, D.M.; Javaherian, A.; Skibinski, G.; Lipnick, S.; Mount, E.; O’neil, A.; Shah, K.; Lee, A.K. In silico labeling: Predicting fluorescent labels in unlabeled images. Cell 2018, 173, 792–803.e719. [Google Scholar] [CrossRef]
- Edlund, C.; Jackson, T.R.; Khalid, N.; Bevan, N.; Dale, T.; Dengel, A.; Ahmed, S.; Trygg, J.; Sjögren, R. LIVECell—A large-scale dataset for label-free live cell segmentation. Nat. Methods 2021, 18, 1038–1045. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, D.; Zhou, Y.; Yang, Y.; Liang, J.; Zhou, Y.; Li, N.; Liu, Y.; Yang, G.; Li, W. Deep learning-based morphological classification of endoplasmic reticulum under stress. Front. Cell Dev. Biol. 2022, 9, 767866. [Google Scholar] [CrossRef]
- Sarti, M.; Parlani, M.; Diaz-Gomez, L.; Mikos, A.G.; Cerveri, P.; Casarin, S.; Dondossola, E. Deep Learning for Automated Analysis of Cellular and Extracellular Components of the Foreign Body Response in Multiphoton Microscopy Images. Front. Bioeng. Biotechnol. 2022, 9, 797555. [Google Scholar] [CrossRef]
- Niioka, H.; Asatani, S.; Yoshimura, A.; Ohigashi, H.; Tagawa, S.; Miyake, J. Classification of C2C12 cells at differentiation by convolutional neural network of deep learning using phase contrast images. Hum. Cell 2018, 31, 87–93. [Google Scholar] [CrossRef]
- Buggenthin, F.; Buettner, F.; Hoppe, P.S.; Endele, M.; Kroiss, M.; Strasser, M.; Schwarzfischer, M.; Loeffler, D.; Kokkaliaris, K.D.; Hilsenbeck, O. Prospective identification of hematopoietic lineage choice by deep learning. Nat. Methods 2017, 14, 403–406. [Google Scholar] [CrossRef]
- Ugawa, M.; Kawamura, Y.; Toda, K.; Teranishi, K.; Morita, H.; Adachi, H.; Tamoto, R.; Nomaru, H.; Nakagawa, K.; Sugimoto, K. In silico-labeled ghost cytometry. eLife 2021, 10, e67660. [Google Scholar] [CrossRef]
- Juhola, M.; Joutsijoki, H.; Varpa, K.; Saarikoski, J.; Rasku, J.; Iltanen, K.; Laurikkala, J.; Hyyrö, H.; Ávalos-Salguero, J.; Siirtola, H. On computation of calcium cycling anomalies in cardiomyocytes data. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1444–1447. [Google Scholar]
- Fan, K.; Zhang, S.; Zhang, Y.; Lu, J.; Holcombe, M.; Zhang, X. A machine learning assisted, label-free, non-invasive approach for somatic reprogramming in induced pluripotent stem cell colony formation detection and prediction. Sci. Rep. 2017, 7, 13496. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Gerlich, D.W. Machine learning in cell biology–teaching computers to recognize phenotypes. J. Cell Sci. 2013, 126, 5529–5539. [Google Scholar] [CrossRef] [PubMed]
- Schaub, N.J.; Hotaling, N.A.; Manescu, P.; Padi, S.; Wan, Q.; Sharma, R.; George, A.; Chalfoun, J.; Simon, M.; Ouladi, M. Deep learning predicts function of live retinal pigment epithelium from quantitative microscopy. J. Clin. Investig. 2020, 130, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, M.S.; Kurita, T.; Park, S.-Y.; Chien, S.-I.; Bae, J.-S.; Ahn, B.-C. Deep vector-based convolutional neural network approach for automatic recognition of colonies of induced pluripotent stem cells. PLoS ONE 2017, 12, e0189974. [Google Scholar] [CrossRef]
- D’Sa, K.; Evans, J.R.; Virdi, G.S.; Vecchi, G.; Adam, A.; Bertolli, O.; Fleming, J.; Chang, H.; Leighton, C.; Horrocks, M.H. Prediction of mechanistic subtypes of Parkinson’s using patient-derived stem cell models. Nat. Mach. Intell. 2023, 5, 933–946. [Google Scholar] [CrossRef]
- Datta, S.; Barua, R.; Das, J. Application of artificial intelligence in modern healthcare system. In Alginates-Recent Uses of This Natural Polymer; InTechOpen: London, UK, 2019. [Google Scholar]
- Sarraf, S.; Tofighi, G. Classification of alzheimer’s disease using fmri data and deep learning convolutional neural networks. arXiv 2016, arXiv:1603.08631. [Google Scholar]
- Ma, H.; Liu, Z.-X.; Zhang, J.-J.; Wu, F.-T.; Xu, C.-F.; Shen, Z.; Yu, C.-H.; Li, Y.-M. Construction of a convolutional neural network classifier developed by computed tomography images for pancreatic cancer diagnosis. World J. Gastroenterol. 2020, 26, 5156. [Google Scholar] [CrossRef] [PubMed]
- Rakhlin, A.; Shvets, A.; Iglovikov, V.; Kalinin, A.A. Deep convolutional neural networks for breast cancer histology image analysis. In Proceedings of the Image Analysis and Recognition: 15th International Conference, ICIAR 2018, Proceedings 15, Póvoa de Varzim, Portugal, 27–29 June 2018; pp. 737–744. [Google Scholar]
- Hosseini-Asl, E.; Gimel’farb, G.; El-Baz, A. Alzheimer’s disease diagnostics by a deeply supervised adaptable 3D convolutional network. arXiv 2016, arXiv:1607.00556. [Google Scholar]
- Zhang, Y.-D.; Pan, C.; Chen, X.; Wang, F. Abnormal breast identification by nine-layer convolutional neural network with parametric rectified linear unit and rank-based stochastic pooling. J. Comput. Sci. 2018, 27, 57–68. [Google Scholar] [CrossRef]
- Yang, S.J.; Lipnick, S.L.; Makhortova, N.R.; Venugopalan, S.; Fan, M.; Armstrong, Z.; Schlaeger, T.M.; Deng, L.; Chung, W.K.; O’Callaghan, L. Applying deep neural network analysis to high-content image-based assays. SLAS Discov. Adv. Life Sci. R D 2019, 24, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjini, S.; Sujatha, C. Deep learning based diagnosis of Parkinson’s disease using convolutional neural network. Multimed. Tools Appl. 2020, 79, 15467–15479. [Google Scholar] [CrossRef]
- Xie, F.; Yang, J.; Liu, J.; Jiang, Z.; Zheng, Y.; Wang, Y. Skin lesion segmentation using high-resolution convolutional neural network. Comput. Methods Programs Biomed. 2020, 186, 105241. [Google Scholar] [CrossRef]
- Imamura, K.; Yada, Y.; Izumi, Y.; Morita, M.; Kawata, A.; Arisato, T.; Nagahashi, A.; Enami, T.; Tsukita, K.; Kawakami, H. Prediction model of amyotrophic lateral sclerosis by deep learning with patient induced pluripotent stem cells. Ann. Neurol. 2021, 89, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Wang, J.; Gong, S. Application of medical imaging based on deep learning in the treatment of lumbar degenerative diseases and osteoporosis with bone cement screws. Comput. Math. Methods Med. 2021, 2021, 2638495. [Google Scholar] [CrossRef]
- Jangir, S.K.; Joshi, N.; Kumar, M.; Choubey, D.K.; Singh, S.; Verma, M. Functional link convolutional neural network for the classification of diabetes mellitus. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3496. [Google Scholar] [CrossRef] [PubMed]
- Schiff, L.; Migliori, B.; Chen, Y.; Carter, D.; Bonilla, C.; Hall, J.; Fan, M.; Tam, E.; Ahadi, S.; Fischbacher, B. Integrating deep learning and unbiased automated high-content screening to identify complex disease signatures in human fibroblasts. Nat. Commun. 2022, 13, 1590. [Google Scholar] [CrossRef]
- Kodipalli, A.; Guha, S.; Dasar, S.; Ismail, T. An inception-ResNet deep learning approach to classify tumours in the ovary as benign and malignant. Expert Syst. 2022, e13215. [Google Scholar] [CrossRef]
- Reis, H.C.; Turk, V. COVID-DSNet: A novel deep convolutional neural network for detection of coronavirus (SARS-CoV-2) cases from CT and Chest X-Ray images. Artif. Intell. Med. 2022, 134, 102427. [Google Scholar] [CrossRef]
- Xu, Y.; He, X.; Xu, G.; Qi, G.; Yu, K.; Yin, L.; Yang, P.; Yin, Y.; Chen, H. A medical image segmentation method based on multi-dimensional statistical features. Front. Neurosci. 2022, 16, 1009581. [Google Scholar] [CrossRef]
- Korda, A.; Ventouras, E.; Asvestas, P.; Toumaian, M.; Matsopoulos, G.; Smyrnis, N. Convolutional neural network propagation on electroencephalographic scalograms for detection of schizophrenia. Clin. Neurophysiol. 2022, 139, 90–105. [Google Scholar] [CrossRef]
- Ackermann, M.; Jiang, J.; Russomanno, E.; Wolf, M.; Kalyanov, A. Hybrid Convolutional Neural Network (hCNN) for Image Reconstruction in Near-Infrared Optical Tomography. In Oxygen Transport to Tissue XLIII; Springer: Berlin/Heidelberg, Germany, 2022; pp. 165–170. [Google Scholar]
- Gharehbaghi, A.; Partovi, E.; Babic, A. Parralel Recurrent Convolutional Neural Network for Abnormal Heart Sound Classification. In Caring Is Sharing–Exploiting the Value in Data for Health and Innovation; IOS Press: Amsterdam, The Netherlands, 2023; p. 526. [Google Scholar]
- Kim, G.H.; Hwang, Y.J.; Lee, H.; Sung, E.-S.; Nam, K.W. Convolutional neural network-based vocal cord tumor classification technique for home-based self-prescreening purpose. BioMed. Eng. OnLine 2023, 22, 81. [Google Scholar] [CrossRef]
- Talevi, A.; Morales, J.F.; Hather, G.; Podichetty, J.T.; Kim, S.; Bloomingdale, P.C.; Kim, S.; Burton, J.; Brown, J.D.; Winterstein, A.G. Machine learning in drug discovery and development part 1: A primer. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 129–142. [Google Scholar] [CrossRef]
- Dara, S.; Dhamercherla, S.; Jadav, S.S.; Babu, C.M.; Ahsan, M.J. Machine learning in drug discovery: A review. Artif. Intell. Rev. 2022, 55, 1947–1999. [Google Scholar] [CrossRef]
- Heikamp, K.; Bajorath, J. Support vector machines for drug discovery. Expert Opin. Drug Discov. 2014, 9, 93–104. [Google Scholar] [CrossRef]
- Shi, H.; Liu, S.; Chen, J.; Li, X.; Ma, Q.; Yu, B. Predicting drug-target interactions using Lasso with random forest based on evolutionary information and chemical structure. Genomics 2019, 111, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Hammann, F.; Gutmann, H.; Vogt, N.; Helma, C.; Drewe, J. Prediction of adverse drug reactions using decision tree modeling. Clin. Pharmacol. Ther. 2010, 88, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bai, M.; Zhao, H.; Qiu, S.; Wang, Z.; Zhao, H. Drug Toxicity Classification Based on ReliefF and K-means Algorithm. In Proceedings of the 2024 12th International Conference on Intelligent Control and Information Processing (ICICIP), Nanjing, China, 8–10 March 2024; pp. 95–99. [Google Scholar]
- Yoo, C.; Shahlaei, M. The applications of PCA in QSAR studies: A case study on CCR5 antagonists. Chem. Biol. Drug Des. 2018, 91, 137–152. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Zang, T. CNN-DDI: A learning-based method for predicting drug–drug interactions using convolution neural networks. BMC Bioinform. 2022, 23, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Piao, C.; Huang, Y.; Zhang, Y.; Zhang, C.; Lu, Y.-J.; Liu, D. LDS-CNN: A deep learning framework for drug-target interactions prediction based on large-scale drug screening. Health Inf. Sci. Syst. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, Y.; Zhou, H.; Sun, Q.; Su, R. DNN-PNN: A parallel deep neural network model to improve anticancer drug sensitivity. Methods 2023, 209, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Müller, A.T.; Huisman, B.J.; Fuchs, J.A.; Schneider, P.; Schneider, G. Generative recurrent networks for de novo drug design. Mol. Inform. 2018, 37, 1700111. [Google Scholar] [CrossRef]
- Kavipriya, G.; Manjula, D. Drug–Target Interaction Prediction Model Using Optimal Recurrent Neural Network. Intell. Autom. Soft Comput. 2023, 35, 1676–1689. [Google Scholar] [CrossRef]
- Blanchard, A.E.; Stanley, C.; Bhowmik, D. Using GANs with adaptive training data to search for new molecules. J. Cheminform. 2021, 13, 14. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Afantitis, A.; Serra, A.; Fratello, M.; Papadiamantis, A.G.; Aidinis, V.; Lynch, I.; Greco, D.; Melagraki, G. Advances in de novo drug design: From conventional to machine learning methods. Int. J. Mol. Sci. 2021, 22, 1676. [Google Scholar] [CrossRef]
- Edvinsson, F.; Jonsson, V. Autonomous Drug Design with Reinforcement Learning. Master’s Thesis, Chalmers University of Technology, Göteborg, Sweden, 2023. [Google Scholar]
- Kraljevic, S.; Stambrook, P.J.; Pavelic, K. Accelerating drug discovery: Although the evolution of ‘-omics’ methodologies is still in its infancy, both the pharmaceutical industry and patients could benefit from their implementation in the drug development process. EMBO Rep. 2004, 5, 837–842. [Google Scholar] [CrossRef]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; De Veij, M.; Félix, E.; Magariños, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M. ChEMBL: Towards direct deposition of bioassay data. Nucleic Acids Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef]
- Chen, J.; Swamidass, S.J.; Dou, Y.; Bruand, J.; Baldi, P. ChemDB: A public database of small molecules and related chemoinformatics resources. Bioinformatics 2005, 21, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT online: Collection of open natural products database. J. Cheminform. 2021, 13, 2. [Google Scholar] [CrossRef]
- Freshour, S.L.; Kiwala, S.; Cotto, K.C.; Coffman, A.C.; McMichael, J.F.; Song, J.J.; Griffith, M.; Griffith, O.L.; Wagner, A.H. Integration of the Drug–Gene Interaction Database (DGIdb 4.0) with open crowdsource efforts. Nucleic Acids Res. 2021, 49, D1144–D1151. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ravikumar, B.; Alam, Z.; Rebane, A.; Vähä-Koskela, M.; Peddinti, G.; van Adrichem, A.J.; Wakkinen, J.; Jaiswal, A.; Karjalainen, E. Drug target commons: A community effort to build a consensus knowledge base for drug-target interactions. Cell Chem. Biol. 2018, 25, 224–229.e222. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, Q.; Meng, F.; Du, P.; Chen, W. INPUT: An intelligent network pharmacology platform unique for traditional Chinese medicine. Comput. Struct. Biotechnol. J. 2022, 20, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Machancoses, Ó.; Fernández-Martínez, J.L. Using artificial intelligence methods to speed up drug discovery. Expert Opin. Drug Discov. 2019, 14, 769–777. [Google Scholar] [CrossRef]
- Dana, D.; Gadhiya, S.V.; St. Surin, L.G.; Li, D.; Naaz, F.; Ali, Q.; Paka, L.; Yamin, M.A.; Narayan, M.; Goldberg, I.D. Deep learning in drug discovery and medicine; scratching the surface. Molecules 2018, 23, 2384. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.-K.; Pichika, M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today 2019, 24, 773–780. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Byrne, R.; Schneider, G.; Yang, S. Concepts of artificial intelligence for computer-assisted drug discovery. Chem. Rev. 2019, 119, 10520–10594. [Google Scholar] [CrossRef]
- Mayr, A.; Klambauer, G.; Unterthiner, T.; Hochreiter, S. DeepTox: Toxicity prediction using deep learning. Front. Environ. Sci. 2016, 3, 80. [Google Scholar] [CrossRef]
- Pu, L.; Naderi, M.; Liu, T.; Wu, H.-C.; Mukhopadhyay, S.; Brylinski, M. etoxpred: A machine learning-based approach to estimate the toxicity of drug candidates. BMC Pharmacol. Toxicol. 2019, 20, 2. [Google Scholar] [CrossRef]
- Basile, A.O.; Yahi, A.; Tatonetti, N.P. Artificial intelligence for drug toxicity and safety. Trends Pharmacol. Sci. 2019, 40, 624–635. [Google Scholar] [CrossRef]
- Lysenko, A.; Sharma, A.; Boroevich, K.A.; Tsunoda, T. An integrative machine learning approach for prediction of toxicity-related drug safety. Life Sci. Alliance 2018, 1. [Google Scholar] [CrossRef]
- Öztürk, H.; Özgür, A.; Ozkirimli, E. DeepDTA: Deep drug–target binding affinity prediction. Bioinformatics 2018, 34, i821–i829. [Google Scholar] [CrossRef]
- Shen, T.; Guo, J.; Han, Z.; Zhang, G.; Liu, Q.; Si, X.; Wang, D.; Wu, S.; Xia, J. AutoMolDesigner for Antibiotic Discovery: An AI-based Open-source Software for Automated Design of Small-molecule Antibiotics. J. Chem. Inf. Model. 2023, 64, 575–583. [Google Scholar] [CrossRef]
- Zang, Q.; Mansouri, K.; Williams, A.J.; Judson, R.S.; Allen, D.G.; Casey, W.M.; Kleinstreuer, N.C. In silico prediction of physicochemical properties of environmental chemicals using molecular fingerprints and machine learning. J. Chem. Inf. Model. 2017, 57, 36–49. [Google Scholar] [CrossRef]
- Wan, F.; Zeng, J. Deep learning with feature embedding for compound-protein interaction prediction. bioRxiv 2016, 086033. [Google Scholar] [CrossRef]
- Panapitiya, G.; Girard, M.; Hollas, A.; Murugesan, V.; Wang, W.; Saldanha, E. Predicting aqueous solubility of organic molecules using deep learning models with varied molecular representations. arXiv 2021, arXiv:2105.12638. [Google Scholar]
- Lee, J.W.; Maria-Solano, M.A.; Vu, T.N.L.; Yoon, S.; Choi, S. Big data and artificial intelligence (AI) methodologies for computer-aided drug design (CADD). Biochem. Soc. Trans. 2022, 50, 241–252. [Google Scholar] [CrossRef]
- Popova, M.; Isayev, O.; Tropsha, A. Deep reinforcement learning for de novo drug design. Sci. Adv. 2018, 4, eaap7885. [Google Scholar] [CrossRef]
- Gómez-Bombarelli, R.; Wei, J.N.; Duvenaud, D.; Hernández-Lobato, J.M.; Sánchez-Lengeling, B.; Sheberla, D.; Aguilera-Iparraguirre, J.; Hirzel, T.D.; Adams, R.P.; Aspuru-Guzik, A. Automatic chemical design using a data-driven continuous representation of molecules. ACS Cent. Sci. 2018, 4, 268–276. [Google Scholar] [CrossRef]
- Mercado, R.; Rastemo, T.; Lindelöf, E.; Klambauer, G.; Engkvist, O.; Chen, H.; Bjerrum, E.J. Graph networks for molecular design. Mach. Learn. Sci. Technol. 2021, 2, 025023. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Liu, Z. Multi-objective de novo drug design with conditional graph generative model. J. Cheminform. 2018, 10, 33. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Sun, H.; Wang, J.; Shen, C.; Weng, G.; Chai, X.; Li, H.; Cao, D.; Hou, T. Deep learning approaches for de novo drug design: An overview. Curr. Opin. Struct. Biol. 2022, 72, 135–144. [Google Scholar] [CrossRef]
- Guo, J.; Janet, J.P.; Bauer, M.R.; Nittinger, E.; Giblin, K.A.; Papadopoulos, K.; Voronov, A.; Patronov, A.; Engkvist, O.; Margreitter, C. DockStream: A docking wrapper to enhance de novo molecular design. J. Cheminform. 2021, 13, 89. [Google Scholar] [CrossRef]
- Wang, M.; Hsieh, C.-Y.; Wang, J.; Wang, D.; Weng, G.; Shen, C.; Yao, X.; Bing, Z.; Li, H.; Cao, D. Relation: A deep generative model for structure-based de novo drug design. J. Med. Chem. 2022, 65, 9478–9492. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Aderinwale, T.; Bharadwaj, V.; Christoffer, C.; Terashi, G.; Zhang, Z.; Jahandideh, R.; Kagaya, Y.; Kihara, D. Real-time structure search and structure classification for AlphaFold protein models. Commun. Biol. 2022, 5, 316. [Google Scholar] [CrossRef]
- Nag, S.; Baidya, A.T.; Mandal, A.; Mathew, A.T.; Das, B.; Devi, B.; Kumar, R. Deep learning tools for advancing drug discovery and development. 3 Biotech 2022, 12, 110. [Google Scholar] [CrossRef]
- Bagherian, M.; Sabeti, E.; Wang, K.; Sartor, M.A.; Nikolovska-Coleska, Z.; Najarian, K. Machine learning approaches and databases for prediction of drug–target interaction: A survey paper. Brief. Bioinform. 2021, 22, 247–269. [Google Scholar] [CrossRef]
- Wen, M.; Zhang, Z.; Niu, S.; Sha, H.; Yang, R.; Yun, Y.; Lu, H. Deep-learning-based drug–target interaction prediction. J. Proteome Res. 2017, 16, 1401–1409. [Google Scholar] [CrossRef]
- Lee, I.; Keum, J.; Nam, H. DeepConv-DTI: Prediction of drug-target interactions via deep learning with convolution on protein sequences. PLoS Comput. Biol. 2019, 15, e1007129. [Google Scholar] [CrossRef]
- Xia, Z.; Wu, L.-Y.; Zhou, X.; Wong, S.T. Semi-supervised drug-protein interaction prediction from heterogeneous biological spaces. BMC Syst. Biol. 2010, 4, S6. [Google Scholar] [CrossRef]
- Dhakal, A.; McKay, C.; Tanner, J.J.; Cheng, J. Artificial intelligence in the prediction of protein–ligand interactions: Recent advances and future directions. Brief. Bioinform. 2022, 23, bbab476. [Google Scholar] [CrossRef]
- Yaseen, B.T. Drug Target Interaction Prediction Using Convolutional Neural Network (CNN). In Proceedings of the 2023 5th International Congress on Human-Computer Interaction, Optimization and Robotic Applications (HORA), Istanbul, Turkey, 8–10 June 2023; pp. 1–5. [Google Scholar]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80. [Google Scholar] [CrossRef]
- Tiwari, P.C.; Pal, R.; Chaudhary, M.J.; Nath, R. Artificial intelligence revolutionizing drug development: Exploring opportunities and challenges. Drug Dev. Res. 2023, 84, 1652–1663. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).