Dose Administration Aid Service in Community Pharmacies: Characterization and Impact Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.4. Data Analysis
2.5. Trustworthiness and Reflexivity
3. Results
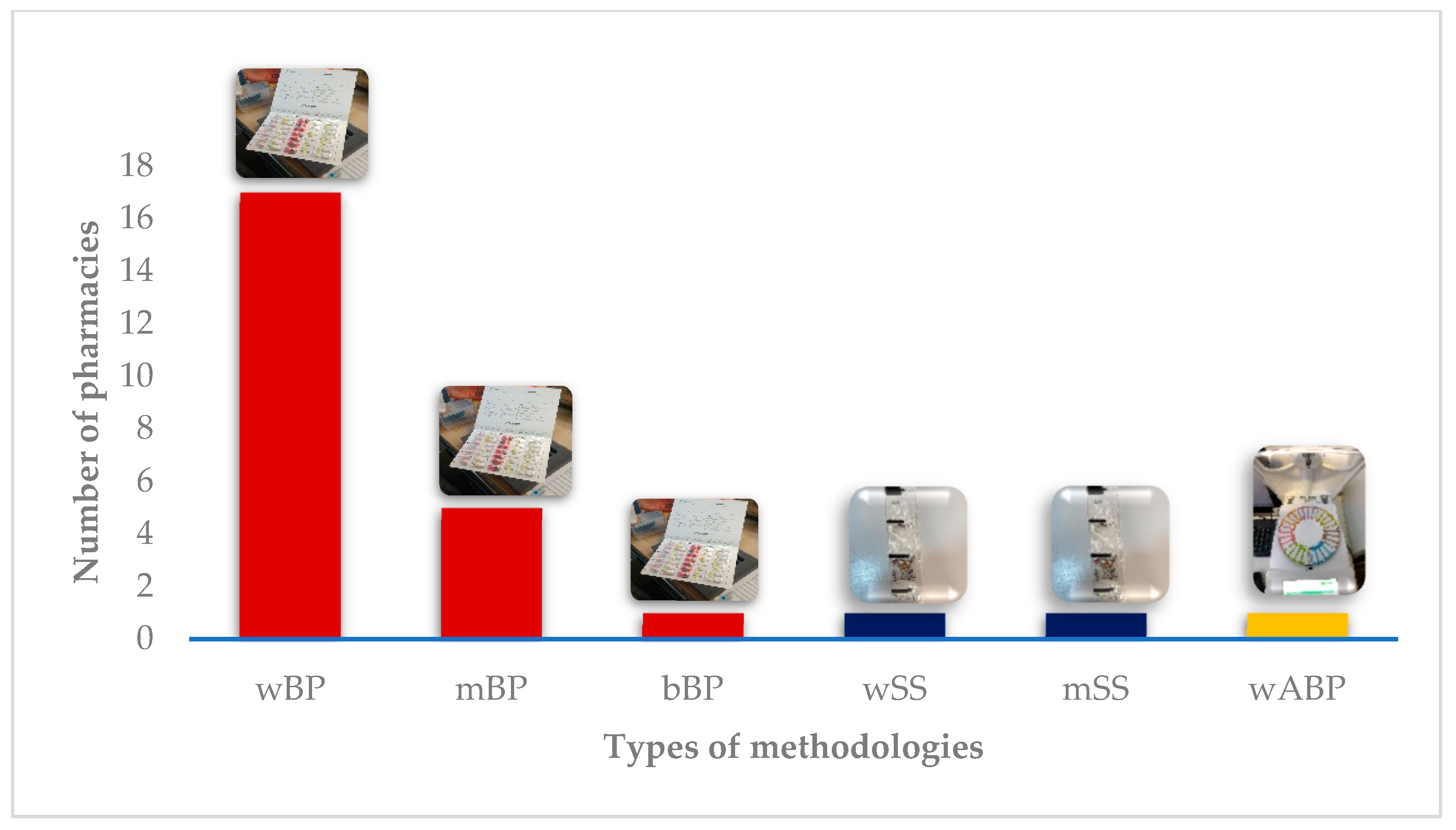
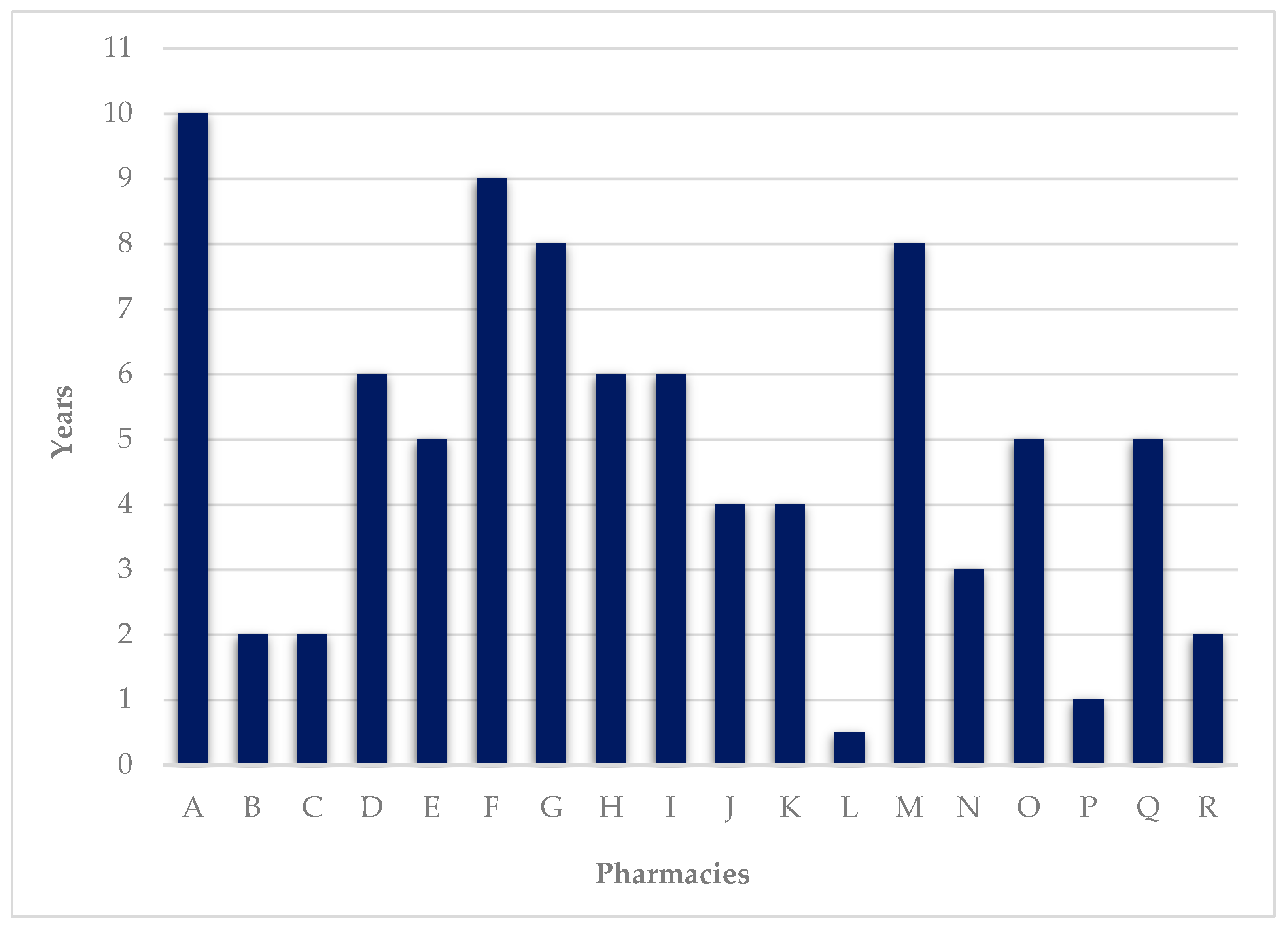
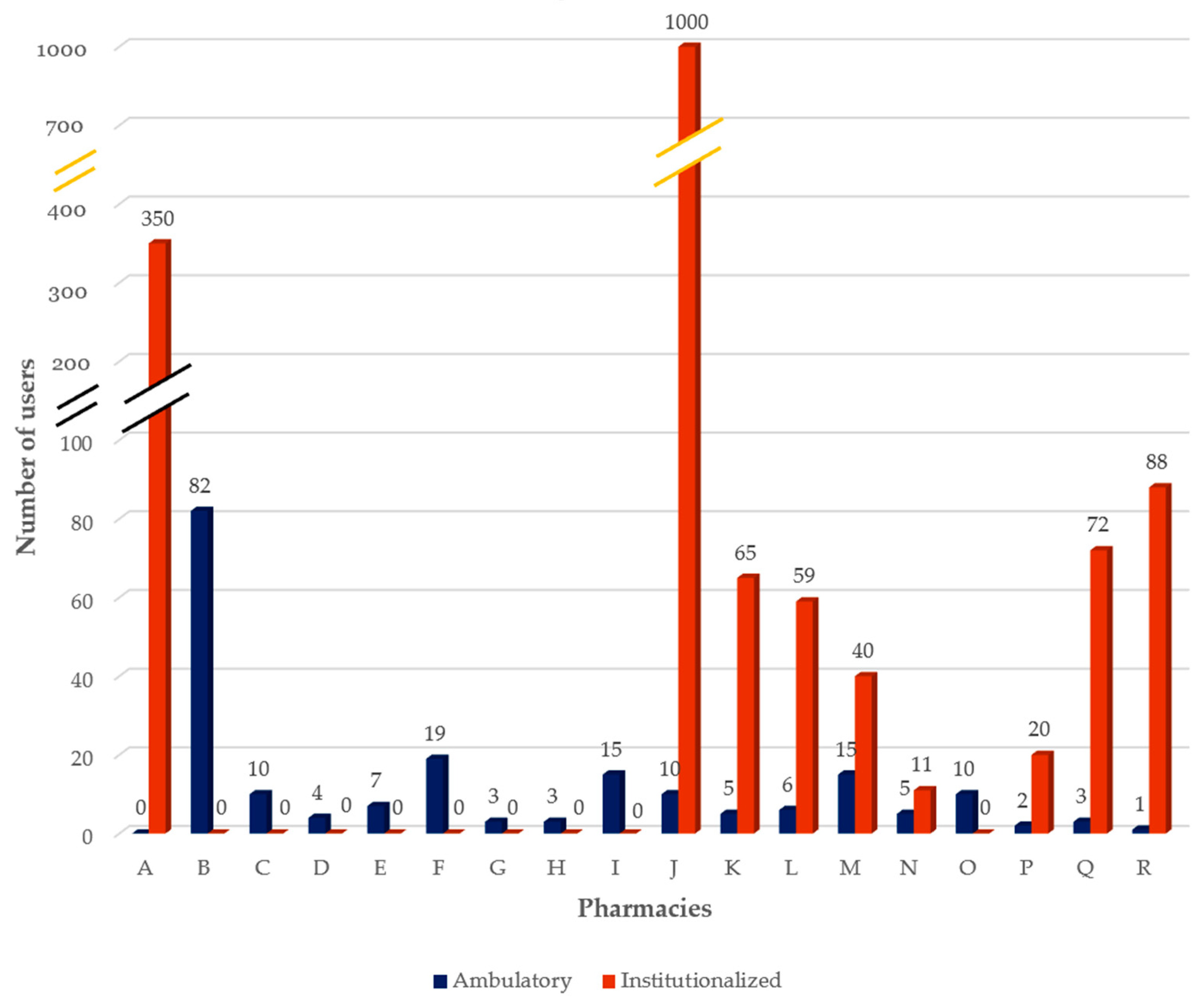
3.1. Characterization of the DAAS
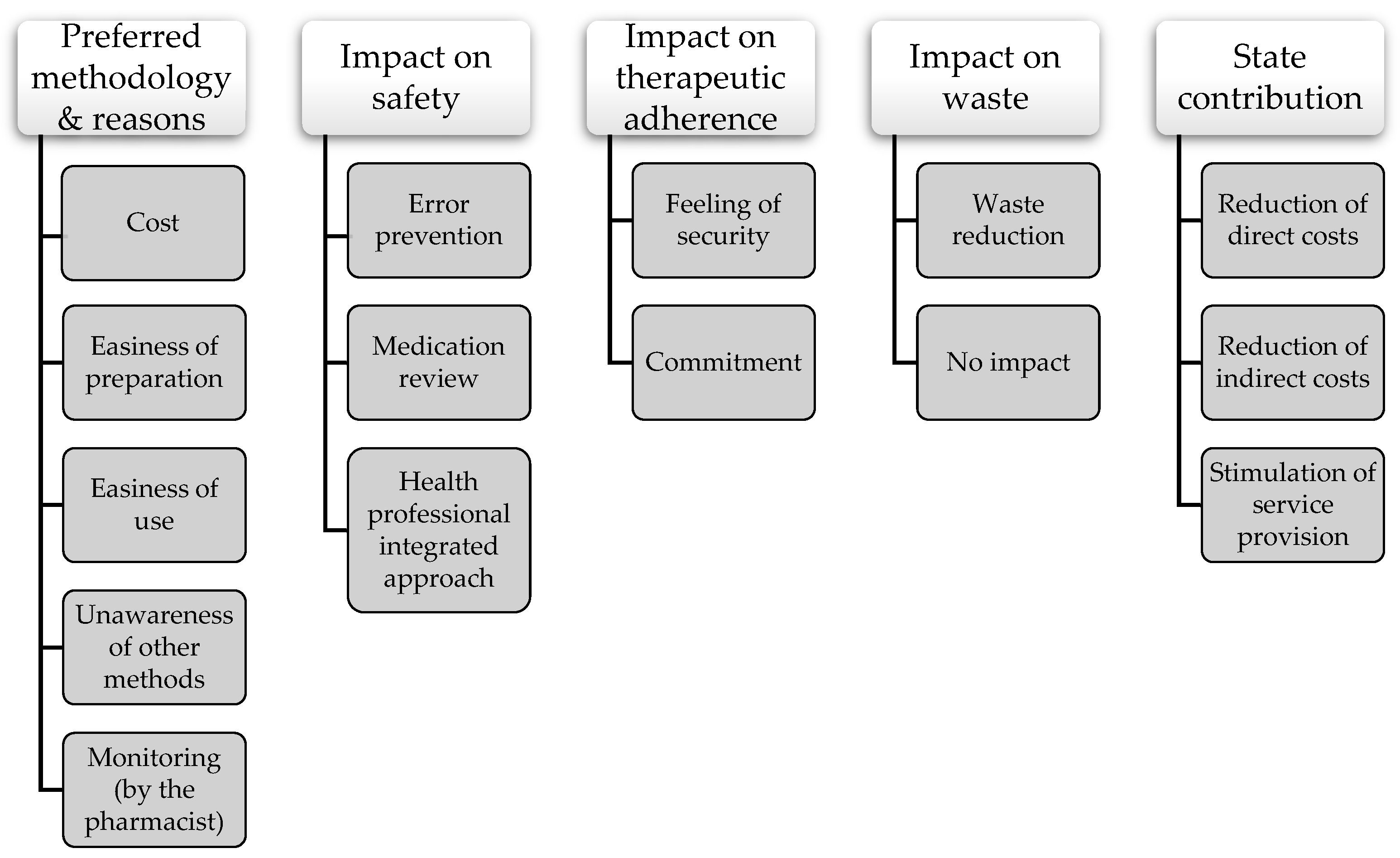
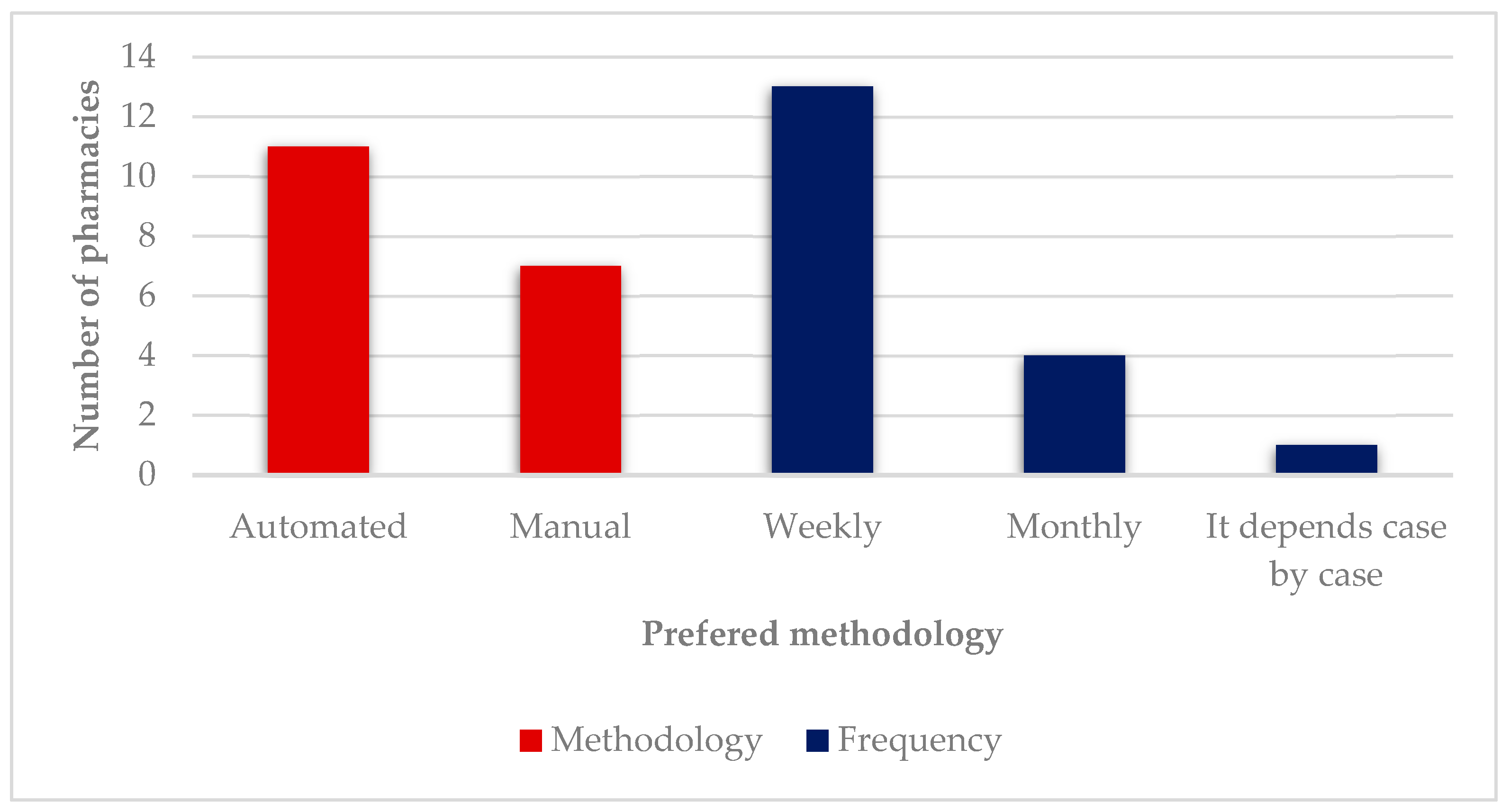
3.2. Preferred Methodology
3.3. Impact on Safety
3.4. Impact on Therapeutic Adherence
3.5. Impact on Waste
3.6. State Contribution
4. Discussion
4.1. Limitations
4.2. Implications for Research and Practice
- DAAS can have a positive impact on safety, adherence, and waste, especially for patients with complicated medication schedules, on multiple drugs, or with some level of cognitive impairment;
- DAAS is useful both for ambulatory users and institutionalized users;
- There is a need to make the service affordable for users, while not too cumbersome for pharmacies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Interview Guide
- For how long has the Dose Administration Aid Service (DAAS) been available?
- How many users currently use the DAAS in your pharmacy?
- What methodology(ies) are available?
- Which of the methodologies do you consider to be the best?
- What is the average cost per month for users?
- What is the average cost per user per month for the pharmacy?
- Do you think that it makes sense to have a State contribution?
- Is therapeutic reconciliation associated with DAAS?
- What impact does this system have on user safety, bearing in mind that it is possible to associate therapy reconciliation?
- What impact does DAAS have on reducing waste at this time?
- What impact do you feel DAAS has on therapy adherence?
- The legal framework in which this service is inserted follows an after-sales service. What do you think about making this pre-sale, that is, within a unitary distribution system?
- With this system, would it be possible to reduce medicine waste?
- Why did your establishment not participate in the experimental regime introduced by the government in 2010?
Appendix B. COREQ Checklist
| Topic | Item No. | Guide Questions/Description | Location in Manuscript/ Reported on Page No |
|---|---|---|---|
| Domain 1: Research team and reflexivity | |||
| Personal Characteristics | |||
| Interviewer/facilitator | 1 | Which author/s conducted the interview or focus group? AV conducted all interviews alone | Materials and Methods/page 5 |
| Credentials | 2 | What are the researcher’s credentials? AV MSc candidate BM MSc ML PhD OL PhD | Materials and Methods/page 6 |
| Occupation | 3 | What was their occupation at the time of the study? AV Medicine master’s student BM Consultant pharmacist ML Researcher OL Assistant Professor | Materials and Methods/page 6 |
| Gender | 4 | Was the researcher male or female? AV male BM, ML, and OL females | Materials and Methods/page 6 |
| Experience and training | 5 | What experience or training did the researcher have? AV took a graduate course on quantitative and qualitative research ML has over 15 years of experience as a qualitative researcher OL has moderate level of experience with qualitative research | Materials and Methods/page 6 |
| Relationship with participants | |||
| Relationship established | 6 | Was a relationship established prior to study commencement? No | |
| Participants’ knowledge of the interviewer | 7 | What did the participants know about the researcher? Participants were briefed on the purpose of the study Participants also reviewed the study information sheet before they gave written informed consent to be involved in the study | Materials and Methods/page 4 |
| Interviewer characteristics | 8 | What characteristics were reported about the interviewer/facilitator? AV acknowledged being a Medicine master’s student with an interest in improving medication adherence | Materials and Methods/page 6 |
| Domain 2: Study design | |||
| Theoretical framework | |||
| Methodological Orientation and Theory | 9 | What methodological orientation was stated to underpin the study? Qualitative descriptive methodology with qualitative content analysis | Materials and Methods/page 5 |
| Participant selection | |||
| Sampling | 10 | How were the participants selected? Convenience | Materials and Methods/page 3 |
| Method of approach | 11 | How were the participants approached? Recruitment involved email and telephone invitations sent to all community pharmacies that according to national data had the DAAS available | Materials and Methods/page 3 |
| Sample size | 12 | How many participants were in the study? 18 | Materials and Methods/page 3 |
| Non-participation | 13 | How many people refused to participate or dropped out? Reasons? 433 pharmacies were contacted, but only 18 agreed to participate, 7 refused to participate for not having the service available at the time and the others did not respond after being contacted twice | Materials and Methods/page 3 |
| Setting | |||
| Setting of data collection | 14 | Where was the data collected? The participants were interviewed by videoconference in their workplaces | Materials and Methods/page 4 |
| Presence of non-participants | 15 | Was anyone else present besides the participant and researchers? No | |
| Description of sample | 16 | What are the important characteristics of the sample? Interviews were conducted between 16 March 2021 and 7 July 2021. Eighteen participants (17 females and 1 male) were technical directors, pharmacists responsible for the service, and/or owners of community pharmacies located in mainland Portugal from 11 different districts. In all cases, the service was available at least for six months with at least one user. | Materials and Methods/page 4 |
| Data collection | |||
| Interview guide | 17 | Were questions, prompts, guides provided by the authors? Was it pilot-tested? Interviews were semi-structured, using a guide, which is attached as an appendix The guide was tested for face and content validity by a panel of experts | Appendix and Materials and Methods/page 4 |
| Repeat interviews | 18 | Were repeat interviews carried out? If yes, how many? No | |
| Audio/visual recording | 19 | Did the researcher use audio or visual recording to collect the data? No | Materials and Methods/page 5 |
| Field notes | 20 | Were field notes made during and/or after the interview or focus group? Field notes were made during and immediately after the interviews | Materials and Methods/page 5 |
| Duration | 21 | What was the duration of the interviews or focus group? The semi-structured interviews took around 40 min | Materials and Methods/page 4 |
| Data saturation | 22 | Was data saturation discussed? In the limitations section, we discussed that data saturation would have been reached with 20 interviews | Limitations/page 15 |
| Transcripts returned | 23 | Were transcripts returned to participants for comment and/or correction? No, but the manuscript was sent to the participants before submission | Limitations/page 16 |
| Domain 3: analysis and findings | |||
| Data analysis | |||
| Number of data coders | 24 | How many data coders coded the data? AV and ML discussed consistency of the codes and the coding process. AV coded all the transcripts. | Materials and Methods/page 5 |
| Description of the coding tree | 25 | Did authors provide a description of the coding tree? Yes | Materials and Methods/page 5 |
| Derivation of themes | 26 | Were themes identified in advance or derived from the data? Themes derived from the data | Materials and Methods/page 5 |
| Software | 27 | What software, if applicable, was used to manage the data? None | |
| Participant checking | 28 | Did participants provide feedback on the findings? No | Limitations/page 16 |
| Reporting | |||
| Quotations presented | 29 | Were participant quotations presented to illustrate the themes/findings? Was each quotation identified? Comments were supported with direct quotes from the participants who were anonymized by participant number | Results/pages 6 to 14 |
| Data and findings consistent | 30 | Was there consistency between data presented and findings? Yes | Results/pages 6 to 14 |
| Clarity of major themes | 31 | Were major themes clearly presented in the findings? Yes | Results/pages 6 to 14 |
| Clarity of minor themes | 32 | Is there a description of diverse cases or discussion of minor themes? No |
References
- Cross, A.J.; Elliott, R.; Petrie, K.; Kuruvilla, L.; George, J. Interventions for improving medication-taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst. Rev. 2020, 2020, CD012419. [Google Scholar] [CrossRef]
- Mair, A.; Fernandez-Llimos, F.; Alonso, A.; Harrison, C.; Hurding, S.; Kempen, T.; Kinnear, M.; Michael, N.; McIntosh, J.; Wilson, M.; et al. Polypharmacy Management by 2030: A Patient Safety Challenge; Simpathy Consortium: Edinburgh, UK, 2017. [Google Scholar]
- Tafreshi, M.J.; Melby, M.J.; Kaback, K.R.; Nord, T.C. Medication-Related Visits to the Emergency Department: A Prospective Study. Ann. Pharmacother. 1999, 33, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Leendertse, A.J.; Egberts, A.C.; Stoker, L.J.; van den Bemt, P.M.; Group, H.S. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch. Intern. Med. 2008, 168, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.L.; Avery, A.J.; Howard, P.D.; Partridge, M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: Observational study. Qual. Saf. Health Care 2003, 12, 280–285. [Google Scholar] [CrossRef]
- DiMatteo, M.R.; Lepper, H.S.; Croghan, T.W. Depression Is a Risk Factor for Noncompliance With Medical Treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch. Intern. Med. 2000, 160, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Col, N.; Fanale, J.; Kronholm, P. The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly. Arch. Intern. Med. 1990, 150, 841–845. [Google Scholar] [CrossRef]
- Balkrishnan, R.; Rajagopalan, R.; Camacho, F.T.; Huston, S.; Murray, F.T.; Anderson, R.T. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: A longitudinal cohort study. Clin. Ther. 2003, 25, 2958–2971. [Google Scholar] [CrossRef]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Hersberger, K.; Boeni, F.; Arnet, I. Dose-dispensing service as an intervention to improve adherence to polymedication. Expert Rev. Clin. Pharmacol. 2013, 6, 413–421. [Google Scholar] [CrossRef]
- Allemann, S.S.; Arnet, I.; Hersberger, K. Patient views on an electronic dispensing device for prepackaged polypharmacy: A qualitative assessment in an ambulatory setting. Integr. Pharm. Res. Pract. 2015, 4, 167–174. [Google Scholar] [CrossRef][Green Version]
- Cramer, J.A. Enhancing Patient Compliance in the Elderly. Role of packaging aids and monitoring. Drugs Aging 1998, 12, 7–15. [Google Scholar] [CrossRef]
- Schneider, P.J.; Murphy, J.E.; Pedersen, C.A. Impact of medication packaging on adherence and treatment outcomes in older ambulatory patients. J. Am. Pharm. Assoc. 2008, 48, 58–63. [Google Scholar] [CrossRef]
- Laufs, U.; Rettig-Ewen, V.; Böhm, M. Strategies to improve drug adherence. Eur. Heart J. 2011, 32, 264–268. [Google Scholar] [CrossRef]
- Osterberg, L.; Blaschke, T. Adherence to Medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Gould, O.N.; Todd, L.; Irvine-Meek, J. Adherence Devices in a Community Sample: How are Pillboxes Used? Can. Pharm. J. 2009, 142, 28–35. [Google Scholar] [CrossRef]
- Stewart, D.C.; McDonald, C.; MacLeod, J.; Maclure, K.; Gray, G.; McIntosh, T. The behaviors and experiences of the community pharmacy team on the provision of multi-compartment compliance aids. Res. Soc. Adm. Pharm. 2018, 14, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Barton, E.; Twining, L.; Walters, L. Understanding the decision to commence a dose administration aid. Aust. Fam. Physician 2017, 46, 943–947. [Google Scholar] [PubMed]
- Elliott, R. Appropriate use of dose administration aids. Aust. Prescr. 2014, 37, 46–50. [Google Scholar] [CrossRef]
- Watson, S.J.; Aldus, C.F.; Bond, C.; Bhattacharya, D. Systematic review of the health and societal effects of medication organisation devices. BMC Health Serv. Res. 2016, 16, 202. [Google Scholar] [CrossRef] [PubMed]
- Sinnemäki, J.; Sihvo, S.; Isojärvi, J.; Blom, M.; Airaksinen, M.; Mäntylä, A. Automated dose dispensing service for primary healthcare patients: A systematic review. Syst. Rev. 2013, 2, 1. [Google Scholar] [CrossRef]
- Sng, Y.; Ong, C.K.; Lai, Y.F. Approaches to outpatient pharmacy automation: A systematic review. Eur. J. Hosp. Pharm. 2019, 26, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J. Qualitative Inquiry and Research Design: Choosing among Five Approaches, 3rd ed.; SAGE: Los Angeles, CA, USA, 2013. [Google Scholar]
- Colorafi, K.J.; Evans, B. Qualitative Descriptive Methods in Health Science Research. HERD Health Environ. Res. Des. J. 2016, 9, 16–25. [Google Scholar] [CrossRef]
- Sandelowski, M. Whatever happened to qualitative description? Res. Nurs. Health 2000, 23, 334–340. [Google Scholar] [CrossRef]
- Sandelowski, M. What’s in a name? Qualitative description revisited. Res. Nurs. Health 2010, 33, 77–84. [Google Scholar] [CrossRef]
- Atif, M.; Razzaq, W.; Mushtaq, I.; Malik, I.; Razzaq, M.; Scahill, S.; Babar, Z.-U.-D. Pharmacy Services beyond the Basics: A Qualitative Study to Explore Perspectives of Pharmacists towards Basic and Enhanced Pharmacy Services in Pakistan. Int. J. Environ. Res. Public Health 2020, 17, 2379. [Google Scholar] [CrossRef]
- Ramos, S.F.; Santos, G.A.D., Jr.; Pereira, A.M.; Dosea, A.S.; Rocha, K.S.S.; Pimentel, D.M.M.; De Lyra, D.P., Jr. Facilitators and strategies to implement clinical pharmacy services in a metropolis in Northeast Brazil: A qualitative approach. BMC Health Serv. Res. 2018, 18, 632. [Google Scholar] [CrossRef]
- Alcantara, T.D.S.; Onozato, T.; Neto, F.D.C.A.; Dosea, A.S.; Cunha, L.C.; De Araújo, D.C.S.A.; Pimentel, D.; Junior, D.P.L. Perceptions of a group of hospital pharmacists and other professionals of the implementation of clinical pharmacy at a high complexity public hospital in Brazil. BMC Health Serv. Res. 2018, 18, 242. [Google Scholar] [CrossRef]
- Bolarinwa, O.A. Principles and methods of validity and reliability testing of questionnaires used in social and health science researches. Niger. Postgrad. Med. J. 2015, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.; Pietilä, A.-M.; Johnson, M.; Kangasniemi, M. Systematic methodological review: Developing a framework for a qualitative semi-structured interview guide. J. Adv. Nurs. 2016, 72, 2954–2965. [Google Scholar] [CrossRef]
- Hsieh, H.-F.; Shannon, S.E. Three Approaches to Qualitative Content Analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, Y.S.; Guba, E.G. Naturalistic Inquiry; SAGE: Newbury Park, CA, USA, 1985. [Google Scholar]
- Lamb, G.S.; Huttlinger, K. Reflexivity in Nursing Research. West. J. Nurs. Res. 1989, 11, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Portaria, N. 97/2018, de 9 de Abril, Publicada no Diário da República n.o 69/2018, Série I 2018. Available online: https://dre.pt/dre/detalhe/portaria/97-2018-115006162 (accessed on 23 November 2021).
- Farmacêuticos, Grupo Profissional de Farmácia Comunitária da Ordem dos. Preparação Individualizada da Medicação (PIM). 2018, N° 30-NGE-00-010-02. Available online: https://www.ordemfarmaceuticos.pt/fotos/documentos/norma_pim_vfinal_30_nge_00_010_02_1834827175bf58d479434f.pdf (accessed on 23 November 2021).
- Miranda, I.; Costa, F. Willingness To Pay (Vontade Para Pagar) Por Um Serviço De Preparação Individualizada da Medicação. Rev. Port. Farmacoter 2014, 6, 151–160. [Google Scholar] [CrossRef]
- Lee, J.K.; Grace, K.A.; Taylor, A.J. Effect of a Pharmacy Care Program on Medication Adherence and Persistence, Blood Pressure, and Low-Density Lipoprotein Cholesterol: A randomized controlled trial. JAMA 2006, 296, 2563–2571. [Google Scholar] [CrossRef]
- Mendes, Z.; Crisóstomo, S.; Marques, F.B.; Martins, A.P.; Rodrigues, V.; Ribeiro, C.F. Desperdício de medicamentos no ambulatório em Portugal. Rev. Port. Med. Geral Fam. 2010, 26, 12–20. [Google Scholar] [CrossRef]
- Tan, J.Z.Y.; Kwan, Y.H. Stability of chronic medicines in dosage administration aids. How much have been done? Saudi Pharm. J. 2016, 24, 21–28. [Google Scholar] [CrossRef]
- Vasileiou, K.; Barnett, J.; Thorpe, S.; Young, T. Characterising and justifying sample size sufficiency in interview-based studies: Systematic analysis of qualitative health research over a 15-year period. BMC Med. Res. Methodol. 2018, 18, 148. [Google Scholar] [CrossRef] [PubMed]

| Participant | Pharmacy | Role | Sex |
|---|---|---|---|
| #1 | A | Pharmacist | Female |
| #2 | B | Owner | Male |
| #3 | C | Technical director | Female |
| #4 | D | Pharmacist | Female |
| #5 | E | Pharmacist | Female |
| #6 | F | Pharmacist | Female |
| #7 | G | Pharmacist | Female |
| #8 | H | Pharmacist | Female |
| #9 | I | Pharmacist | Female |
| #10 | J | Technical director | Female |
| #11 | K | Technical director | Female |
| #12 | L | Technical director | Female |
| #13 | M | Technical director and owner | Female |
| #14 | N | Technical director | Female |
| #15 | O | Pharmacist | Female |
| #16 | P | Pharmacist | Female |
| #17 | Q | Technical director | Female |
| #18 | R | Pharmacist | Female |
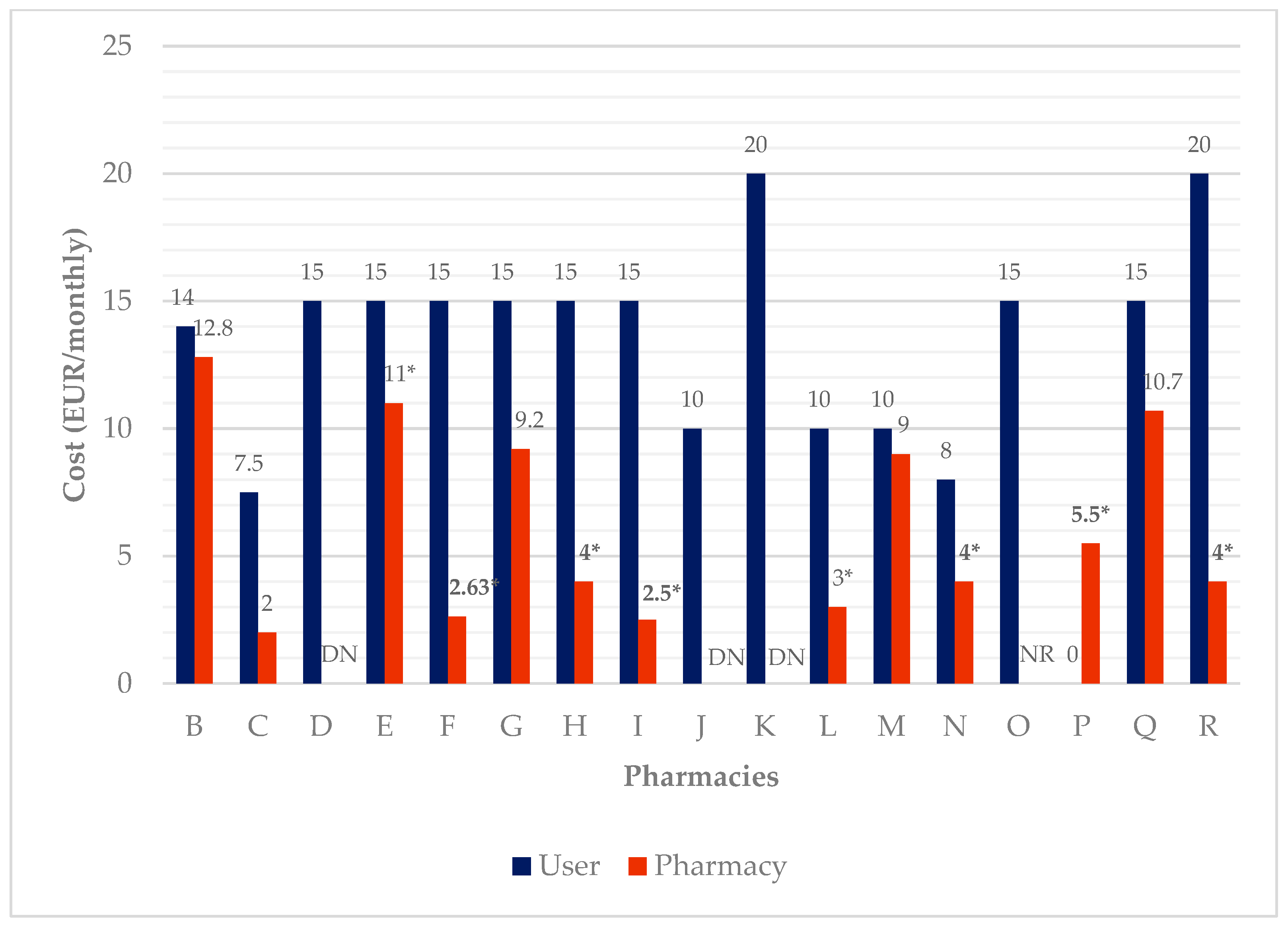
| Pharmacy | For How Long Has the Service Been Provided? (Years) | Number of Current Users | DAAS’ Cost (EUR/Monthly) | Methodology | ||
|---|---|---|---|---|---|---|
| Institutionalized | Ambulatory | User | Pharmacy | |||
| A | 10 | 350 | 0 | N/A | N/A | Weekly BP and monthly SS |
| B | 2 | 0 | 82 | 14 | 12.8 | Weekly BP |
| C | 2 | 0 | 10 | 7.5 | 2 | Weekly BP |
| D | 6 | 0 | 4 | 15 | DK | Monthly BP |
| E | 5 | 0 | 7 | 15 | 11 * | Weekly BP |
| F | 9 | 0 | 19 | 15 | 2.63 * | Weekly, biweekly, or monthly BP |
| G | 8 | 0 | 3 | 15 | 9.2 | Weekly or monthly BP |
| H | 6 | 0 | 3 | 15 | 4 * | Weekly or monthly BP |
| I | 6 | 0 | 15 | 15 | 2.5 * | Weekly BP |
| J | 4 | 1000 | 10 | 10 | DK | Weekly BP or SS |
| K | 4 | 65 | 5 | 20 | DK | Weekly BP |
| L | 0.5 | 59 | 6 | 10 | 3 * | Weekly BP |
| M | 8 | 40 | 15 | 10 | 9 | Weekly BP |
| N | 3 | 11 | 5 | 8 | 4 * | Weekly BP |
| O | 5 | 0 | 10 | 15 | NR | Monthly BP |
| P | 1 | 20 | 2 | 0 | 5.5 * | Weekly BP |
| Q | 5 | 72 | 3 | 15 | 10.7 | Weekly BP |
| R | 2 | 88 | 1 | 20 | 4 * | Weekly ABP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, A.; Mónico, B.; Lourenço, M.; Lourenço, O. Dose Administration Aid Service in Community Pharmacies: Characterization and Impact Assessment. Pharmacy 2021, 9, 190. https://doi.org/10.3390/pharmacy9040190
Vicente A, Mónico B, Lourenço M, Lourenço O. Dose Administration Aid Service in Community Pharmacies: Characterization and Impact Assessment. Pharmacy. 2021; 9(4):190. https://doi.org/10.3390/pharmacy9040190
Chicago/Turabian StyleVicente, André, Beatriz Mónico, Mónica Lourenço, and Olga Lourenço. 2021. "Dose Administration Aid Service in Community Pharmacies: Characterization and Impact Assessment" Pharmacy 9, no. 4: 190. https://doi.org/10.3390/pharmacy9040190
APA StyleVicente, A., Mónico, B., Lourenço, M., & Lourenço, O. (2021). Dose Administration Aid Service in Community Pharmacies: Characterization and Impact Assessment. Pharmacy, 9(4), 190. https://doi.org/10.3390/pharmacy9040190







