Phosphating Modification with Metal Ions of Carbon Steel Surface to Improve the Influence of Anticorrosion Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Substrate Pre-Treatment
2.2.1. Modification of the Phosphate Film by Ni2+, Ce3+ i Ti2+ Ions
2.2.2. Preparing of Coating System with Modified Phosphate Surface
2.3. Surface Analysis Techniques
3. Results and Discussion
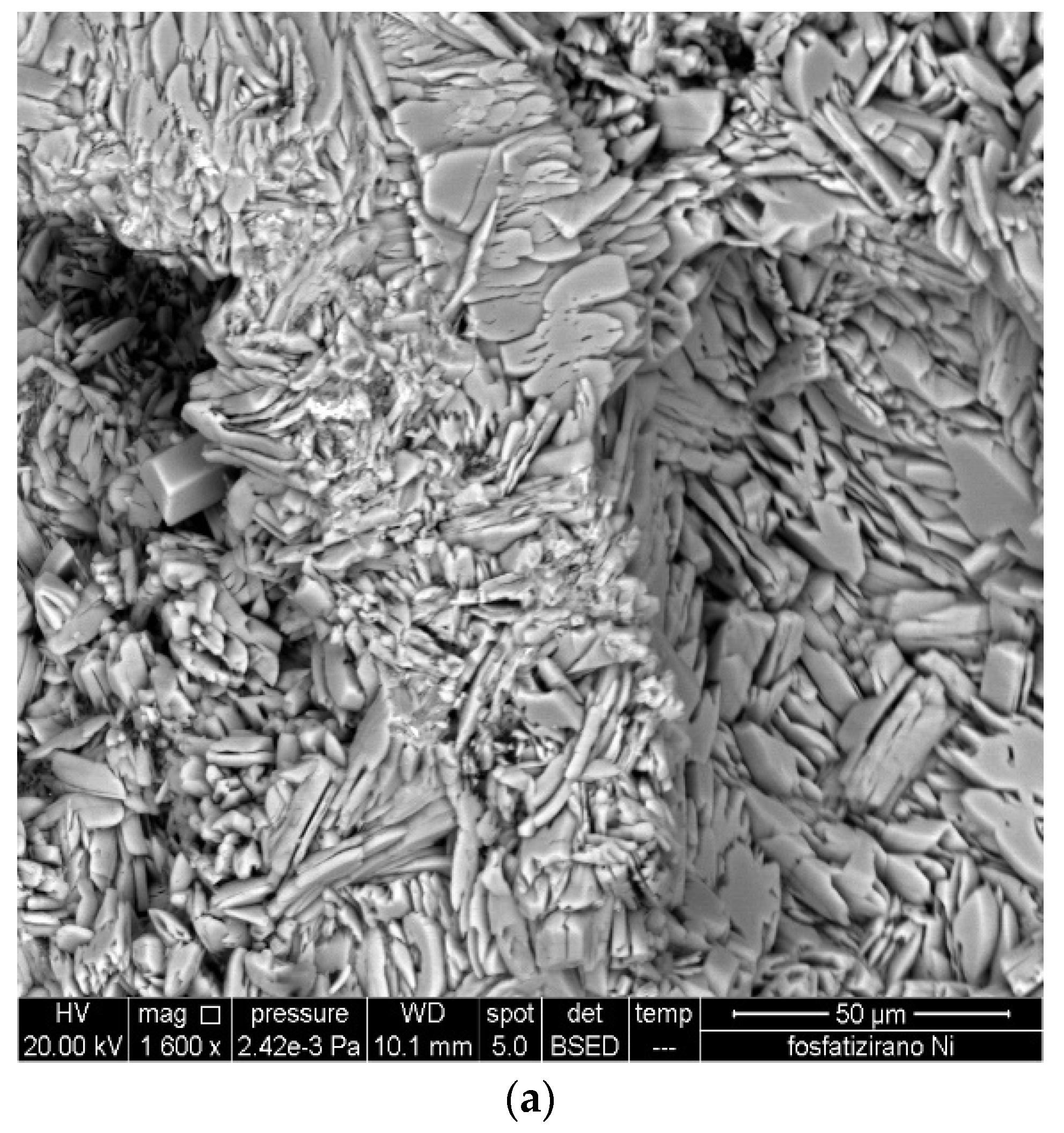
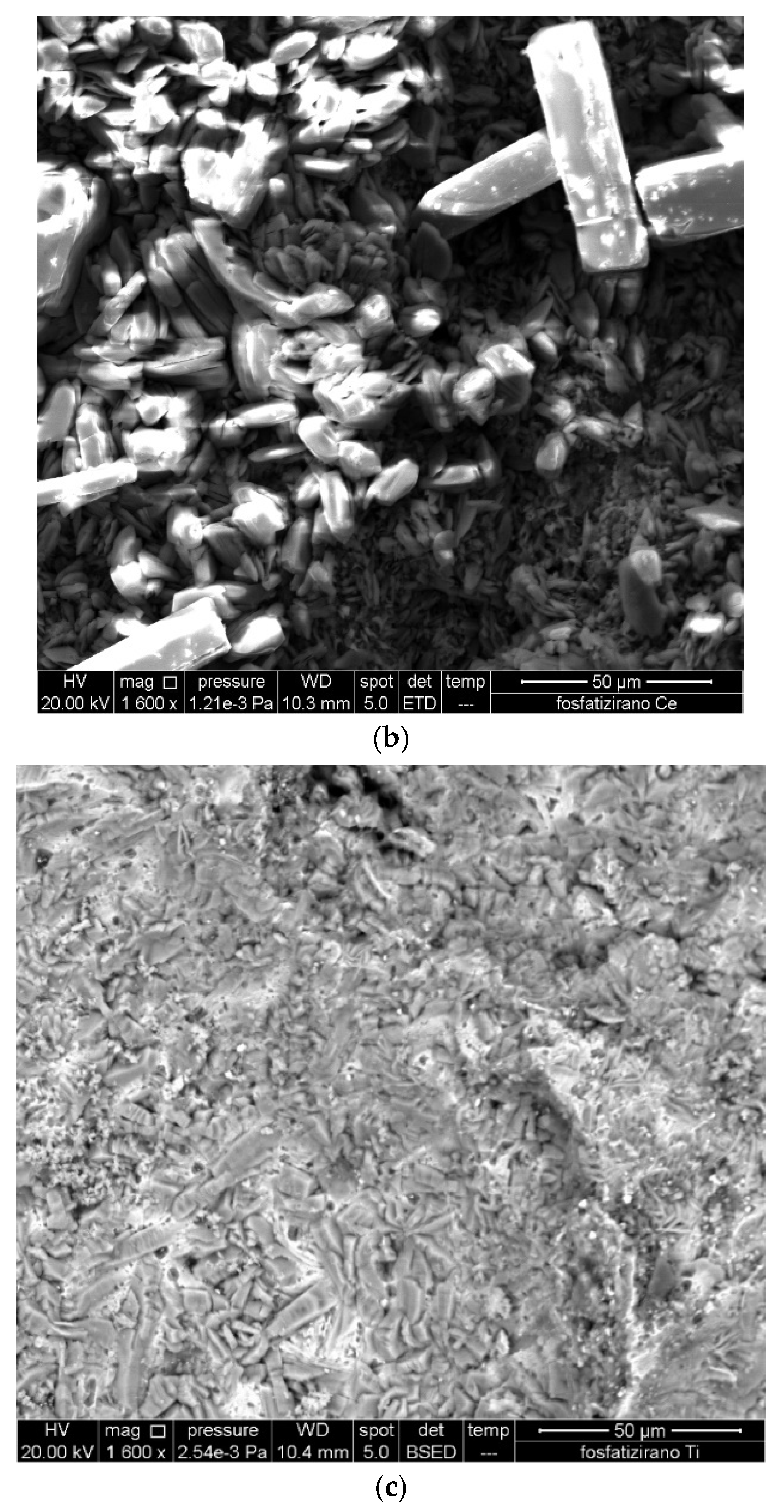
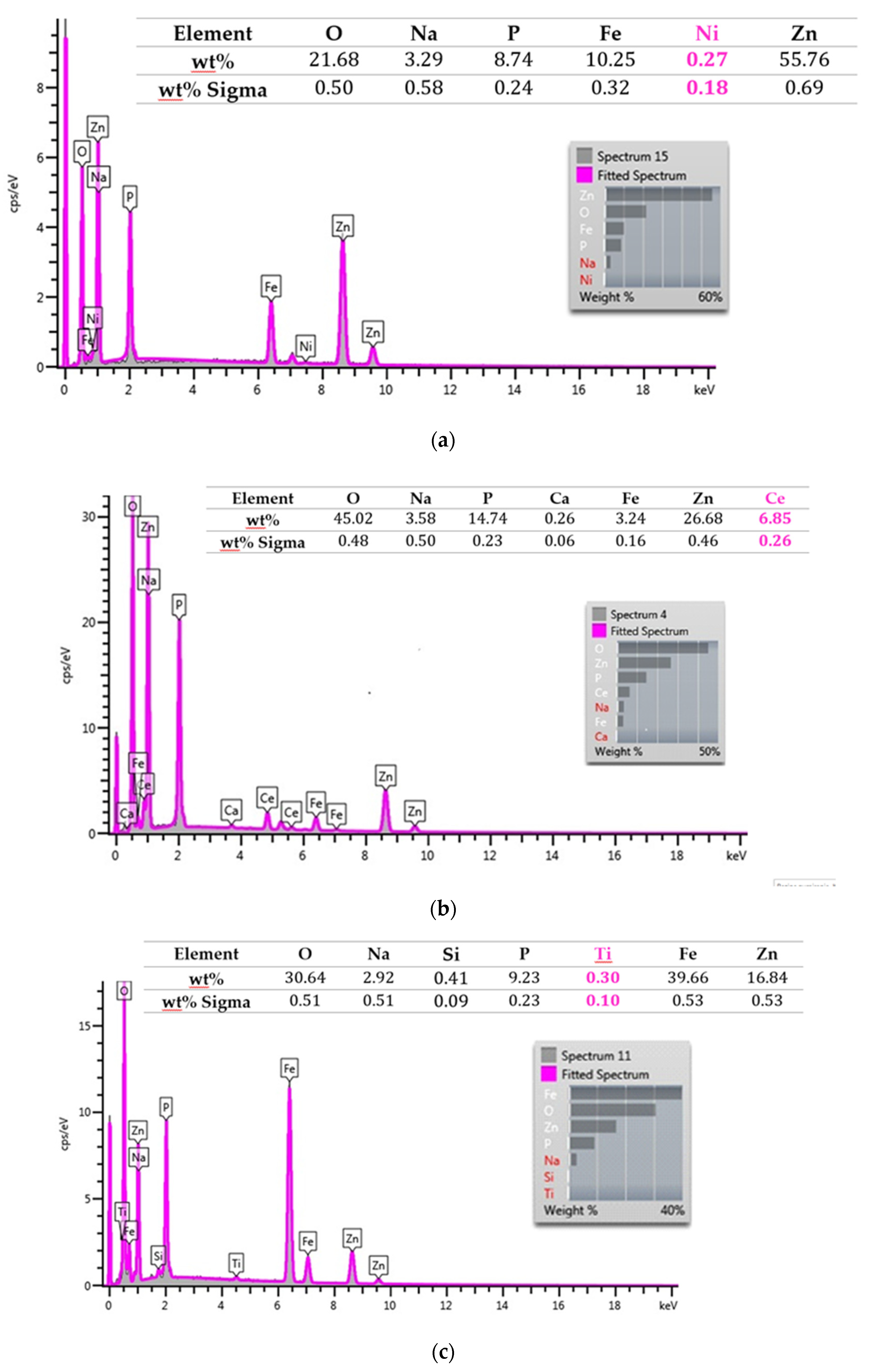
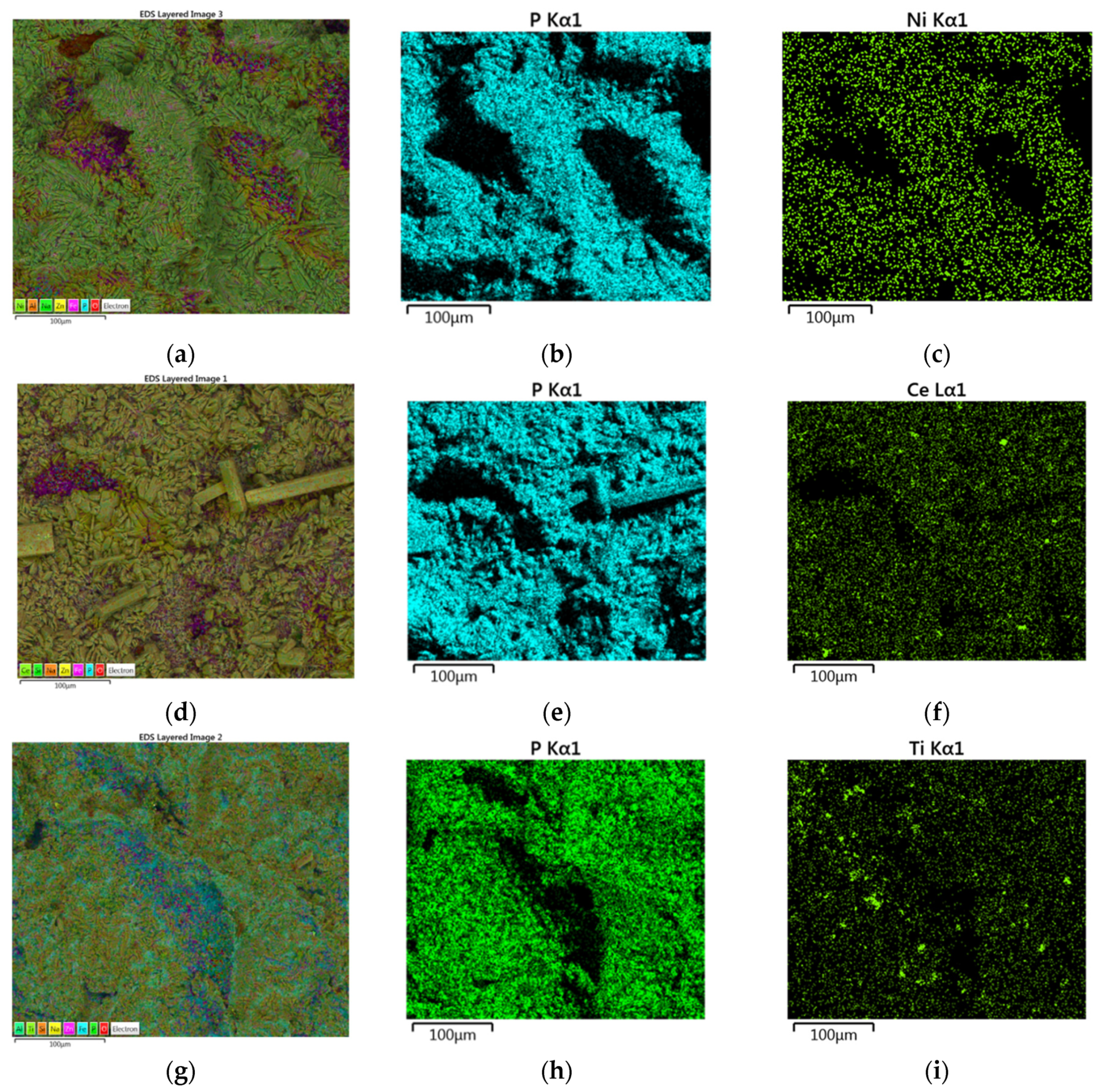
3.1. Surface Morphology of Carbon Steel
3.2. Modification of the Phosphate Film by Ce3+ Ions
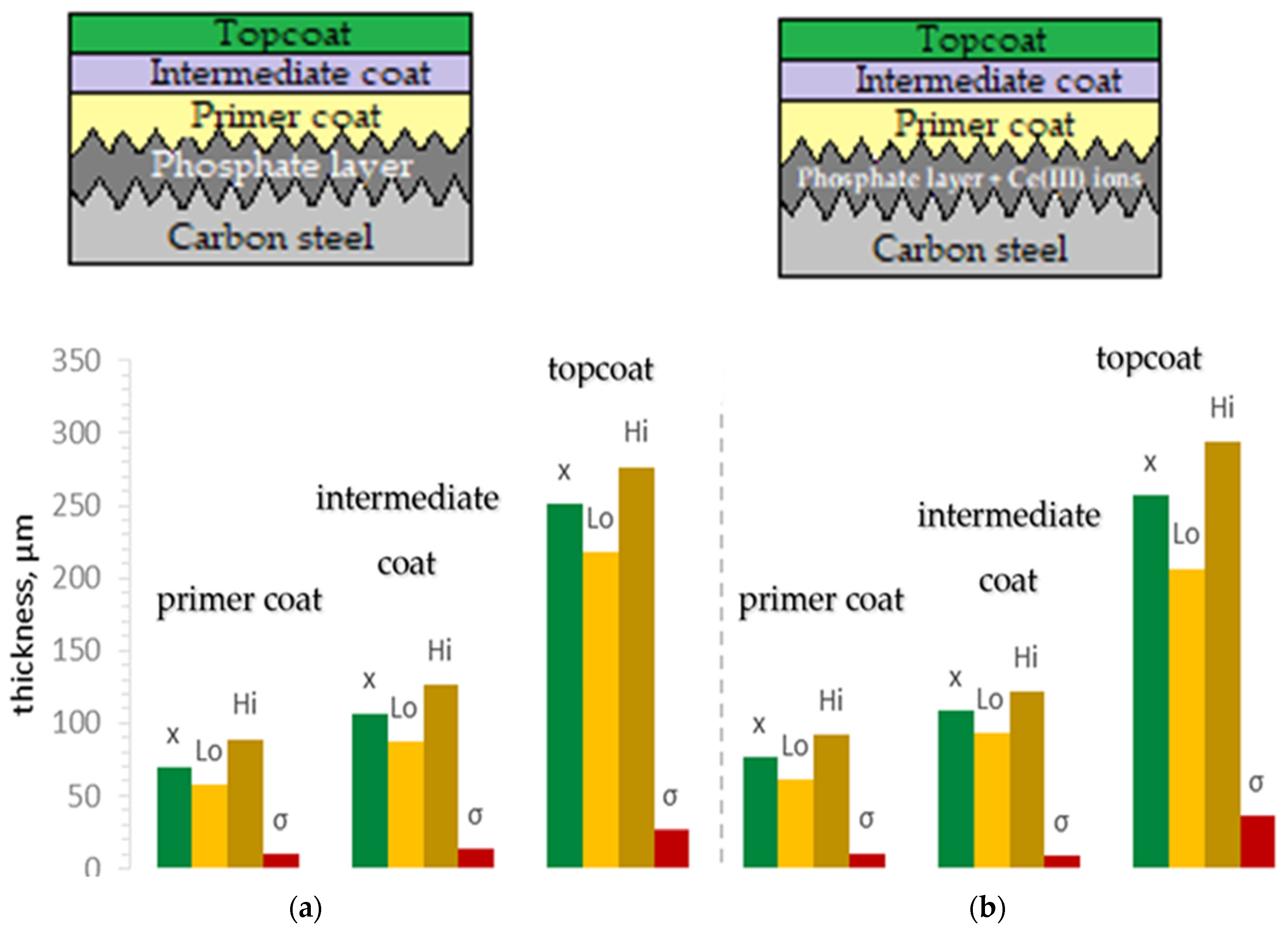
3.3. Coating Thickness Measurement
3.4. Accelerated Corrosion Tests
3.5. Adhesion Measurements
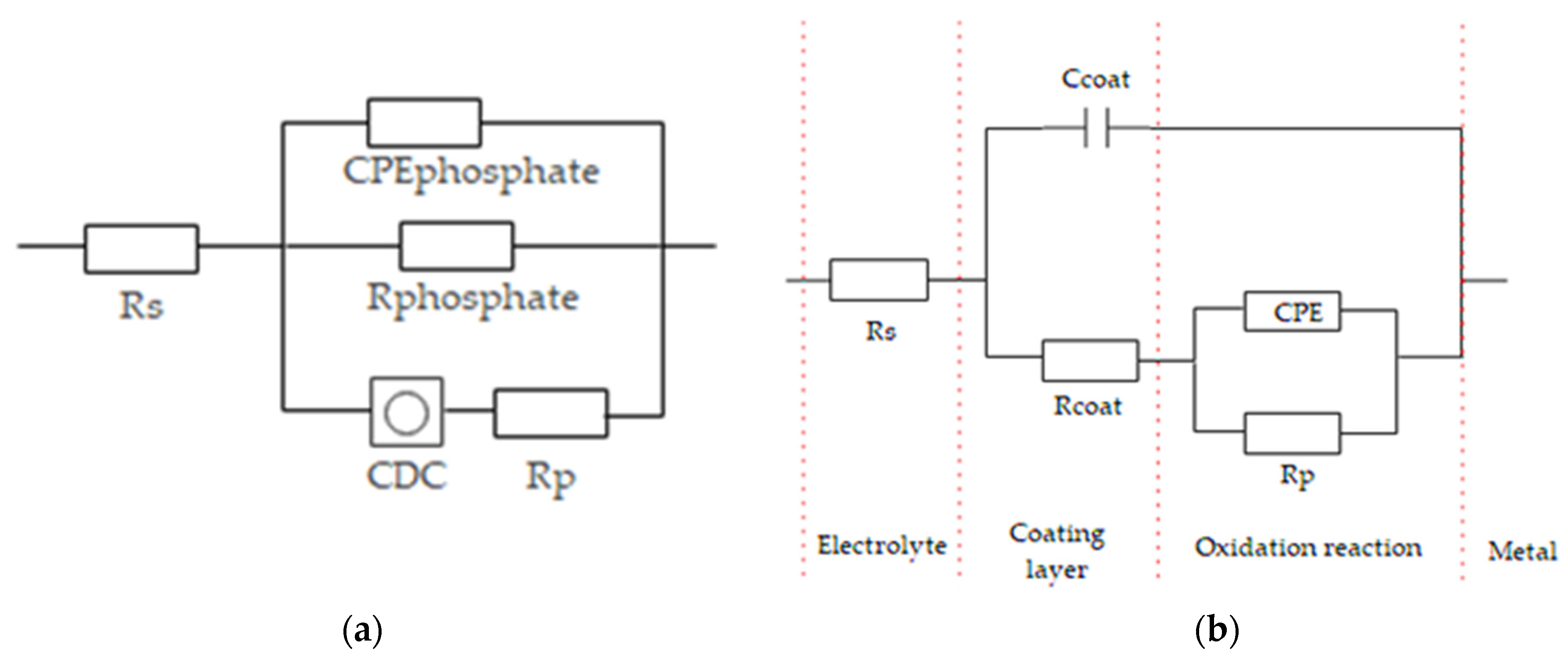
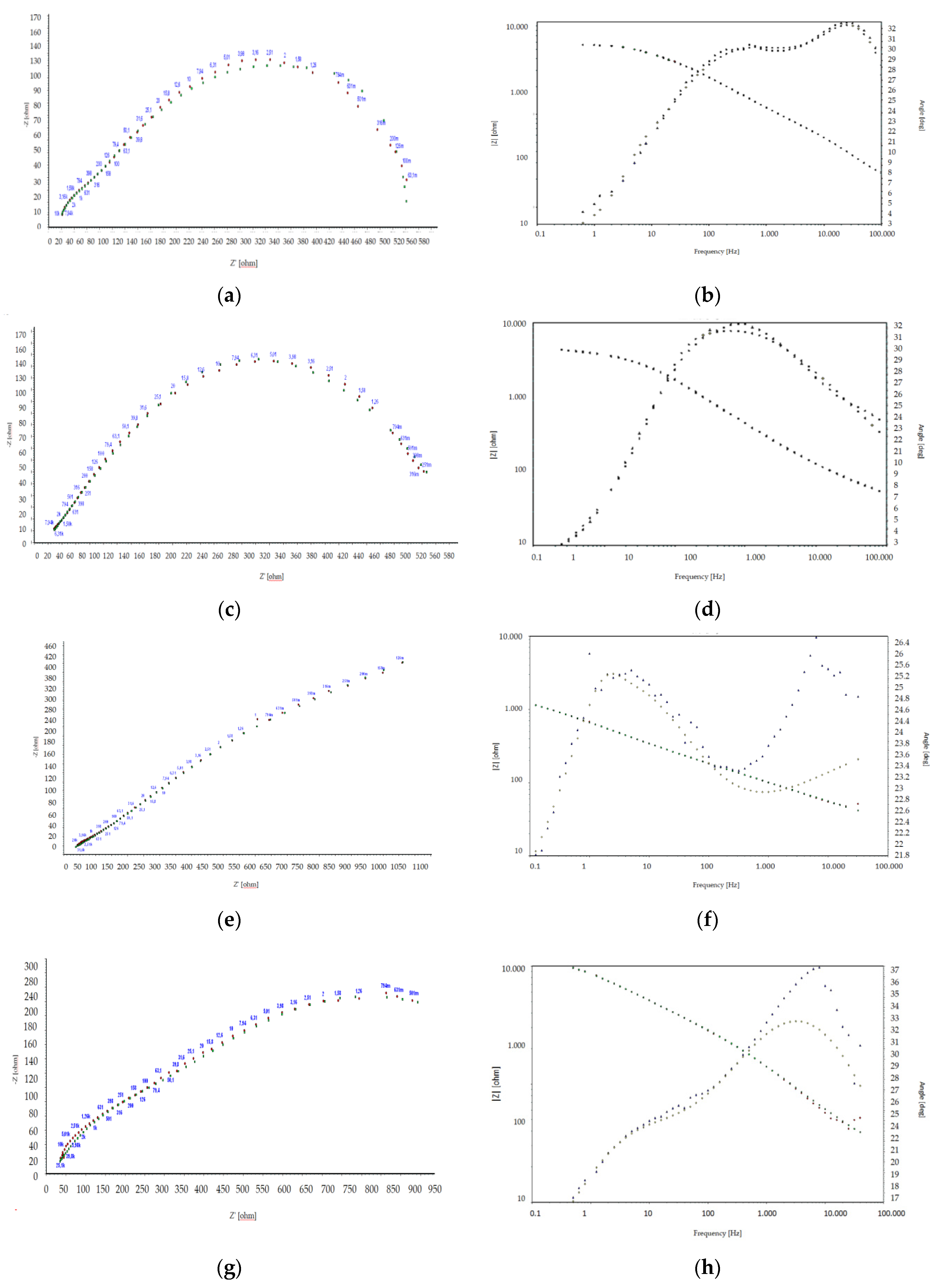
3.6. Electrochemical Impedance Spectroscopy
4. Conclusions
- Phosphate steel and phosphate steel with Ni2+, Ce3+, and Ti2+ ions have the same value of roughness because all samples were prepared with abrasive blasting.
- All samples show a similar value of thickness and confirm uniformly applied layers.
- A gray color with a bright metallic luster of samples did not change after being exposed to corrosion conditions.
- Surface hardness of samples is still characterized as medium-hard, although it takes time for this type of topcoat to develop the appropriate degree of hardness.
- Due to loss of adhesion in the intermediate coating, samples did not comply with EN ISO 4624:2016. There is no difference in the pull-off values between the phosphate and the phosphate carbon surfaces with Ce3+ ions.
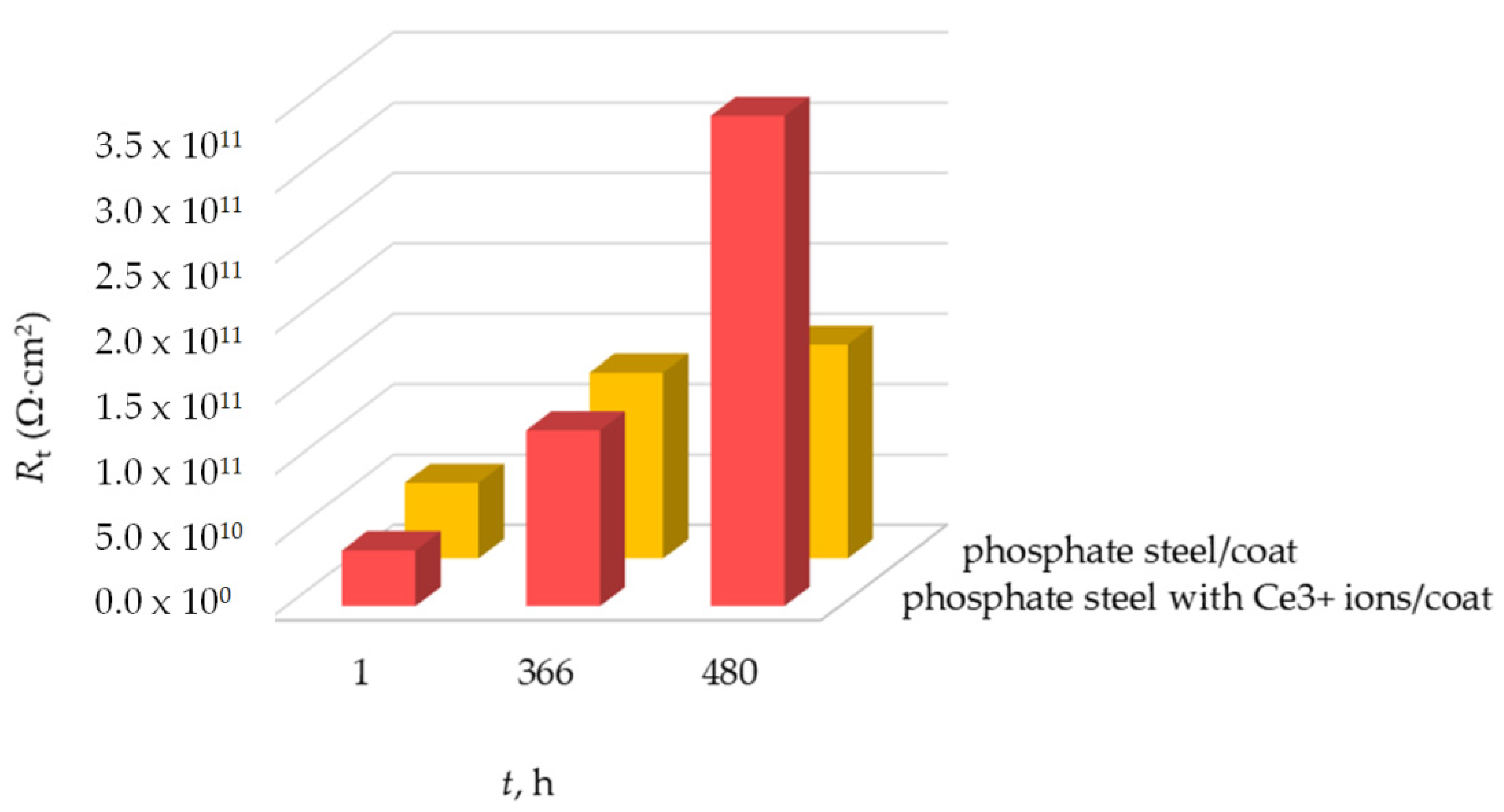
- An analysis of the EIS measurement results showed equal diffusion through all modified layers. Double layer capacitance showed low values, indicating the formation of sparingly soluble phosphate salts on the steel substrate, which results in an increase in the value of Rp and better corrosion protection. Ce3+ ions in the form of phosphate salts bind to the carbon steel surface and thus act as an inhibitor for the phosphate surface.
- After a 20-day immersion, the EIS measurement confirmed that epoxy coating and polyurethane have a good protection effect, and the electrolyte solution had not been diffused on the steel substrate. This is a short period of coating exposure to water penetration, but we can conclude that a long-term corrosion behavior of phosphate carbon steel could offer better corrosion protection due to a significant increase of the Rp value.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ISO | International Organization for Standardization |
| SCE | Saturated calomel electrode |
| EIS | Electrochemical impedance spectroscopy |
| CDC | Circuit description code |
| EEC | Equivalent electric circuit |
| CPE | Double layer capacitance |
References
- Ubong, M.E.; Mazen, M.K. Corrosion protection of non-alloyed AIAI 316L concrete steel metalgrade in aqueous H2SO4. Electroanalytical and surface analyses with Metiamide. Constr. Build. Mater. 2014, 68, 285–290. [Google Scholar]
- Morozov, Y.; Calado, L.M.; Shakoor, R.A.; Kahraman, R.; Taryba, M.G.; Montemor, M.F. Epoxy coatings modified with a new cerium phosphate inhibitor for smart corrosion protection of steel. Corros. Sci. 2019, 159, 108–128. [Google Scholar] [CrossRef]
- Naeem, M.; Shafiq, M.; Zaka-ul-Islamc, M.; Bashir, M.I.; Díaz-Guillénd, J.C.; Lopez-Badilloe, C.M.; Zakaullah, M. Novel duplex cathodic cage plasma nitriding of non-alloyed steel using aluminum and austenite steel cathodic cages. J. Alloy. Compd. 2017, 721, 307–311. [Google Scholar] [CrossRef]
- Francis, R.; Byrne, G. Duplex Stainless Steel–Alloys for the 21st Century. Metals 2021, 11, 836. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Wenbin, H. Preparation and corrosion behavior of Cu-8-HQ@HNTs/epoxy coating. Prog. Org. Coat. 2020, 139, 105–434. [Google Scholar] [CrossRef]
- Finšar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef] [Green Version]
- López-Ortega, A.; Bayón, R.; Arana, J.L. Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications. Materials 2019, 12, 1325. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.G.; Garbatov, Y.; Zayed, A.; Wang, G. Influence of environmental factors on corrosion of ship structures in marine atmosphere. Corros. Sci. 2009, 9, 2014–2026. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Ramezanzadeh, B.; Sari, M.G.; Saeb, M.R. Corrosion resistance of epoxy coating on mild steel through polyamidoamine dendrimer-covalently functionalized graphene oxide nanosheets. J. Ind. Eng. Chem. 2020, 82, 290–302. [Google Scholar] [CrossRef]
- Chandler, K.A. Marine and Offshore Corrosion; Butterworth-Heinemann: Oxford, UK, 1985; ISBN 978-0-408-01175-4. [Google Scholar]
- Silva, E.; Fedel, M.; Deflorian, F.; Cotting, F.; Lins, V. Properties of Post-Consumer Polyethylene Terephtalate Coating Mechanically deposited on Middle Steel. Coatings 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Khodaei, P.; Shabani-Nooshabadi, M.; Behpour, M. Epoxy-Based nanocomposite coating reinforced by a zeolite complex: Its anticorrosion properties on mild steel in 3.5 wt% NaCl media. Prog. Org. Coat. 2019, 136, 105–254. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Ghasem, B.; Ramezanzadeh, B. Development of a nanostructured Ce(III)-Pr(III) film for excellently corrosion resistance improvement of epoxy/polyamide coating on carbon steel. J. Alloy. Compd. 2019, 792, 375–388. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Yao, D.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- ISO 12944:1-8. Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Rahmania, P.; Shojaeia, A.; Tavandashti, N.P. Nanodiamond loaded with corrosion inhibitor as efficient nanocarrier to improve anticorrosion behavior of epoxy coating. J. Ind. Eng. Chem. 2020, 83, 153–163. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Jiang, L.; Tan, Q. Enhancing corrosion resistance of epoxy coating on steel reinforcement by aminobenzoate intercalated layered double hydroxides. Prog. Org. Coat. 2019, 134, 288–296. [Google Scholar] [CrossRef]
- Bajat, J.B.; Mišković-Stanković, V.B.; Popić, J.P.; Dražić, D.M. Adhesion characteristics and corrosion stability of epoxy coatings electrodeposited on phosphate hot-dip galvanized steel. Prog. Org. Coat. 2008, 63, 201–208. [Google Scholar] [CrossRef]
- Alvarado-Macías, G.; Fuentes-Aceituno, J.C.; Salinas-Rodríguez, A.; Rodríguez-Varela, F.J. Understanding the Nature of the Manganese Hot Dip Phosphatizing Process of Steel. J. Mex. Chem. Soc. 2013, 57, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Gang, L.; Wanger, S.; Yanrong, C.; Shili, Z. Quick organic phosphating at room temperature. Met. Finish. 1997, 95, 54–57. [Google Scholar]
- Jegannathana, S.; Sankara Narayanan, T.S.N.; Ravichandran, K.; Rajeswar, S. Formation of zinc phosphate coating by anodic electrochemical treatment. Surf. Coat. Technol. 2006, 200, 6014–6020. [Google Scholar] [CrossRef]
- Jegannathana, S.; Arumugam, T.K.; Sankara Narayanan, T.S.N.; Ravichandran, K. Formation and characteristics of zinc phosphate coatings obtained by electrochemical treatment: Cathodic vs. anodic. Prog. Org. Coat. 2009, 65, 229–236. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, X. Anti-corrosion zinc phosphate coating on building steel via a facile one-step brushing method. Electrochem. Commun. 2019, 109, 106596. [Google Scholar] [CrossRef]
- Yongsheng, H.; Fuchun, L.; En-Hou, H.; Saima, A.; Guobao, X. The mechanism of inhibition by zinc phosphate in an epoxy coating. Corros. Sci. 2013, 69, 77–86. [Google Scholar]
- Naderi, R.; Attar, M.M. The inhibitive performance of polyphosphate-based anticorrosion pigments using electrochemical techniques. Dye. Pigment. 2009, 80, 349–354. [Google Scholar] [CrossRef]
- ISO 2360. Non-Conductive Coatings on Non-Magnetic Electrically Conductive Base Metals–Measurement of Coating Thickness–Amplitude-Sensitive Eddy-Current Method; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Powder Coating Consultants, Machu Test: Accelerated Salt Sprey Test. Available online: https://powdercc.com/pdf/Machu%20Test.pdf (accessed on 8 December 2021).
- ISO 6270-2. Paints and Varnishes–Determination of Resistance to Humidity–Part 2: Condensation (In-Cabinet Exposure with Water Reservoir); International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- ISO 8503-1. Preparation of Steel Substrates before Application of Paints and Related Products–Surface Roughness Characteristics of Blast-Cleaned Steel Substrates–Part 1: Specifications and Definitions for ISO Surface Profile Comparators for the Assessment of Abrasive Blast-Cleaned Surfaces; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Spahiu, K.; Jordi, B. A selected thermodynamic database for REE to be used in HLNW performance assessment exercises. Tech. Rep. 1995, 28, 29. [Google Scholar]
- Dagdag, O.; El Harfi, A.; El Gana, L.; Hlimi, Z.; Erramli, H.; Hamed, O.; Jodeh, S. The Role of Zinc Phosphate Pigment in the Anticorrosion Properties of Bisphenol A Diglycidyl Ether-Polyaminoamide Coating for Aluminum Alloy AA2024-T3. J. Bio- Tribo- Corros. 2018, 5, 7. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Zhang, X.; Zhang, M. The growth of zinc phosphate coating on 6061-Al alloy. Surf. Coat. Technol. 2008, 202, 1674–1680. [Google Scholar]
- Hiromoto, S. 3-Chemical solution deposition of hydroxyapatite and octacalcium phosphate coating for magnesium and its alloys to improve biocompatibility. Woodhead Publ. Ser. Biomater. 2015, 2, 59–80. [Google Scholar]
- ASTM B117. Standard Practice for Operating Salt Spray (Fog) Apparatus; American Society for Testing and Material: West Conshohocken, PL, USA, 2019. [Google Scholar]
- ISO 4624. Paints and Varnishes–Pull-Off Test for Adhesion; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- Fu, X.; Shen, Z.; Chen, X.; Lin, J.; Cao, H. Influence of Element Penetration Region on Adhesion and Corrosion Performance of Ni-Base Coatings. Coatings 2020, 10, 895. [Google Scholar] [CrossRef]
- Bejinuriu, C.; Burduhos-Nergis, D.-P.; Cimpoesu, N. Immersion Behaviour of Carbon Steel, Phosphate Carbon Steel and Phosphate and Painted Carbon Steel in Saltwater. Materials 2021, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, I.; Šimunović, V.; Alar, V.; Kapor, F. Experimental Evaluation of Polyester and Epoxy-Polyester Polyester Powder Coatings in Aggressive Media. Coatings 2018, 8, 98. [Google Scholar] [CrossRef] [Green Version]
| Element | C | Si | Mn | P | S | Cr | Mo | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Percent (%) | 0.33 | 0.16 | 0.55 | 0.019 | 0.019 | 0.06 | 0.01 | 0.03 | 0.04 | balance |
| Bath | Bath 1 | Bath 2 | Bath 3 | Bath 4 |
|---|---|---|---|---|
| H3PO4, mL/L | 14 | 14 | 14 | 14 |
| ZnO, g/L | 12 | 12 | 12 | 12 |
| HNO3, mL/L | 12 | 12 | 12 | 12 |
| NaNO2, g/L | 2 | 2 | 2 | 2 |
| NaOH, g/L | 3 | 3 | 3 | 3 |
| NiSO4∙7H2O, g/L | - | 1.25 | - | - |
| CeCl3, g/L | - | - | 1.25 | - |
| TiO2, g/L | - | - | - | 1.25 |
| Temperature, °C | 70 | 70 | 70 | 70 |
| Duration, min | 15 | 15 | 15 | 15 |
| Coat | Binder | Solvent |
|---|---|---|
| primer coat | epoxy resin | xylene, but-1-ol and toluene |
| intermediate coat | epoxy resin | xylene, but-1-ol and toluene |
| topcoat | polyurethane | n-butil-acetat, petrol |
| Samples | Phosphating Solution Modified with | Three-Layer Coating System |
|---|---|---|
| Sample 1 | - | primer, intermediate and topcoat |
| Sample 2 | Ni2+ ions | primer, intermediate and topcoat |
| Sample 3 | Ce3+ ions | primer, intermediate and topcoat |
| Sample 4 | Ti2+ ions | primer, intermediate and topcoat |
| Phosphate Sample | Phosphate Samples with Ni2+ | Phosphate Samples with Ce3+ | Phosphate Samples with Ti2+ | |
|---|---|---|---|---|
| Thickness (μm) | 15.03 | 15.92 | 21.69 | 14.01 |
| Samples | Phosphate Samples | Phosphate Samples with Ni2+ | Phosphate Samples with Ce3+ | Phosphate Samples with Ti2+ |
|---|---|---|---|---|
| Rz (μm) | 53.29 | 50.63 | 55.86 | 51.49 |
| Ra (μm) | 8.70 | 8.91 | 9.25 | 9.03 |
| Reaction | log K |
|---|---|
| Ce3+ + H2PO4−→ CeH2PO42+ | −2.43 |
| Ce3+ + HPO42−→ CeHPO4+ | −4.98 |
| Ce3+ + 2HPO42−→ Ce(HPO4)2− | −8.34 |
| Ce3+ + PO43−→ CePO4 | −11.35 |
| Ce3+ + 2PO43−→ Ce(PO4)23− | −18.48 |
| Day | Phosphate Steel/Coat | Phosphate Steel with Ce3+/Coat | ||||
|---|---|---|---|---|---|---|
| Initial Sample | Humidity Chamber | Immersed in Sea Water | Initial Sample | Humidity Chamber | Immersed in Sea Water | |
| 1. | RAL 6013 | RAL 6013 | RAL 6013 | RAL 6013 | RAL 6013 | RAL 6013 |
| 23. | RAL 6013 | RAL 6013 | RAL 6013 | RAL 6013 | RAL 6013 | RAL 6013 |
| Day | Phosphate Steel/Coat | Phosphate Steel with Ce3+/Coat | ||||
|---|---|---|---|---|---|---|
| Initial Sample | Humidity Chamber | Immersed in Sea Water | Initial Sample | Humidity Chamber | Immersed in Sea Water | |
| 1. | B | F | HB | HB | F | HB |
| 13. | F | F | - | F | F | - |
| 23. | F | F | F | F | F | F |
| Initial Samples (MPa) | Samples in Humidity Chamber (MPa) | Samples Immersed in 5% NaCl Solution (MPa) | |
|---|---|---|---|
| phosphate steel/coat | - | 1.67 | 2.56 |
| - | - | 3.94 | |
| phosphated steel with Ce3+/coat | 2.59 | 12.15 | 4.81 |
| 2.06 | 12.34 | 7.31 |
| Samples | Rs (Ω) | CPEcoat (F) | ncoat | Rcoat (Ω) | CPEphosphate (F) | Rphosphate (Ω) | CPE (F) | Rp (Ω) | Yo (S∙s5) | B (s5) |
|---|---|---|---|---|---|---|---|---|---|---|
| phosphate steel | 15.26 | - | - | - | 9.9 × 10−6 | 52.26 | 9.9 × 10−5 | 647.2 | 3.8 × 10−4 | 1.35 |
| phosphate steel with Ni2+ | 15.31 | - | - | - | 1.9 × 10−4 | 57.3 | - | 925.1 | 1.4 × 10−4 | 1.25 |
| phosphate steel with Ce3+ | 15.01 | - | - | - | 7.9 × 10−4 | 901.5 | - | 1.8 × 104 | 6.6 × 10−5 | 1.36 |
| phosphate steel with Ti2+ | 17.48 | - | - | - | 7.1 × 10−5 | 582.3 | - | 1.4 × 103 | 1.7 × 10−4 | 1.23 |
| immersion after 1 h | ||||||||||
| phosphate steel/coat | 15.87 | 5.9 × 10−11 | 0.99 | 1.1 × 108 | - | - | 2.1 × 10−10 | 5.4 × 1010 | - | - |
| phosphate steel with Ce3+/coat | 16.34 | 2.7 × 10−11 | 1 | 3.5 × 109 | - | - | 1.4 × 10−10 | 3.6 × 1010 | - | - |
| immersion after 14 days | ||||||||||
| phosphate steel/coat | 12.44 | 3.2 × 10−11 | 1 | 3.9 × 109 | - | - | 8.7 × 10−11 | 1.3 × 1011 | - | - |
| phosphate steel with Ce3+/coat | 14.54 | 1.1 × 10−11 | 1 | 5.9 × 109 | - | - | 3.8 × 10−11 | 1.2 × 1011 | - | - |
| immersion after 20 days | ||||||||||
| phosphate steel/coat | 17.47 | 1.2 × 10−11 | 1 | 7.8 × 109 | - | - | 2.3 × 10−10 | 1.4 × 1011 | ||
| phosphate steel with Ce3+/coat | 15.39 | 6.4 × 10−12 | 1 | 2.6 × 1010 | - | - | 1.1 × 10−11 | 3.2 × 1011 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samardžija, M.; Alar, V.; Špada, V.; Kapor, F. Phosphating Modification with Metal Ions of Carbon Steel Surface to Improve the Influence of Anticorrosion Properties. Technologies 2022, 10, 3. https://doi.org/10.3390/technologies10010003
Samardžija M, Alar V, Špada V, Kapor F. Phosphating Modification with Metal Ions of Carbon Steel Surface to Improve the Influence of Anticorrosion Properties. Technologies. 2022; 10(1):3. https://doi.org/10.3390/technologies10010003
Chicago/Turabian StyleSamardžija, Marina, Vesna Alar, Vedrana Špada, and Frankica Kapor. 2022. "Phosphating Modification with Metal Ions of Carbon Steel Surface to Improve the Influence of Anticorrosion Properties" Technologies 10, no. 1: 3. https://doi.org/10.3390/technologies10010003
APA StyleSamardžija, M., Alar, V., Špada, V., & Kapor, F. (2022). Phosphating Modification with Metal Ions of Carbon Steel Surface to Improve the Influence of Anticorrosion Properties. Technologies, 10(1), 3. https://doi.org/10.3390/technologies10010003






