1. Introduction
Three-dimensional (3D) printing has paved a way forward through the technological paradigm shift by creating massive opportunities in diverse fields. The pharmaceutical field has faced slow innovation progress since the 1960s, and drug development procedures are time-consuming and require enormous resources [
1]. Further, industrially produced drugs mostly fall under the principle of one-size-fits-all, where only a few dosage forms and strengths are produced, limiting the patient’s benefit and increasing associated side effects. Individual variation in drug response is due to the genetic and metabolic differences between patients [
2]. This dilemma led to the emergence of the Precision Medicine Initiative in 2015 in the USA, which shifted the demand from mass production of medicine to personalized medicine. Personalized medicine tailors a drug dose for a patient based on their needs while increasing treatment efficacy and reducing side effects [
3].
The recent innovation of 3D printing has presented immense prospects for revolutionizing the personalization of drug dispensing. The FDA approval of the levetiracetam (Spritam
®) tablet in 2015 indirectly led to a tremendous increase in 3D printing research initiatives for the production of personalized medication and dose tailoring. Spritam
® is the first commercially available 3D printed drug; it is a highly porous tablet that disintegrates within seconds, and it is useful in epileptic seizures in adult and children [
2].
Three-dimensional (3D) printing is the fabrication of a 3D structure into any shape and size by depositing materials layer-by-layer, and it is designed by computer-aided design (CAD) software. The current 3D printing technologies being investigated to fabricate medications vary from powder solidification (drop on solid, selective laser sintering), liquid solidification (stereolithography (SLA), microneedles, and drop on drop), and extrusion-based methods (fused deposition modeling (FDM) and extrusion at room temperature). The dominant techniques used are powder solidification and extrusion-based printing [
2,
4].
This technology can provide solutions to drug formulations that are not approachable by conventional manufacturing technologies. For example, the conventional fixed-strength production of drugs that have a narrow therapeutic index is commonly associated with fluctuating efficacy and toxicity. Three-dimensional (3D) printing can produce the exact dose needed by the patient, improving effectiveness and reducing toxicity [
5]. Polypill is a pharmaceutical pill that contains a combination of drugs targeting a specific chronic disease such as hypertension and aims to reduce the number of tablets consumed by the patient to increase adherence to the therapeutic regimen. Shaban et al. successfully 3D printed a cardiac polypill consisting of five drugs, including an immediate-release compartment containing aspirin and hydrochlorothiazide, and a controlled release compartment containing atenolol, pravastatin, and ramipril. This ability to produce a complex system allows customization of the dose and release of each drug in the polypill system [
6].
The Saudi Ministry of Health (MoH) established a new vision in 2012 aimed to improve the clinical pharmacy and pharmaceutical care services and reduce drug-related problems. This vision resulted in several initiatives that promote personalized medicine such as special pharmacy clinics that are run by specialized pharmacists. The anticoagulant clinic is an example of the special pharmacy where the pharmacists have the authority to adjust and change anticoagulant drugs when required. Other specialized pharmacy clinics include cardiology, solid organ transplant, pain, oncology, and infectious diseases [
7]. The Saudi Food and Drug Authority (SFDA), which has the role to regulate food, drugs, and medical devices in Saudi Arabia, has issued general guidance for the use of 3D printing in the production of personalized medical devices. This guidance includes basic regulatory requirements needed to approve the use of 3D printing for the production of patient personalized medical devices [
8]. We hope that the SFDA issue guidance and regulations specific for the use of 3D printing in the production of personalized medicine.
Even though the concept of 3D printing has been revolutionary, there are certain challenges for its implementation into the clinical pharmacy setting. The lack of knowledge and experience of 3D printing compared to the conventional manufacturing process poses several challenges, and many questions related to regulations, quality, and safety still remain. Implementing 3D printing in the pharmacy is complex and requires the development of effective strategies for effective outcomes. These implementation strategies require cooperation between practicing pharmacists, legislative bodies, and manufacturers.
The present study aimed to assess knowledge and attitude among pharmacists in Saudi Arabia about 3D printing technology as an innovative dispensing method for personalized medicine, and assess the readiness and possible barriers for implementation.
2. Materials and Method
This observational cross-sectional designed survey was conducted to explore the knowledge and the attitude of the pharmacists working in Saudi hospitals toward the future use of 3D printing technique in the dispensing of personalized medicine. In addition, the study evaluates the readiness to implement such innovative technology in clinical practice. The questions were informed by reviewing the latest literature of 3D printing in the field of personalized medicine and previous surveys that studied the implementation of personalized medicine. The survey questionnaire was validated by a committee of three experts in the field.
2.1. Participants
The study sample consisted of 156 practicing pharmacists working in Saudi Arabia hospitals. Hospitals in Saudi Arabia are divided into three types: MoH hospitals, other governmental hospitals, and private hospitals. Participating in the survey was voluntary, and participants were free to leave at any point of the survey. The identity of the participants was kept anonymous with the full confidentiality of their responses. The agreement to participate in the survey was considered as consent. Approval of the study protocol was obtained from Najran University Research Ethical Committee on 20 January 2020 with Ref. No: 20-1-2-20 ET.
2.2. Procedure
Google forms was used to construct and generate a hyperlink to the survey. Practicing pharmacists in Saudi hospitals were invited to participate in the study survey through the Twitter platform. This platform was used to invite the participants, as there was no accessible database to reach the pharmacists working in Saudi hospitals. Invitation messages were sent to users who identified themselves in their account bio as a hospital pharmacist working in a Saudi hospital. Then, the invited pharmacists were asked to distribute the survey among their colleagues in the hospital.
The invitation message consisted of the 3D printing definition, the study objective, survey hyperlink, and a video showing the process of fabrication of a dosage form using a 3D printer. The survey was open for participation between 10 May through 4 June 2020.
2.3. Materials
The survey consisted of 32 questions divided into five sections as following:
First section: Socio-demographic profile. Pharmacists were asked about their educational and professional qualifications, the type of hospital they work in, and the number of years since completing their latest degree.
Second section: Each pharmacist’s knowledge was evaluated regarding the 3D printing technology and their applications in the medical and the pharmaceutical field.
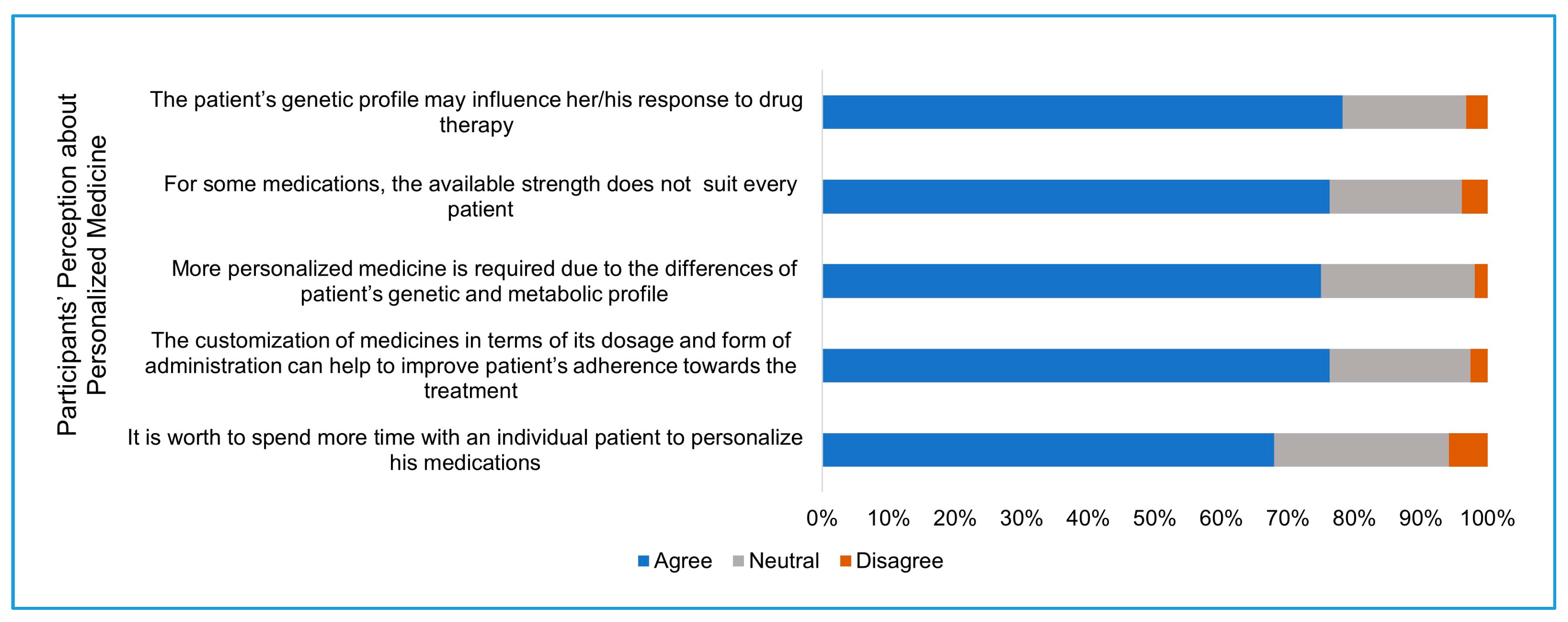
Third section: Participants were asked about their perception on the extent to which a patient’s genetic profile affects their response to drugs, the need for more personalization of medicine, and if the personalization of medicine will enhance patient adherence to the treatment.
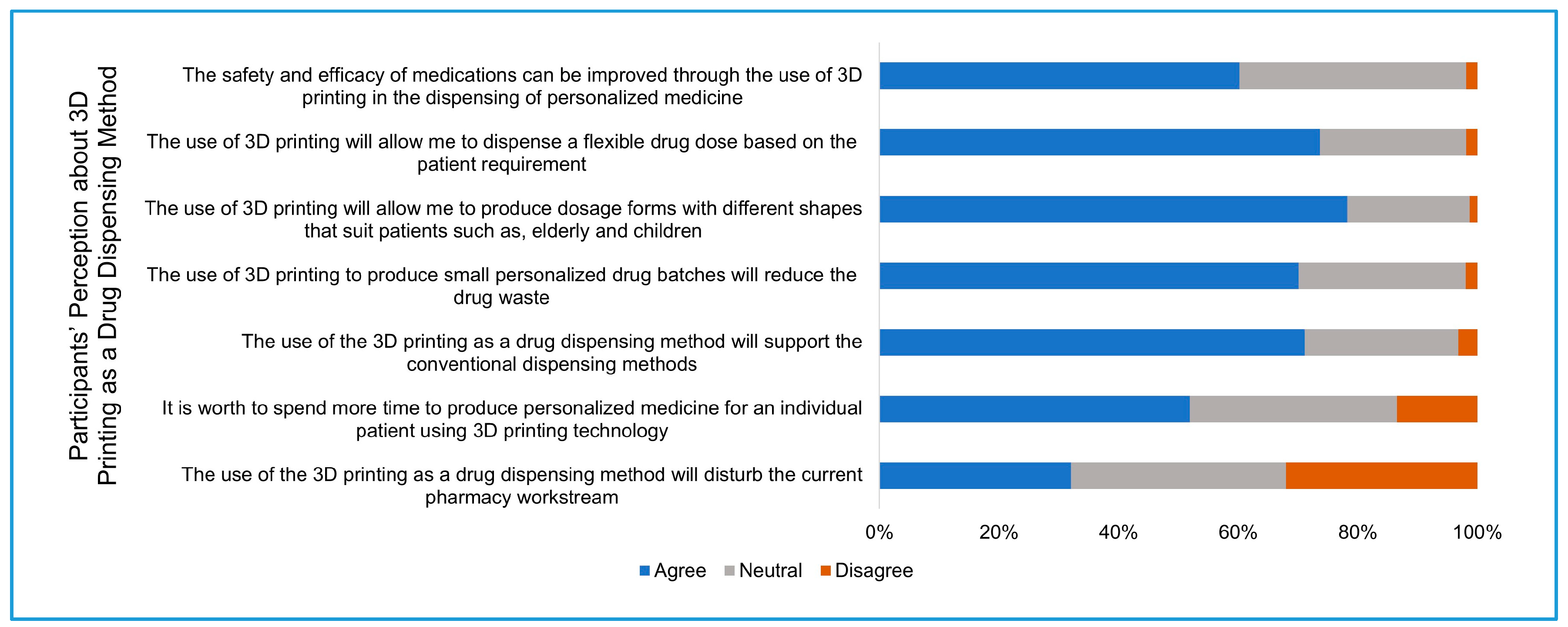
Section four: Pharmacists were asked about their perception regarding the future use of 3D printing as an innovative method of drug dispensing. This perception was evaluated in terms of the role of 3D printing in increasing the efficacy and safety of medication and the possibility of producing different dosages and dosage forms, and the effect of implementing 3D printing on pharmacy workflow.
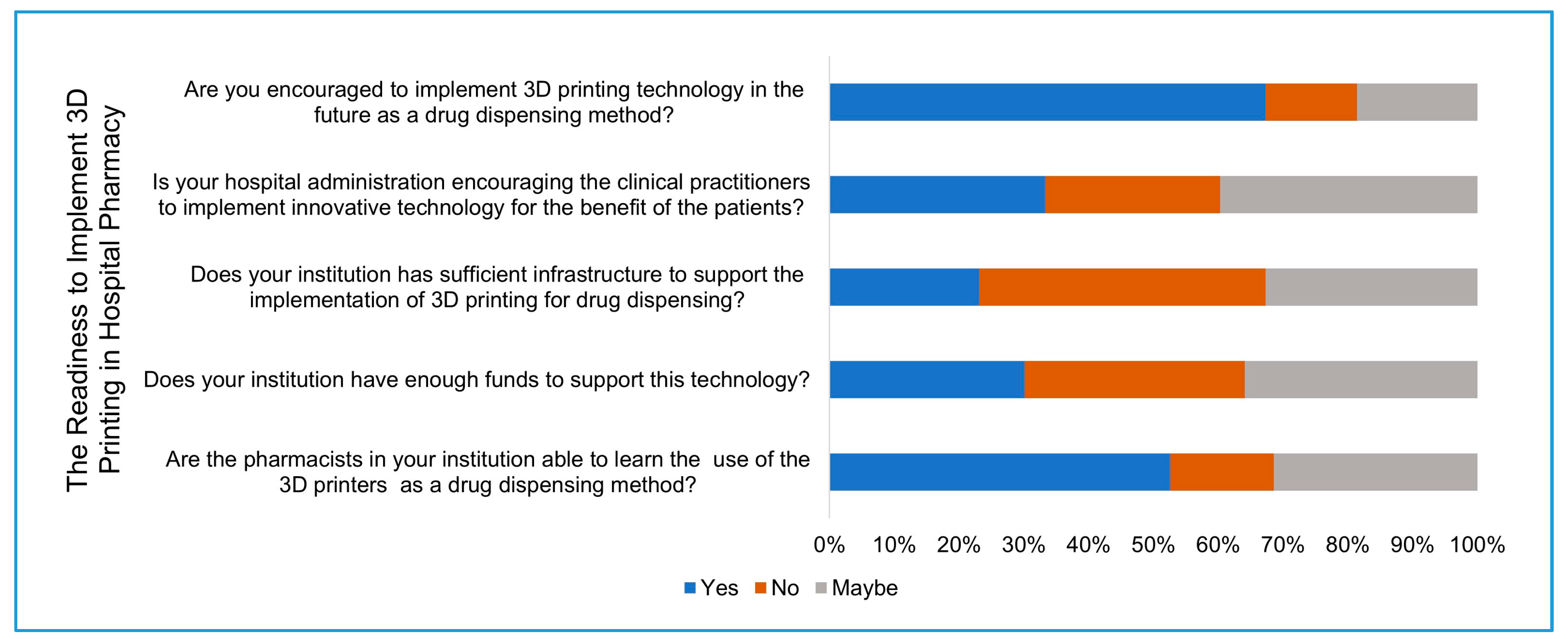
Section five: Participants were asked about the availability of automated drug dispensing systems and the availability of 3D printing in their institution for any medical application and their support of implementing 3D printing in the pharmacy. At the end of the questionnaire, the participants were asked about the expected barriers that might limit the implementation of 3D printing.
2.4. Data Analysis and Statistics
Statistical Package for Social Sciences (SPSS) software (ver. 23; IBM SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The Chi-square test was used to assess associations between categorical variables. Statistical significance was set at p < 0.05.
4. Discussion
This study was designed to evaluate the hospital pharmacist’s knowledge about 3D printing technology and their expectations from this technology as a new method of drug dispensing in pharmacy practice. Three-dimensional (3D) printing has made a big impact in many areas of life, including engineering, educational, and clinical applications [
9]. Pharmaceutical research has been predicted to significantly benefit from 3D printing in the field of drug production. This research has proven that this technology can produce a drug in personalized doses and customize drug releases. Three-dimensional (3D) printing is a promising solution to personalized medication to provide better therapeutic efficacy and less adverse effects [
4]. Despite the tremendous number of studies testing 3D printing technology for the future use in drug dispensing, the pharmacist is still disconnected from this progress; hence, this study was conducted.
The common trend globally is the transformation from general pharmaceutical services to more customized clinical and specialized pharmaceutical care. Pharmacy practice in Saudi is no exception, as it is well-established and has dramatically improved in recent years with accreditation from the Council for Pharmacy Education and the American Society of Health-System Pharmacists [
10]. Several initiatives and practices have been introduced into pharmaceutical services to promote medication therapy management, such as electronic prescriptions, automated dispensing systems, and automated I.V. compounding systems. In addition, there has been an initiation of special pharmacy clinics managed by specialized pharmacists to enhance the treatment efficiency and reduce associated medication errors through a cost-effective approach. Anticoagulant clinics are an example of specialized pharmacies managed by pharmacists with the authority to adjust doses, change medications, and add other anticoagulants when needed. In addition, other specialized pharmacies such as cardiology, solid organ transplant, pain, and oncology have been implemented in several Saudi hospitals [
7]. All of these advanced practices reflect the government’s keenness to improve pharmaceutical services, which suggests that innovative methods such as drug dispensing using 3D printing technology may be one of the future practices in this sector.
The FDA approval of the first drug (Spritam
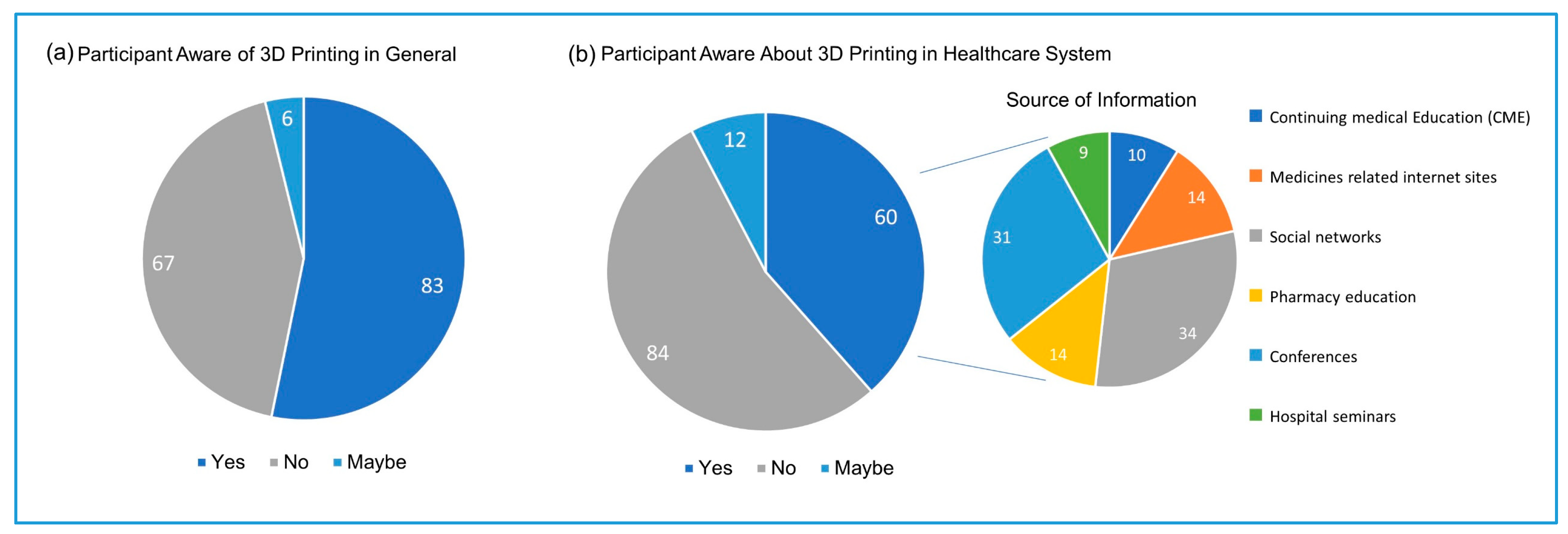
®) produced by 3D printing in 2015 stimulated the enthusiasm to explore the possibility of using this technology in drug dispensing. Despite the high trend of 3D printing in recent years, only 38% of the study participants were aware of the using of this technology in healthcare, and less (22%) were aware that this technology can produce drugs in precise doses. Only 22 out of 156 participants were aware of Spritam
®. Pharmacy education was the source of this awareness of 3D printing use in the healthcare system to only 14 participants, indicating that the traditional pharmacy education in Saudi is lagging behind the trends in the pharmaceutical research. Participants indicated that the main source of awareness about the 3D printing applications in the healthcare systems is the social networks, followed by scientific conferences. Social media has provided a unique opportunity to share new research and clinical guidelines that were once only available through scientific conferences and journals or professional organizations. A systemic review conducted by Benetoli et al. found that the social networks have facilitated professional communications and interaction between the healthcare providers [
11]. Therefore, pharmaceutical researchers and pharmacists are encouraged to share knowledge and communicate recent advances in research and practices through social media guided by professionalism and ethics. Conferences were selected by the participants as the second source of awareness about the use of 3D printing in the healthcare system.
The common practice in pharmaceutical industries is to produce a few discrete drug strengths and forms for all consumers. However, individual variability related to genetic, ethnic, gender, age, and weight makes the concept of one-size-fits-all challenging to achieve in real practice, and some consumers will be exposed to high doses and more side effects and the others exposed to under-therapeutic doses. The idea of personalized medicine has grown dramatically in recent years where dose adjustments are made according to pharmacokinetic and pharmacogenetic profiles of the patient at the point of care in order to improve the efficacy and to reduce the toxicity of the drug. The flexibility of 3D printing to produce a small scale of customized doses and release profiles provides a promising solution to implement personalized medicine practice in the healthcare sector [
12]. The personalization of medicine has been implemented to some extent by pharmaceutical services in Saudi hospitals, such as in the anticoagulant clinic where the doses or drugs are adjusted frequently based on the patient blood profile [
7]. Participants of this study had a positive perception about the concept of personalized medicine. A similar percentage of respondents believe in the need for more drug personalization and believe that it will increase the patient’s commitment to his treatment plan. Interestingly, a high percentage of participants (76%) are willing to spend more time to personalize treatment for individual patients. This additional time could be used to prepare and set up the 3D printer to fabricate the individual patient drug batch. The high perception presented by pharmacists working in Saudi hospitals about the importance of pharmacogenomics [
13], in addition to the high perception given at this study about the importance of personalized medicine, increases the future role of 3D printing in the production of effective and safe medicine.
Three-dimensional (3D) printing presents a promising solution to tailor dosage forms for specific doses and release profiles. Various startup and pharmaceutical companies have started prototyping 3D printers and automated systems specific for the production of pharmaceutical dosage forms. For example, FabRx is a startup biotech company specializing in 3D printing of medicine that managed to bring the first pharmaceutical 3D printer named M3DIMAKER™. This printer is specialized with a hardware that can use different printing nozzles and software to allow the selection of the necessary dose by the pharmacist based on the given prescription [
14]. In this study, participants had high positive expectations about the future application of 3D printing in the pharmacy practice. More than 60% of the participants expected that the use of 3D printing will improve the efficacy and safety of medications, and around three-quarters of participants expected that this technology will give the opportunity to produce flexible dosage forms.
Drug waste has a great negative impact on the economy and the environment. The World Health Organization (WHO) has reported that half of the medications dispensed are sold improperly, and half of the patients do not adhere to their treatment plan [
15]. Faten Alhomoud conducted a survey to study the waste-reducing activities among the practicing pharmacists in the Gulf region countries. Pharmacists participating in this study believe in the importance of reducing drug-waste; however, waste-reducing activities were not practiced continuously [
16]. A study found that on average, 2–3 drugs are expired per household in Saudi [
17]. The ability of 3D printing to produce personalized medications on a small scale, and the concept of a polypill provide a great solution to reduce the drug waste [
12].
The future of 3D printing in pharmacy practice focuses on dispensing the personalized medicine where specific doses, drug release, or dosage form shape is required. Therefore, this technology is designed to support and not to replace the current practice of dispensing the mass produced medicines by the pharmaceutical industries [
2]. Around 70% of the participants agreed that the use of 3D printing as a drug dispensing method will support the current conventional method of drug dispensing. On the other hand, more than 30% of the participants believed that the introduction of this technology will disturb the current workstream in the pharmacy. Therefore, careful planning is needed to implement this technology into the pharmacy practice with minimal disruption to the current workflow, and it will include technical and legislative considerations, infrastructure changes, and training practicing pharmacists for 3D printing [
18]. Automation in drug dispensing has increased globally to improve dispensing accuracy and to reduce human error, as well as to curb the effect of labor cost [
19]. Additionally, automation has allowed the pharmacist to perform more valued patient care practices such as patient counseling and drug monitoring [
20].
The automated unit dose systems and IV compounding system are examples of automation in pharmacy practice. Thirty percent of the participants stated that their hospitals have automatic unit dose systems, and around the same percentage stated that their hospitals have the I.V. compounding system. The implementation of such technologies into the pharmacy practice reflect the willingness of the hospital and pharmacy administration to accommodate new technologies for the benefit of the patient. Since the early 2000s, 3D printing technology has been applied in different medical fields such as dental implants and prosthetics. The applications have expanded considerably to cover several specialties including tissue and organ bioprinting, anatomical models for complex surgery interventions, drug delivery, and personalized implants [
21]. At this study, only eight participants reported the use of 3D printing at their hospitals.
The role of hospital administration has also expanded widely as healthcare systems continuously innovate and develop to respond to the changes in diseases, the impact of aging populations, and the advances in diagnosis and treatments [
22]. Lack of support from healthcare leaders has been identified as one of the major barriers of implementing new technologies [
23]. Despite the large percentage (67%) of participants who support the implementation of 3D printing, only 33.3% of them believe that their hospital administration is supportive of innovative technologies and encourages clinical practitioners to implement them. Furthermore, more than half of the study participants believe that their colleagues are able to learn the process of 3D printing for drug dispensing. Even so, only 30% of the participants believe that their hospitals have sufficient infrastructure to implement 3D printing, and less than 30% of the participants believe that their hospitals have the sufficient financial support to implement this technology.
The Expert Recommendations for Implementing Change (ERIC) project has provided a consensus of 73 discrete implementation strategies for innovative technologies. Those strategies are used to enhance the adoption, the sustainability, and the spread of an innovation. Assessing the readiness and identifying possible barriers is an implementation strategy and is particularly useful before the formal implementation [
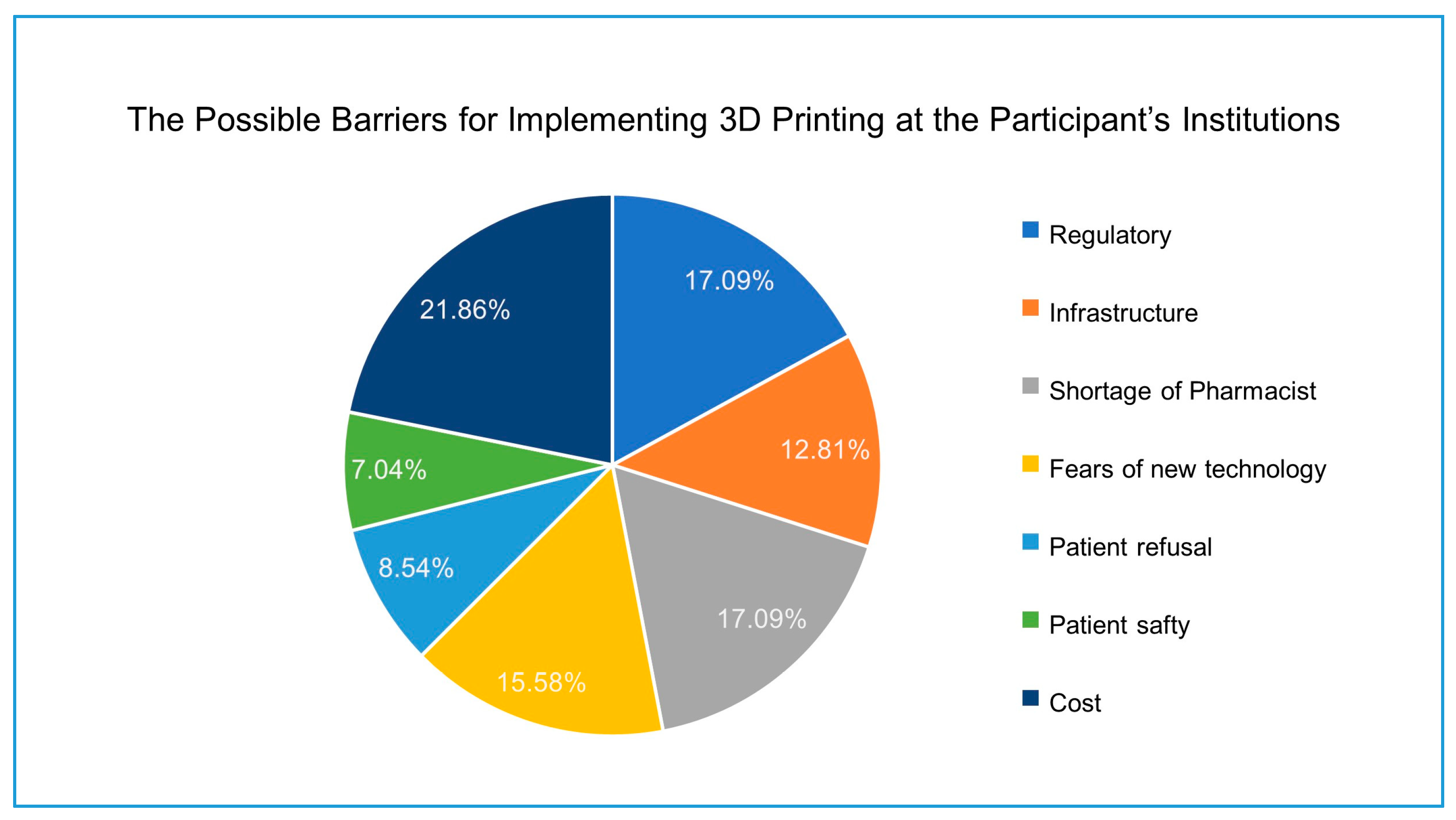
24]. The survey participants assessed the possible barriers that might affect the implementation of 3D printing for drug dispensing. The cost of the technology implementation was selected as the greatest barrier to overcome. Three-dimensional (3D) printing has proven cost-effective, especially for small-scale production. Therefore, 3D printing has a great potential to reduce medical cost. In surgery practice, 3D printing of anatomical models helps the surgeons simulate the surgery to reduce the surgery time, complications, and ultimately reduce the surgery overall cost. The opportunity of producing personalized medicine and polypill formulations will improve the patient compliance and reduce the material costs [
25]. The collaborative effort between the healthcare providers and 3D printer manufacturers, the availability of the printing materials, and the expansion of manufacturing of 3D printing systems designed for medical applications are factors that will reduce the cost of implementation [
26].
Staff shortage was selected by the participants as a barrier for the future implementation of the 3D printing in practice after the cost. The transformation of the general pharmacy practice to a specialized pharmacy practice with more determination on patient counselling and drug monitoring increases the demand for more qualified pharmacists. Albekairy et al. evaluated the sufficiency of the clinical pharmacists in the National Guard Affairs central region hospital, Saudi Arabia. The study indicated that the number of clinical pharmacists is not enough to perform the clinical pharmacy services; the total number of practicing clinical pharmacist was 24 at the time of the study, while the study suggests adding 60 to 65 positions to adequately staff a clinical pharmacy [
27]. The shortage of trained pharmacists is a major reason hindering most pharmacy initiatives or services.
Regulations of pharmacy practice is another possible barrier for the future implementation of 3D printing in pharmacy. The general administration of pharmaceutical care in the MoH and the SFDA are the regulatory bodies for the pharmaceutical services, including clinical pharmacy practice and clinical pharmacy programs. Since 2013, the general administration of pharmaceutical care has implemented a national plan with new set of standards and regulations to meet all the American Society of Health-System Pharmacists (ASHP) standards and regulations [
28]. The application of 3D printing in the medical field is still in its infancy; however, the FDA has been the forerunner on implementation and has issued technical guidance for the additive manufacturing of medical devices on December 2017. The guidance is consists of two sections: Design and manufacturing considerations, and printed device testing considerations [
29]. The FDA Drug Evaluation and Research approved Spritam
® as the first 3D-printed drug in 2015. This medicine complies with the existing chemistry, manufacturing, and control standards as any other solid dosage form [
30]. The uniqueness of the 3D printing process compared to the conventional drug production and the lack of previous clinical experience raises challenges to regulate such practice. One of the challenges is the quality assurance of 3D-printed dosage forms. The current traditional methods used to test the quality of a conventional tablet including the content uniformity, disintegration, hardness, and dissolution test will vary when applied to the 3D printed formulations. In addition, such tests are destructive and impractical in the clinical sitting. Therefore, revised quality tests need to be issued by the FDA and pharmacopoeias to be more applicable for the 3D printed formulations [
12].
Fear of new technology is the next barrier for the future implementation of 3D printing for drug dispensing. Understandably, most of the practicing pharmacists have reservations with any change that can affect the pharmaceutical services workflow. Fear of implementing new technologies such as 3D printing may restrict or slow the development of pharmacy services [
31].
Patient safety and patient refusal were the lowest barriers for the implementation of 3D printing. However, a study by Goyanes et al. conducted a study in 2017 among pediatric patients to evaluate isoleucine blood levels after six months of treatment with two types of formulations: conventional capsules prepared by manual compounding, and personalized chewable formulations prepared by automated 3D printing. Three-dimensional (3D) printing therapy was well-accepted by patients and offered a feasible, rapid, and automated approach to tailor made doses in hospital settings [
32]. Further research and clinical trials exploring patient’s acceptance, response, and safety are necessary.
The researchers estimated a gap of 17 years between a clinical innovation’s proven effectiveness and its routine adoption into the healthcare system [
24]. There is a crucial need to collaborate between the 3D manufacturers, clinical practitioners, and regulatory bodies to develop strategies to accelerate the implementation of 3D printing in drug dispensing. This study is unique, as it for the first time assesses the knowledge and perceptions of the pharmacists along with elucidation of barriers faced by them in 3D printing implementation in their institutions.