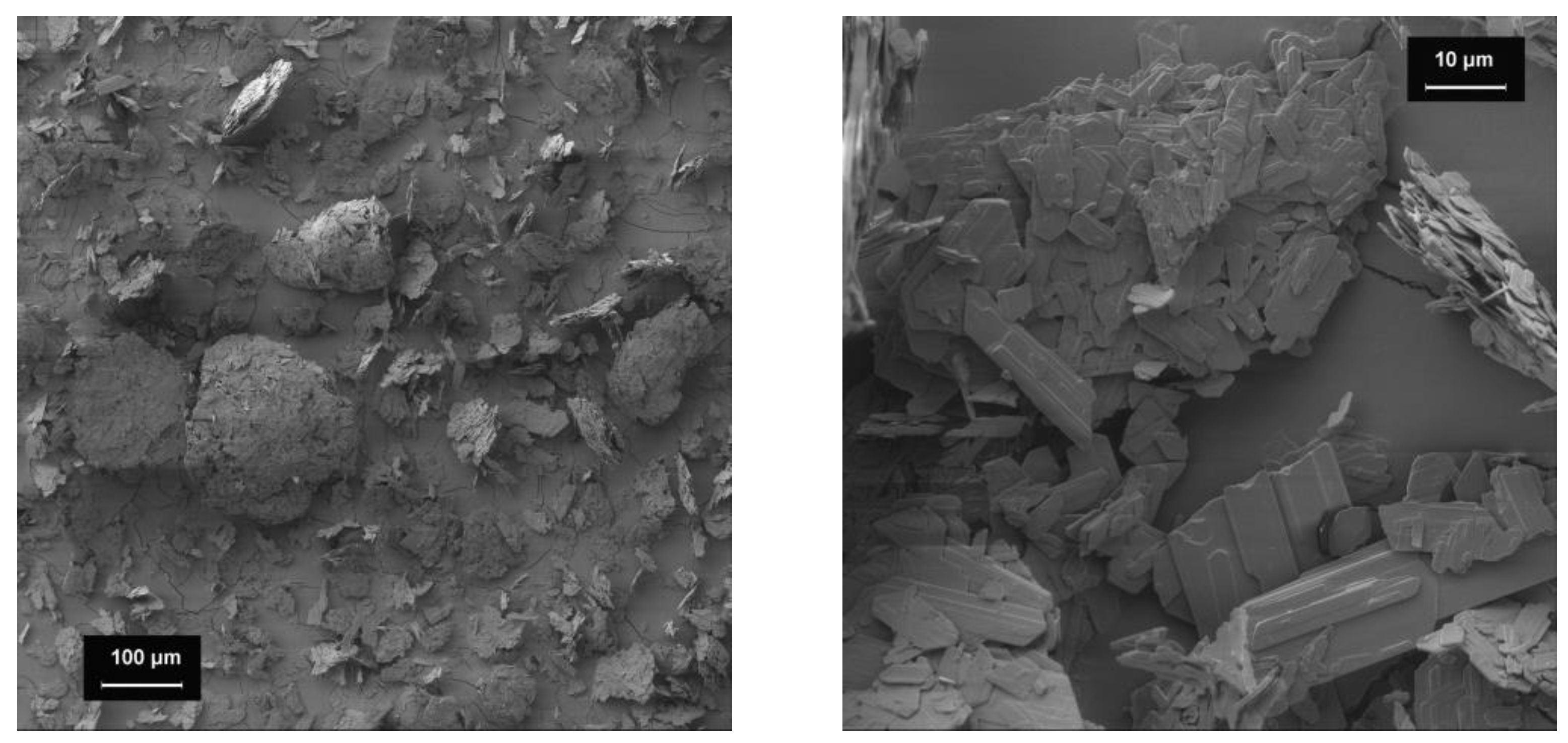
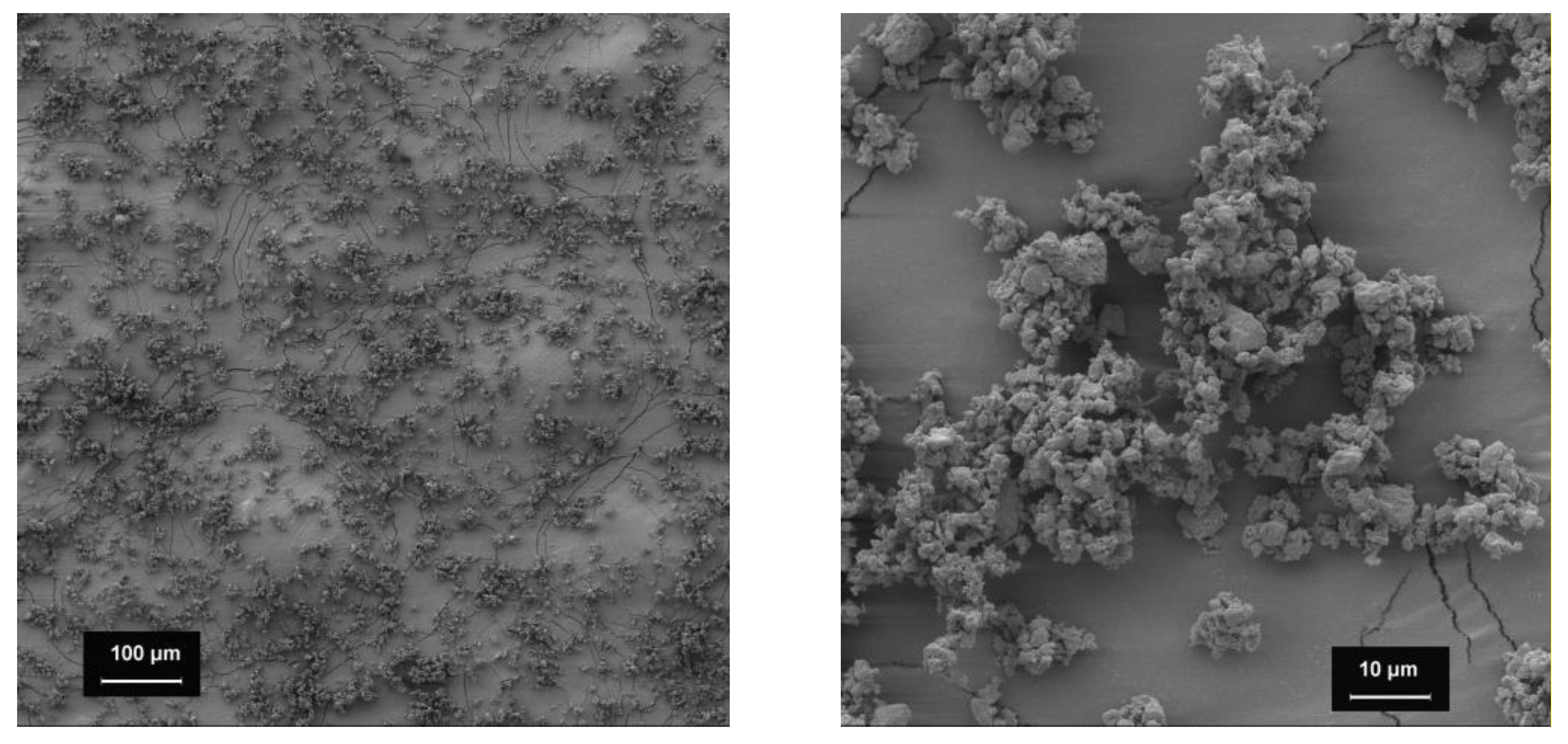
Figure 1.
SEM images of the bulking agents (1000-fold magnification). Bulking agents comprising mcc (top left), la (top right) and m35 (lower middle).
Figure 1.
SEM images of the bulking agents (1000-fold magnification). Bulking agents comprising mcc (top left), la (top right) and m35 (lower middle).
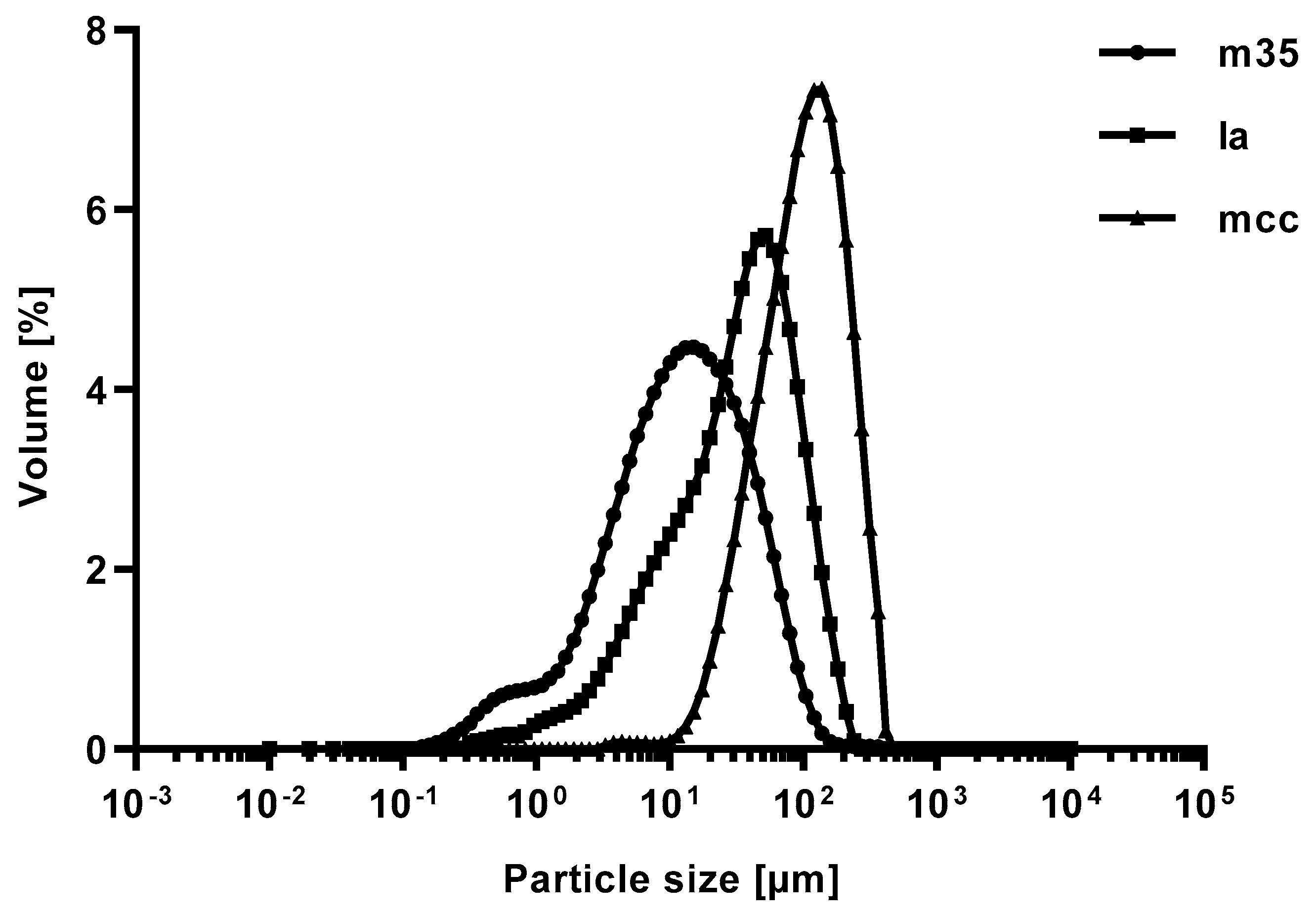
Figure 2.
Volume fraction of the particle size distribution of bulking agents comprising mcc (top left), la (top right) and m35 (lower middle). Measured by laser diffraction.
Figure 2.
Volume fraction of the particle size distribution of bulking agents comprising mcc (top left), la (top right) and m35 (lower middle). Measured by laser diffraction.
Figure 3.
SEM-images of baclofen;100-fold magnification (left), 1000-fold magnification (right).
Figure 3.
SEM-images of baclofen;100-fold magnification (left), 1000-fold magnification (right).
Figure 4.
SEM-images of spironolactone; 100-fold magnification (left), 1000-fold magnification (right).
Figure 4.
SEM-images of spironolactone; 100-fold magnification (left), 1000-fold magnification (right).
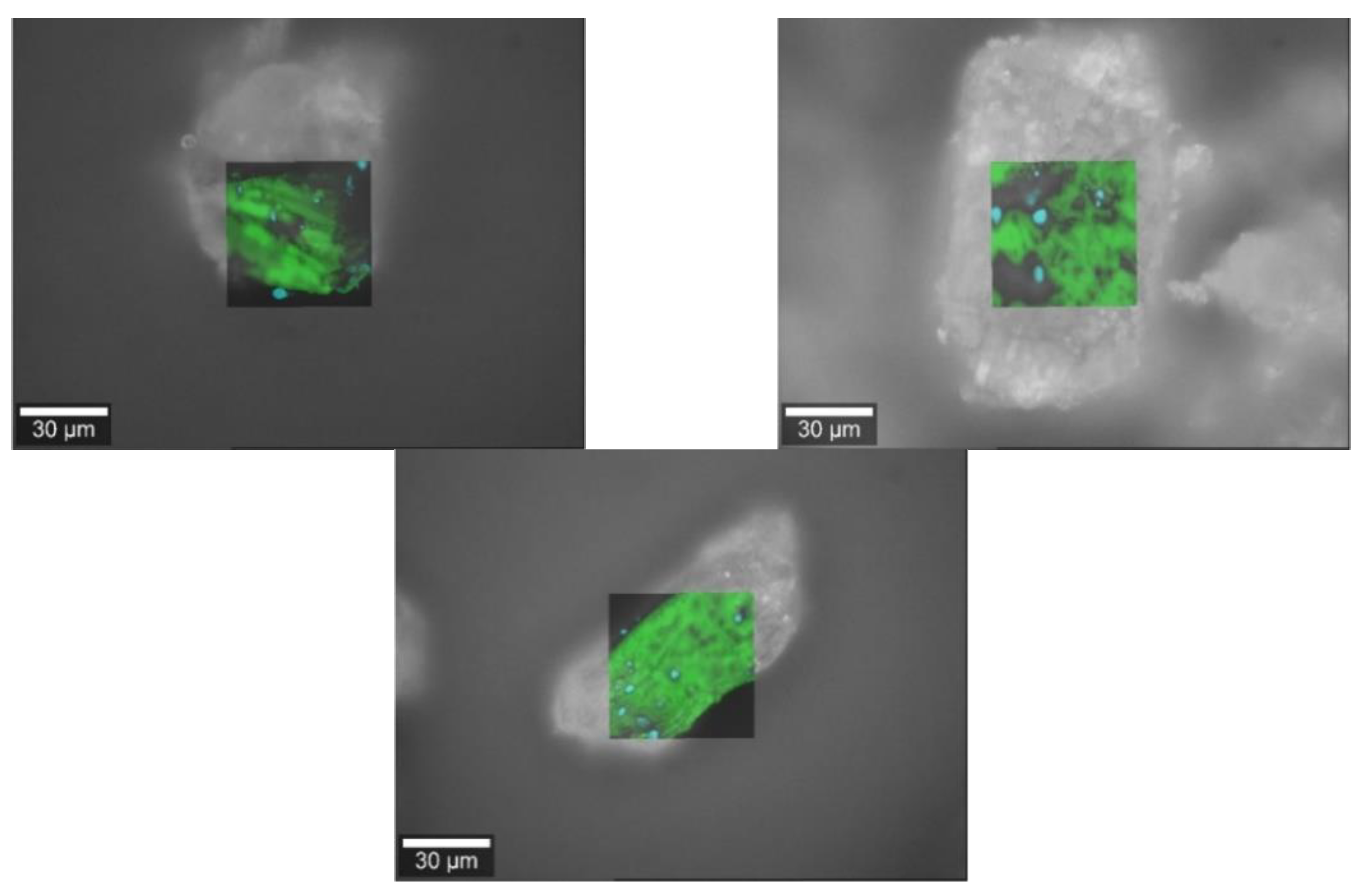
Figure 5.
Raman images of baclofen (yellow) and the bulking agents (green) comprising m35 (top left), la (top right) and mcc (bottom middle).
Figure 5.
Raman images of baclofen (yellow) and the bulking agents (green) comprising m35 (top left), la (top right) and mcc (bottom middle).
Figure 6.
Raman images of spironolactone (cyan) and the bulking agents (green) comprising m35 (top left), la (top right) and mcc (bottom middle).
Figure 6.
Raman images of spironolactone (cyan) and the bulking agents (green) comprising m35 (top left), la (top right) and mcc (bottom middle).
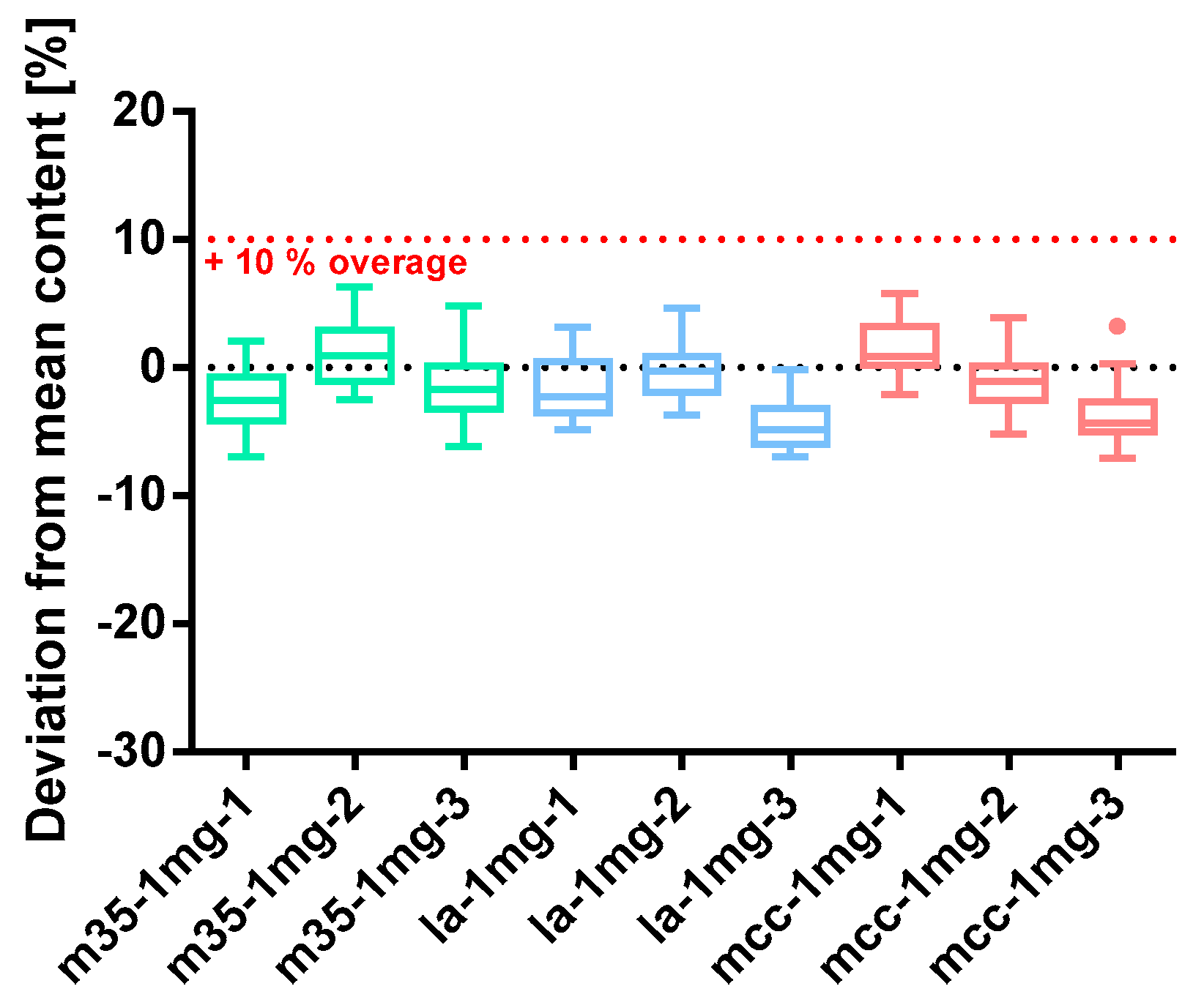
Figure 7.
Deviation from the mean content of baclofen 1 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
Figure 7.
Deviation from the mean content of baclofen 1 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
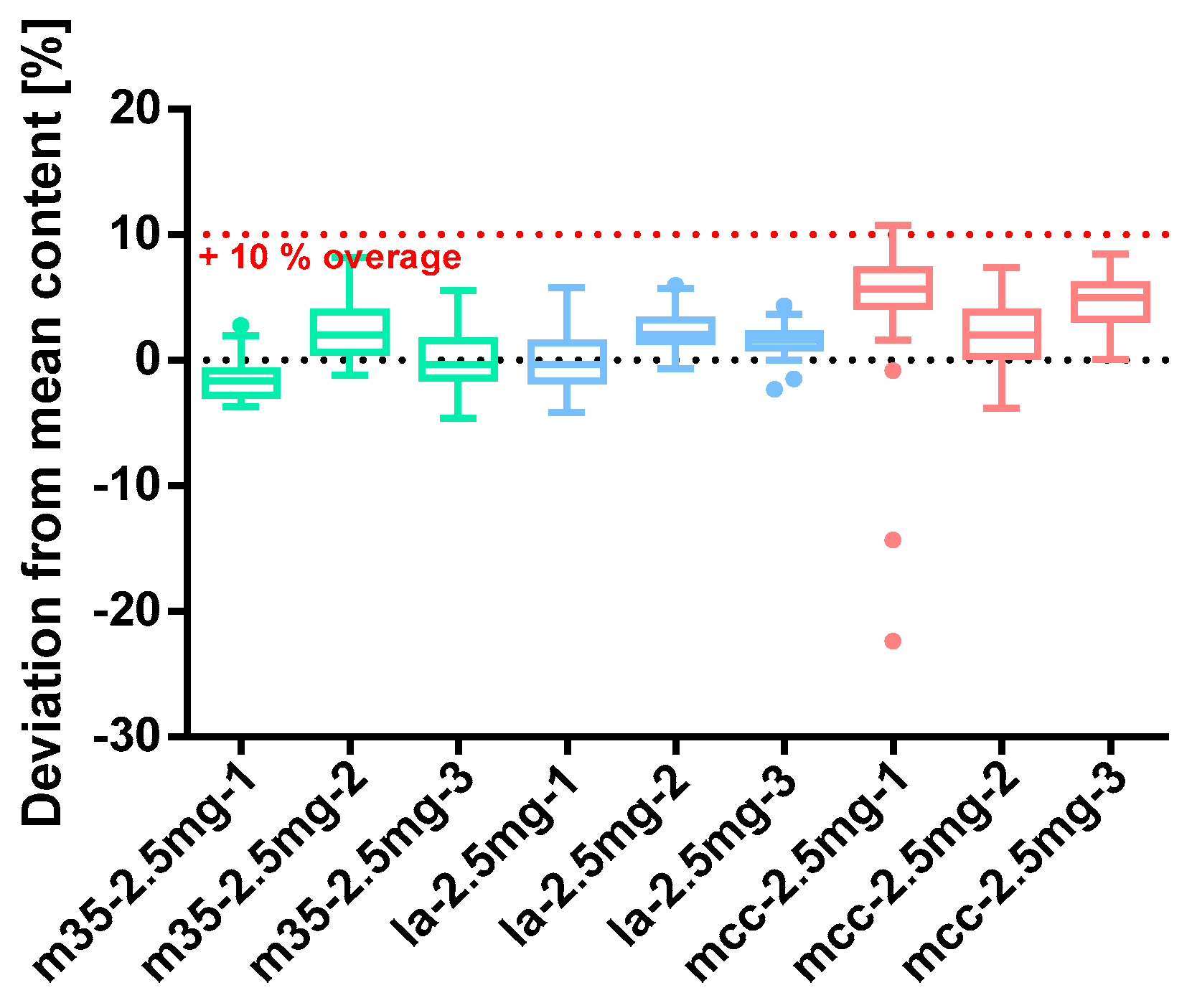
Figure 8.
Deviation from the mean content of baclofen 2.5 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
Figure 8.
Deviation from the mean content of baclofen 2.5 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
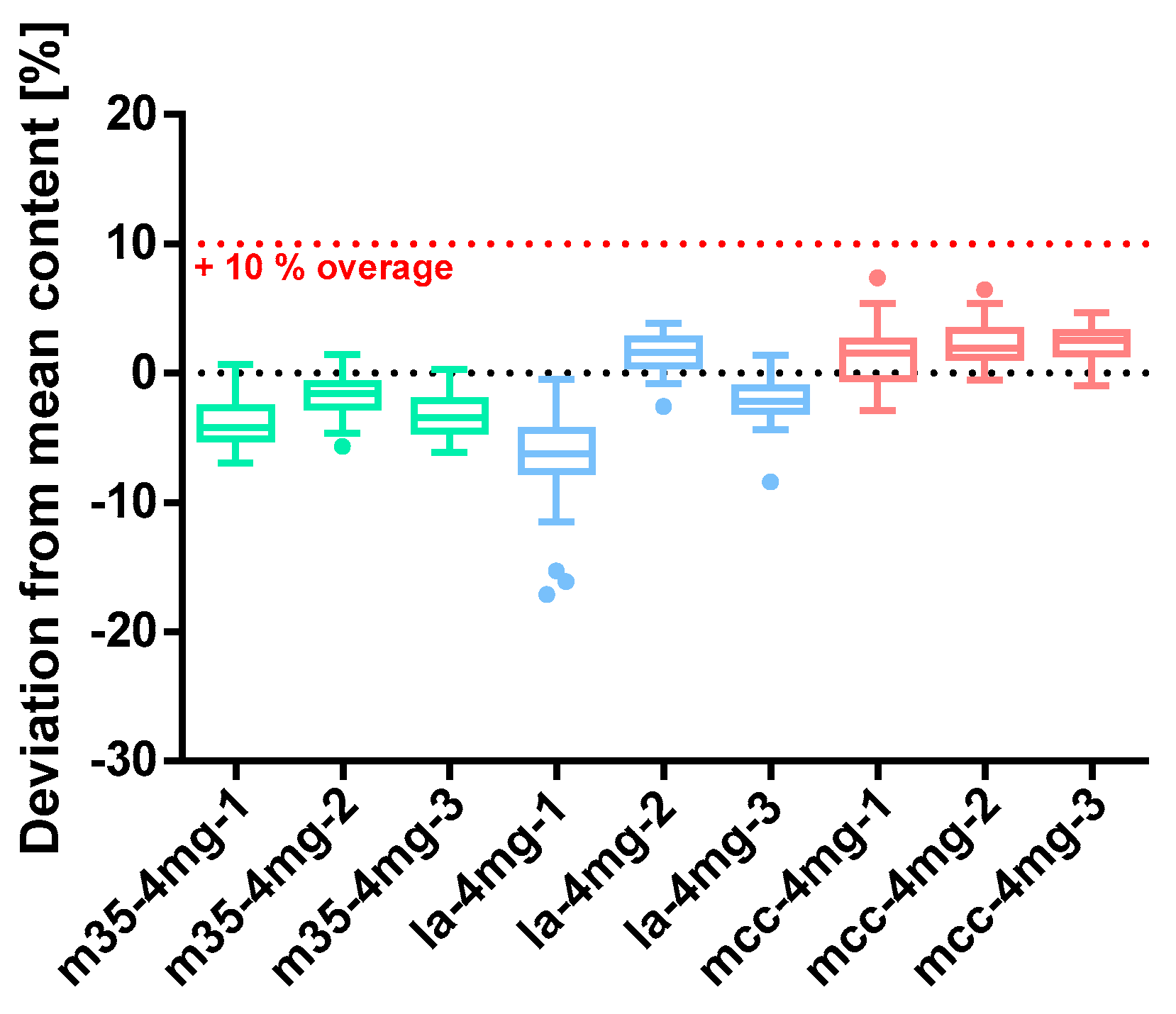
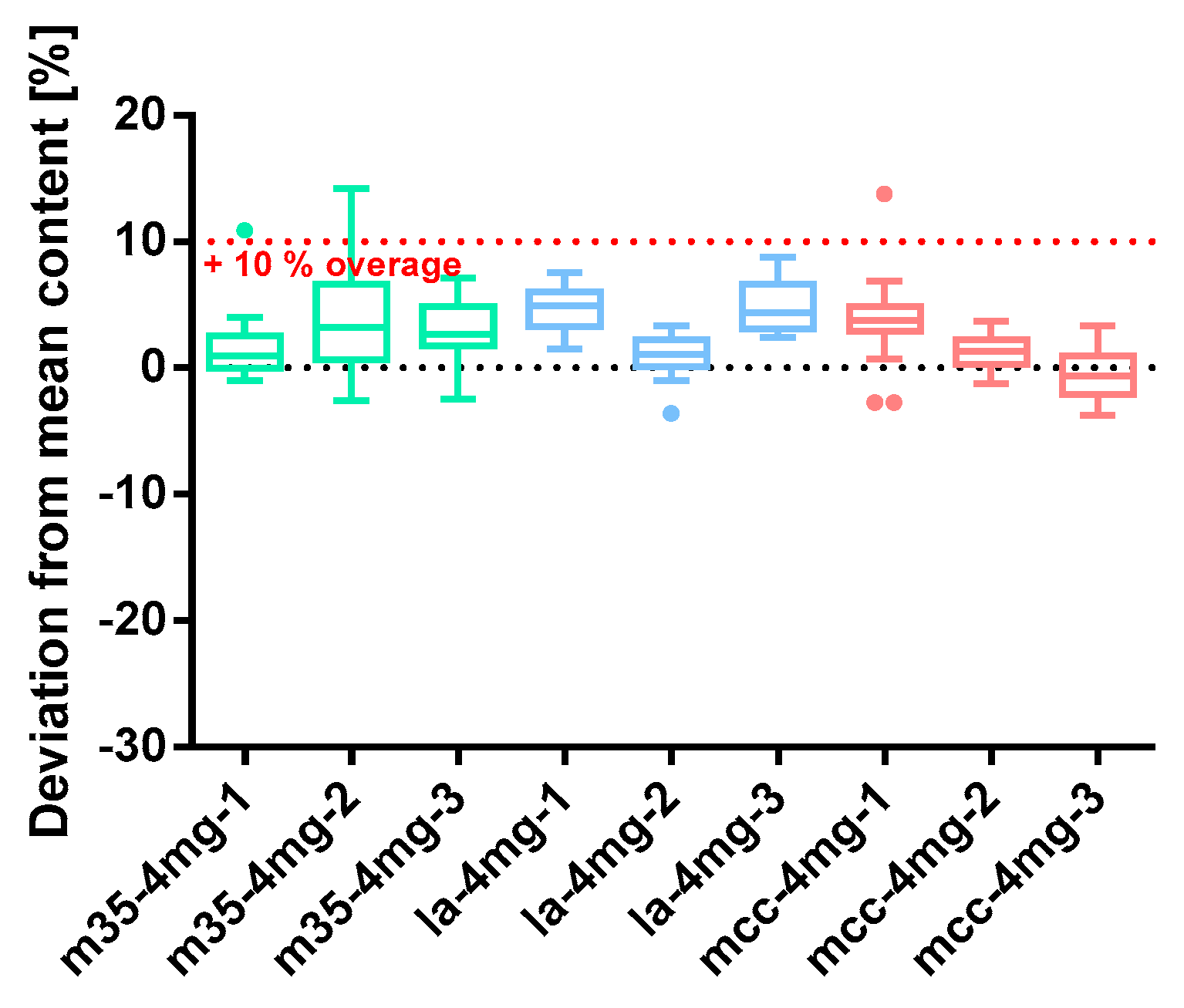
Figure 9.
Deviation from the mean content of baclofen 4 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
Figure 9.
Deviation from the mean content of baclofen 4 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
Figure 10.
Deviation from the mean content of spironolactone 0.5 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50°% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
Figure 10.
Deviation from the mean content of spironolactone 0.5 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50°% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
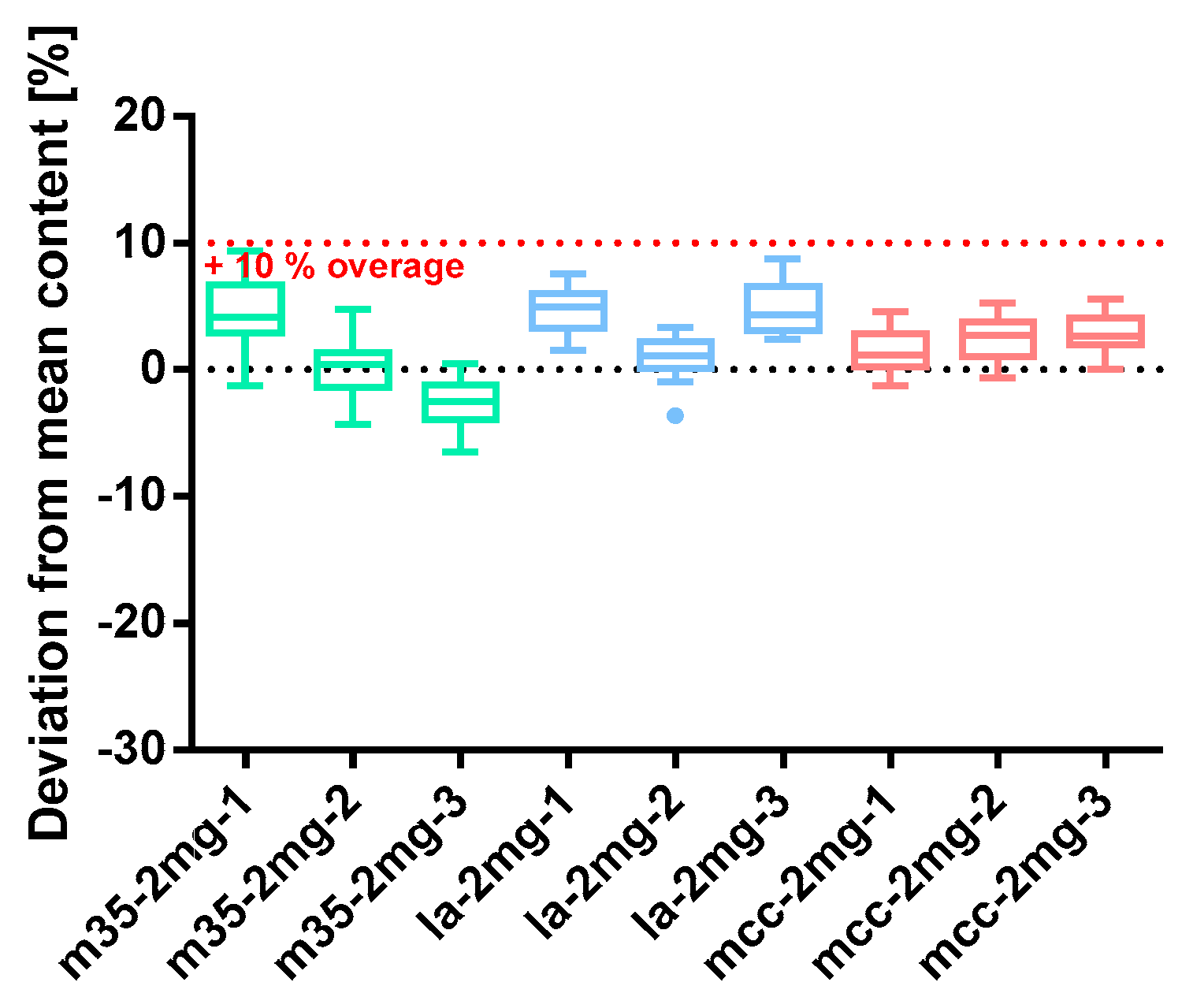
Figure 11.
Deviation from the mean content of spironolactone 2 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50°% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
Figure 11.
Deviation from the mean content of spironolactone 2 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50°% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
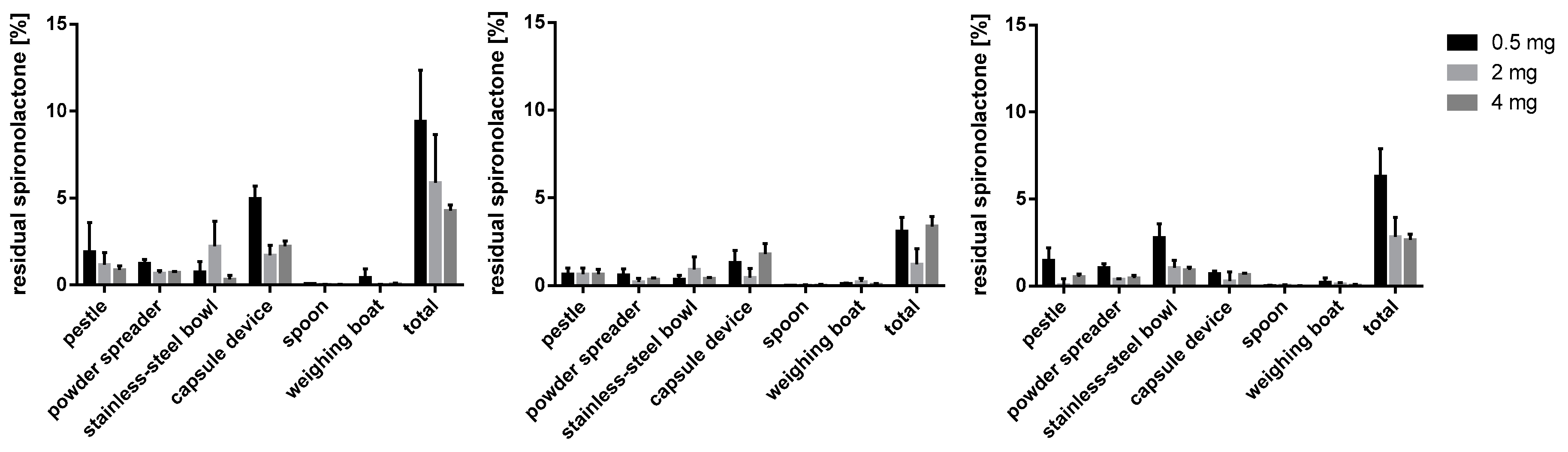
Figure 12.
Deviation from the mean content spironolactone 4 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50°% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
Figure 12.
Deviation from the mean content spironolactone 4 mg capsule batches prepared with bulking agents comprising m35, la and mcc; Tukey’s box plot: □ box—includes 50°% of all data points and shows the median (line in the box), the length of the box resembles the interquartile range (IQR); T whisker—includes values within the 1.5-fold IQR; ● outlier; n = 30.
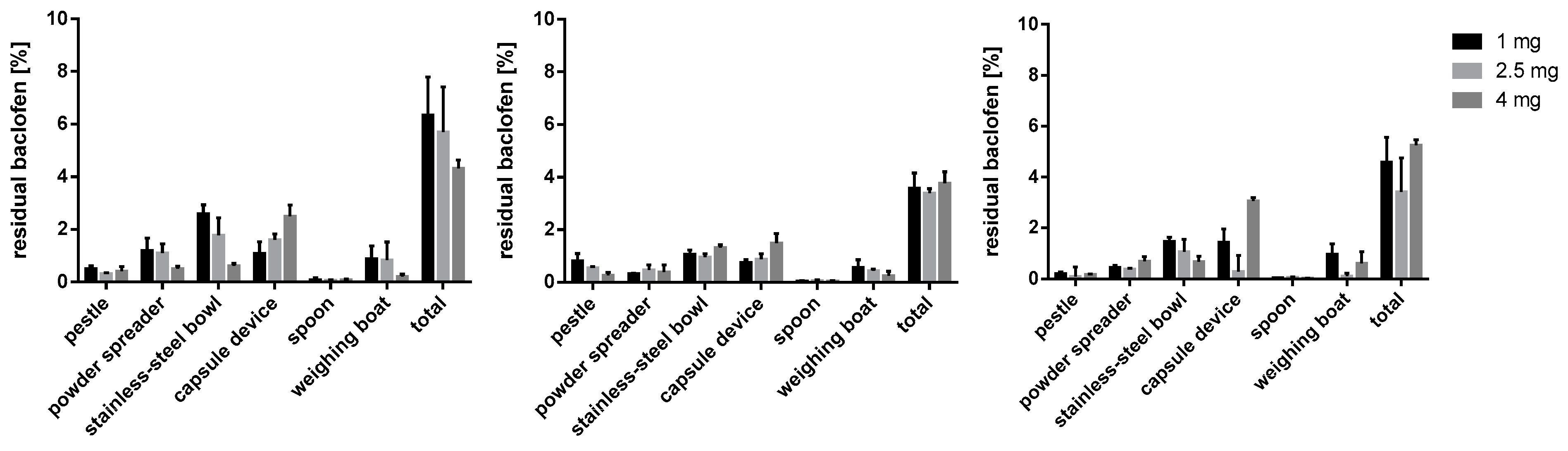
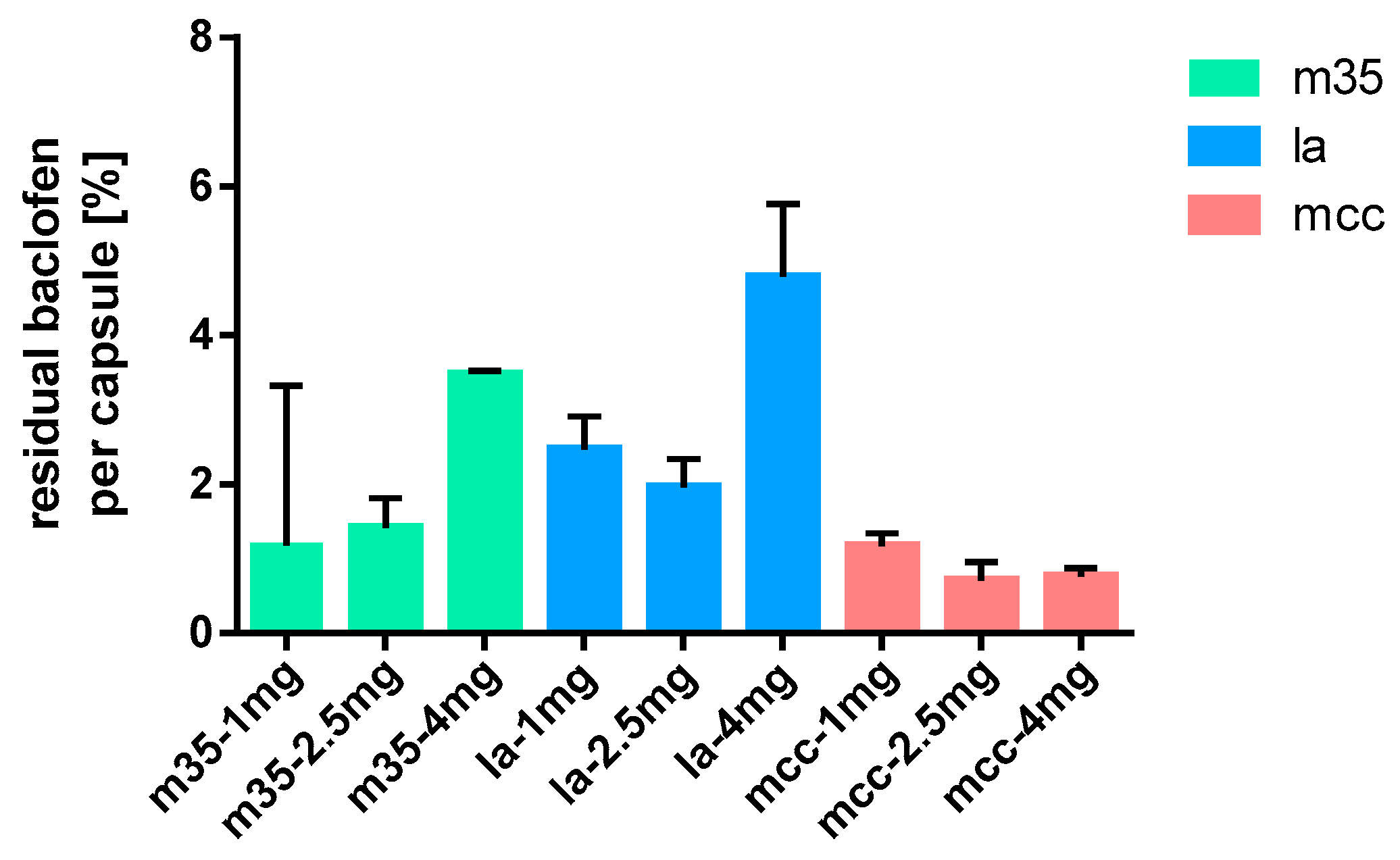
Figure 13.
Baclofen residues on used equipment; m35 (left), la (middle), mcc (right); n = 3.
Figure 13.
Baclofen residues on used equipment; m35 (left), la (middle), mcc (right); n = 3.
Figure 14.
Spironolactone residues on used working materials; m35 (left), la (middle), mcc (right); n = 3.
Figure 14.
Spironolactone residues on used working materials; m35 (left), la (middle), mcc (right); n = 3.
Figure 15.
Baclofen residues on capsule shells after emptying; n = 15.
Figure 15.
Baclofen residues on capsule shells after emptying; n = 15.
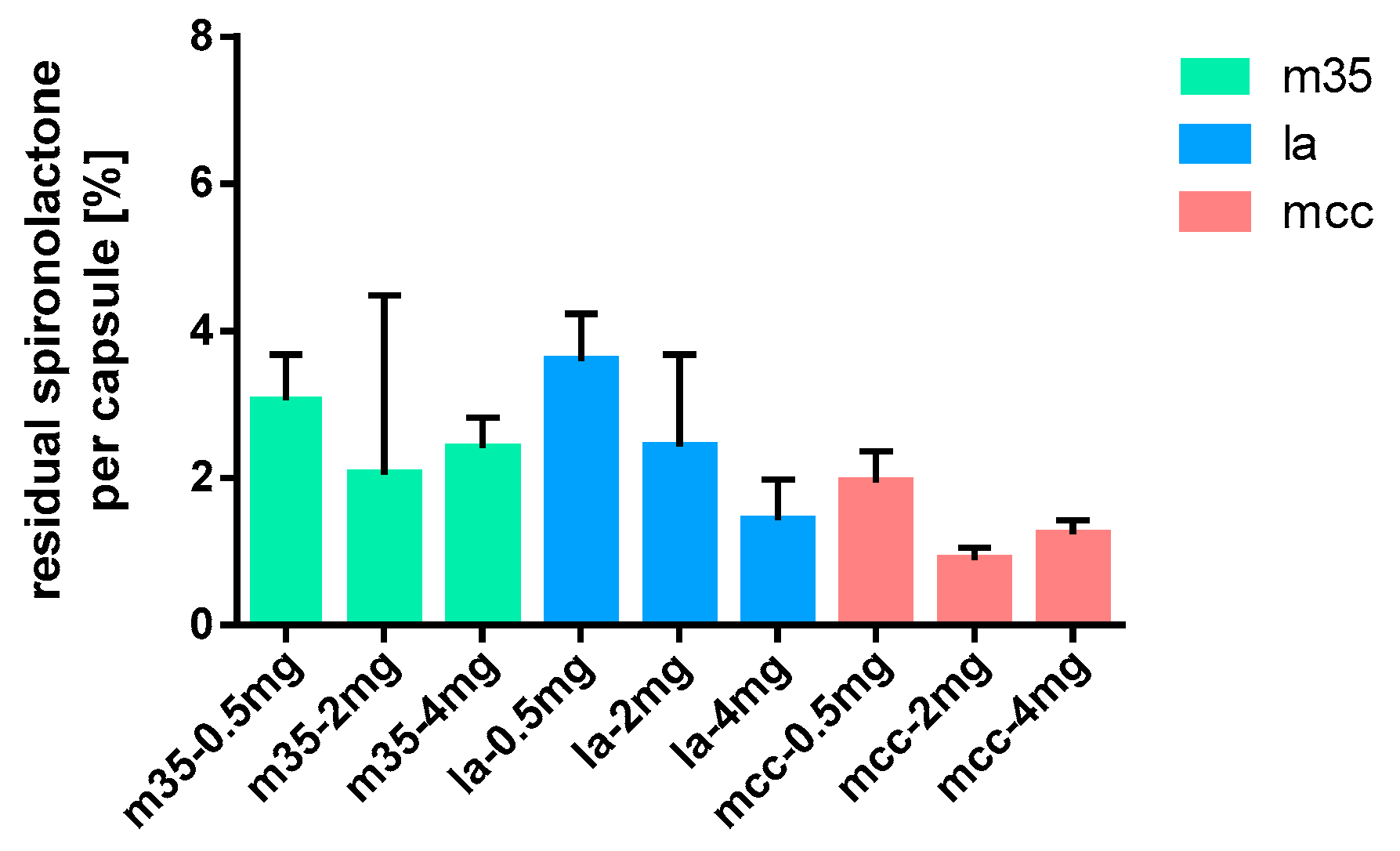
Figure 16.
Spironolactone residues on capsule shells after depletion; n = 15.
Figure 16.
Spironolactone residues on capsule shells after depletion; n = 15.
Table 1.
Composition of the powder blends used to prepare 30 capsules including a 10% overage of the API.
Table 1.
Composition of the powder blends used to prepare 30 capsules including a 10% overage of the API.
| | API [mg] | m35 [mg] | la [mg] | mcc [mg] | Colloidal Silica [mg] |
|---|
| Baclofen 1 mg Capsules | 33.0 | 8574.9 | | | 43.1 |
| Baclofen 1 mg Capsules | 33.0 | | 9227.6 | | 46.6 |
| Baclofen 1 mg Capsules | 33.0 | | | 6040.6 | 30.4 |
| Baclofen 2.5 mg Capsules | 82.5 | 8574.9 | | | 43.1 |
| Baclofen 2.5 mg Capsules | 82.5 | | 9227.6 | | 46.6 |
| Baclofen 2.5 mg Capsules | 82.5 | | | 6040.6 | 30.4 |
| Baclofen 4 mg Capsules | 132.0 | 8574.9 | | | 43.1 |
| Baclofen 4 mg Capsules | 132.0 | | 9227.6 | | 46.6 |
| Baclofen 4 mg Capsules | 132.0 | | | 6040.6 | 30.4 |
| Spironolactone 0.5 mg Capsules | 16.5 | 8574.9 | | | 43.1 |
| Spironolactone 0.5 mg Capsules | 16.5 | | 9227.6 | | 46.6 |
| Spironolactone 0.5 mg Capsules | 16.5 | | | 6040.6 | 30.4 |
| Spironolactone 1 mg Capsules | 33.0 | 8574.9 | | | 43.1 |
| Spironolactone 1 mg Capsules | 33.0 | | 9227.6 | | 46.6 |
| Spironolactone 1 mg Capsules | 33.0 | | | 6040.6 | 30.4 |
| Spironolactone 4 mg Capsules | 132.0 | 8574.9 | | | 43.1 |
| Spironolactone 4 mg Capsules | 132.0 | | 9227.6 | | 46.6 |
| Spironolactone 4 mg Capsules | 132.0 | | | 6040.6 | 30.4 |
Table 2.
On residual API investigated devices with the amount of used extraction volume and cotton swabs.
Table 2.
On residual API investigated devices with the amount of used extraction volume and cotton swabs.
| Device | Extraction Volume [mL] | Number of Used Swabs | Total Volume [mL] |
|---|
| Weighing boat | 5 | - | 5 |
| Spatula | 5 | - | 5 |
| Pestle | 10 | 2 | 12 |
| Powder spreader | 10 | 2 | 12 |
| Mixing bowl | 15 | 3 | 18 |
| Capsule filling machine | 19 | 6 | 25 |
Table 3.
Average particle sizes by volume at the undersize values of 90, 50, and 10 per cent (Dv(0.9), Dv(0.5), Dv(0.1)) of the used bulking agents; n = 3.
Table 3.
Average particle sizes by volume at the undersize values of 90, 50, and 10 per cent (Dv(0.9), Dv(0.5), Dv(0.1)) of the used bulking agents; n = 3.
| Bulking Agent | Dv(0.9) ± SD [µm] | Dv(0.5) ± SD [µm] | Dv(0.1) ± SD [µm] |
|---|
| m35 | 51.96 ± 0.84 | 13.41 ± 0.10 | 2.12 ± 0.05 |
| la | 116.94 ± 1.89 | 39.95 ± 0.37 | 6.13 ± 0.04 |
| mcc | 255.96 ± 3.84 | 113.70 ± 2.36 | 38.27 ± 0.46 |
Table 4.
Bulk density ρb and compressed density ρc of the used bulking agents with the resulting mass of bulking agent mb for 30 size #1 capsules (*) ( = 0.5 mL) (size 1); n = 3.
Table 4.
Bulk density ρb and compressed density ρc of the used bulking agents with the resulting mass of bulking agent mb for 30 size #1 capsules (*) ( = 0.5 mL) (size 1); n = 3.
| Bulking Agent | | | Density Increase ± SD [%] | |
|---|
| m35 | 0.531 ± 0.003 | 0.705 ± 0.011 | 24.7 ± 1.78 | 8.618 ± 0.072 |
| la | 0.564 ± 0.006 | 0.781 ± 0.027 | 27.7 ± 3.15 | 9.274 ± 0.170 |
| mcc | 0.392 ± 0.003 | 0.443 ± 0.002 | 11.3 ± 1.64 | 6.071 ± 0.038 |
Table 5.
Quality parameters of baclofen 1 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
Table 5.
Quality parameters of baclofen 1 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
| Batch | AV | Powder Loss [%] | SDrel Mass Deviation [%] | SDrel Content [%] | Mean Capsule Content [mg] | SDrel Content/Mass [%] |
|---|
| m35-1mg-1 | 5.65 | 1.98 | 2.02 | 2.33 | 0.97 | 1.20 |
| m35-1mg-2 | 4.78 | 2.40 | 1.88 | 2.37 | 1.01 | 1.41 |
| m35-1mg-3 | 4.88 | 2.03 | 1.78 | 2.47 | 0.98 | 1.51 |
| la-1mg-1 | 5.03 | 2.37 | 1.83 | 2.48 | 0.98 | 1.72 |
| la-1mg-2 | 4.18 | 1.63 | 1.50 | 2.10 | 1.00 | 1.31 |
| la-1mg-3 | 6.70 | 2.22 | 1.19 | 2.04 | 0.96 | 1.31 |
| mcc-1mg-1 | 4.02 | 3.75 | 1.35 | 1.98 | 1.01 | 1.66 |
| mcc-1mg-2 | 4.32 | 3.89 | 0.79 | 2.18 | 0.99 | 2.16 |
| mcc-1mg-3 | 6.56 | 7.27 | 1.43 | 2.17 | 0.96 | 2.24 |
Table 6.
Quality parameters of baclofen 2.5 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
Table 6.
Quality parameters of baclofen 2.5 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
| Batch | AV | Powder Loss [%] | SDrel Mass Deviation [%] | SDrel Content [%] | Mean Capsule Content [mg] | SDrel Content/Mass [%] |
|---|
| m35-2.5mg-1 | 3.08 | 4.22 | 1.64 | 1.56 | 2.46 | 0.552 |
| m35-2.5mg-2 | 6.01 | 3.10 | 3.07 | 2.56 | 2.56 | 0.791 |
| m35-2.5mg-3 | 4.92 | 2.53 | 3.02 | 2.46 | 2.51 | 0.962 |
| la-2.5mg-1 | 5.15 | 1.95 | 2.13 | 2.57 | 2.50 | 1.15 |
| la-2.5mg-2 | 3.86 | 2.17 | 1.54 | 1.50 | 2.56 | 1.62 |
| la-2.5mg-3 | 2.66 | 1.69 | 1.41 | 1.30 | 2.54 | 0.947 |
| mcc-2.5mg-1 | 16.07 | 1.31 | 1.43 | 6.36 | 2.61 | 6.44 |
| mcc-2.5mg-2 | 5.54 | 4.67 | 1.36 | 2.50 | 2.55 | 2.13 |
| mcc-2.5mg-3 | 7.42 | 1.93 | 1.24 | 2.03 | 2.62 | 1.78 |
Table 7.
Quality parameters of baclofen 4 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
Table 7.
Quality parameters of baclofen 4 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
| Batch | AV | Powder Loss [%] | SDrel Mass Deviation [%] | SDrel Content [%] | Mean Capsule Content [mg] | SDrel Content/Mass [%] |
|---|
| m35-4mg-1 | 6.04 | 6.01 | 2.93 | 1.98 | 3.85 | 1.80 |
| m35-4mg-2 | 3.50 | 4.72 | 0.79 | 1.63 | 3.93 | 0.891 |
| m35-4mg-3 | 5.23 | 4.77 | 1.87 | 1.85 | 3.87 | 1.59 |
| la-4mg-1 | 13.16 | 3.16 | 1.81 | 4.26 | 3.73 | 3.30 |
| la-4mg-2 | 3.19 | 3.61 | 1.66 | 1.52 | 4.06 | 0.925 |
| la-4mg-3 | 4.22 | 7.07 | 1.69 | 1.81 | 3.91 | 1.41 |
| mcc-4mg-1 | 4.39 | 5.48 | 1.96 | 2.15 | 4.06 | 1.04 |
| mcc-4mg-2 | 4.12 | 5.72 | 2.05 | 1.58 | 4.10 | 0.675 |
| mcc-4mg-3 | 3.61 | 5.80 | 1.64 | 1.41 | 4.09 | 0.841 |
Table 8.
Quality parameters of spironolactone 0.5 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
Table 8.
Quality parameters of spironolactone 0.5 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
| Batch | AV | Powder Loss [%] | SDrel Mass Deviation [%] | SDrel Content [%] | Mean Capsule Content [mg] | SDrel Content/Mass [%] |
|---|
| m35-0.5mg-1 | 8.83 | 6.72 | 1.40 | 2.17 | 0.469 | 1.40 |
| m35-0.5mg-2 | 11.29 | 10.67 | 2.19 | 2.31 | 0.457 | 1.67 |
| m35-0.5mg-3 | 17.00 | 10.84 | 1.98 | 2.28 | 0.427 | 1.38 |
| la-0.5mg-1 | 5.81 | 3.87 | 2.38 | 2.91 | 0.485 | 1.71 |
| la-0.5mg-2 | 3.35 | 2.77 | 1.82 | 1.69 | 0.492 | 1.02 |
| la-0.5mg-3 | 4.37 | 3.26 | 2.12 | 2.25 | 0.483 | 1.03 |
| mcc-0.5mg-1 | 7.51 | 4.54 | 2.17 | 2.30 | 0.455 | 1.88 |
| mcc-0.5mg-2 | 10.40 | 2.50 | 1.27 | 3.12 | 0.448 | 3.05 |
| mcc-0.5mg-3 | 5.87 | 4.14 | 1.85 | 2.99 | 0.475 | 2.32 |
Table 9.
Quality parameters of spironolactone 2 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
Table 9.
Quality parameters of spironolactone 2 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
| Batch | AV | Powder Loss [%] | SDrel Mass Deviation [%] | SDrel Content [%] | Mean Capsule Content [mg] | SDrel Content/Mass [%] |
|---|
| m35-2mg-1 | 8.44 | 3.16 | 2.47 | 2.62 | 2.09 | 0.748 |
| m35-2mg-2 | 4.24 | 4.66 | 2.39 | 2.12 | 2.00 | 0.704 |
| m35-2mg-3 | 4.73 | 4.25 | 1.89 | 1.83 | 1.95 | 1.53 |
| la-2mg-1 | 7.95 | 1.42 | 2.16 | 1.63 | 2.12 | 2.07 |
| la-2mg-2 | 3.87 | 2.41 | 1.72 | 1.51 | 2.05 | 1.05 |
| la-2mg-3 | 8.48 | 2.21 | 1.69 | 1.84 | 2.10 | 0.717 |
| mcc-2mg-1 | 5.36 | 2.69 | 1.40 | 2.62 | 2.03 | 0.760 |
| mcc-2mg-2 | 4.31 | 2.60 | 1.11 | 1.63 | 2.05 | 1.24 |
| mcc-2mg-3 | 4.26 | 3.29 | 1.75 | 1.37 | 2.06 | 1.37 |
Table 10.
Quality parameters of spironolactone 4 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
Table 10.
Quality parameters of spironolactone 4 mg capsule batches prepared with bulking agents comprising m35, la and mcc; n = 30.
| Batch | AV | Powder Loss [%] | SDrel Mass Deviation [%] | SDrel Content [%] | Mean Capsule Content [mg] | SDrel Content/Mass [%] |
|---|
| m35-4mg-1 | 4.54 | 4.46 | 1.89 | 2.27 | 4.06 | 1.98 |
| m35-4mg-2 | 10.59 | 2.44 | 3.90 | 4.06 | 4.16 | 0.821 |
| m35-4mg-3 | 6.18 | 3.03 | 2.83 | 2.26 | 4.13 | 0.611 |
| la-4mg-1 | 13.61 | 2.20 | 1.65 | 3.33 | 4.34 | 1.75 |
| la-4mg-2 | 5.94 | 2.29 | 1.74 | 2.74 | 4.08 | 1.71 |
| la-4mg-3 | 7.57 | 1.55 | 2.30 | 2.56 | 4.16 | 1.07 |
| mcc-4mg-1 | 7.87 | 3.14 | 0.92 | 2.83 | 4.15 | 2.61 |
| mcc-4mg-2 | 2.67 | 3.74 | 0.74 | 1.33 | 4.05 | 1.23 |
| mcc-4mg-3 | 4.54 | 4.46 | 1.89 | 2.27 | 4.06 | 1.77 |