An Observational Cohort Study Evaluating Antimicrobial Use in Peripartum Sepsis: A Tendency towards Overdiagnosis?
Abstract
1. Introduction
2. Materials and Methods
Definitions
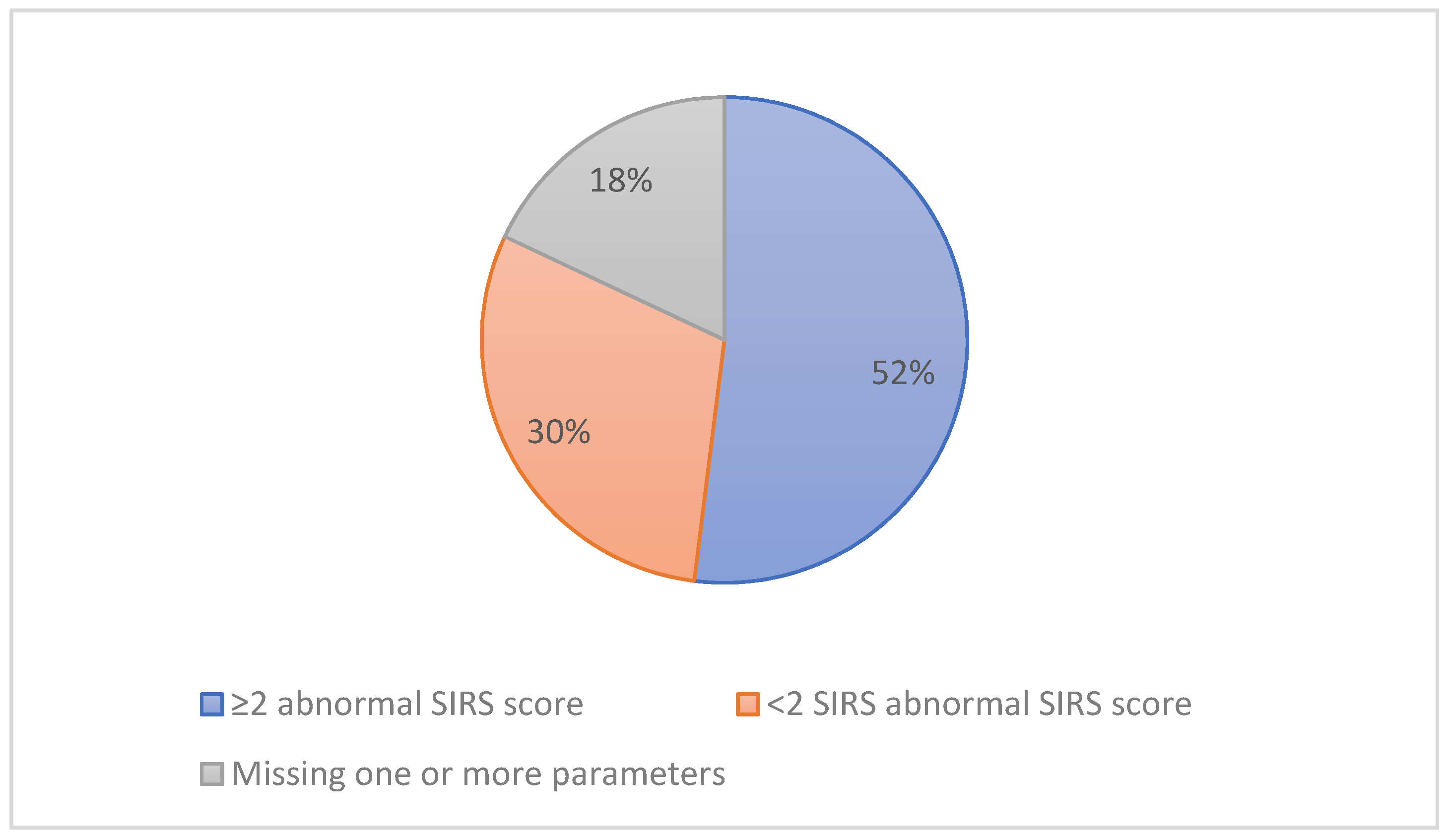
- Sepsis diagnosis: the clinical diagnosis of sepsis according to the written information within a patient’s medical notes and not limited to a positive microbiology report or a patient’s SIRS score.
- Suspected sepsis: women who were diagnosed with sepsis and started on empiric antibiotic therapy regardless of their medical (i.e., their SIRS score) or microbiology status.
- Confirmed sepsis: women who were diagnosed with sepsis and started on empiric antibiotic therapy and have a positive culture.
- Postnatal sepsis: sepsis suspected or diagnosed at least 24 h after delivery, including all readmissions reported during the data collection period and within 42 days postpartum.
3. Results
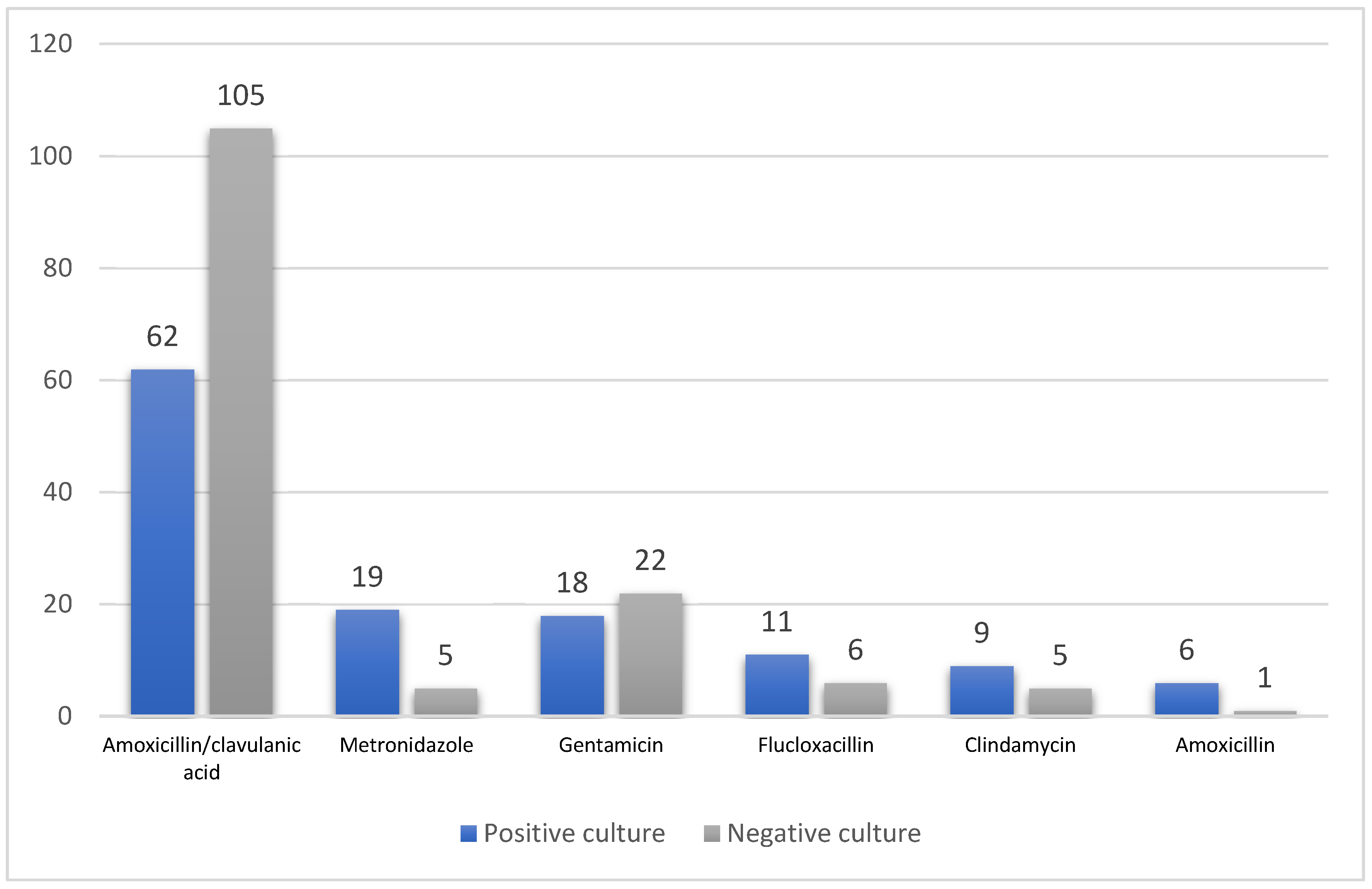
3.1. Antimicrobial Therapy
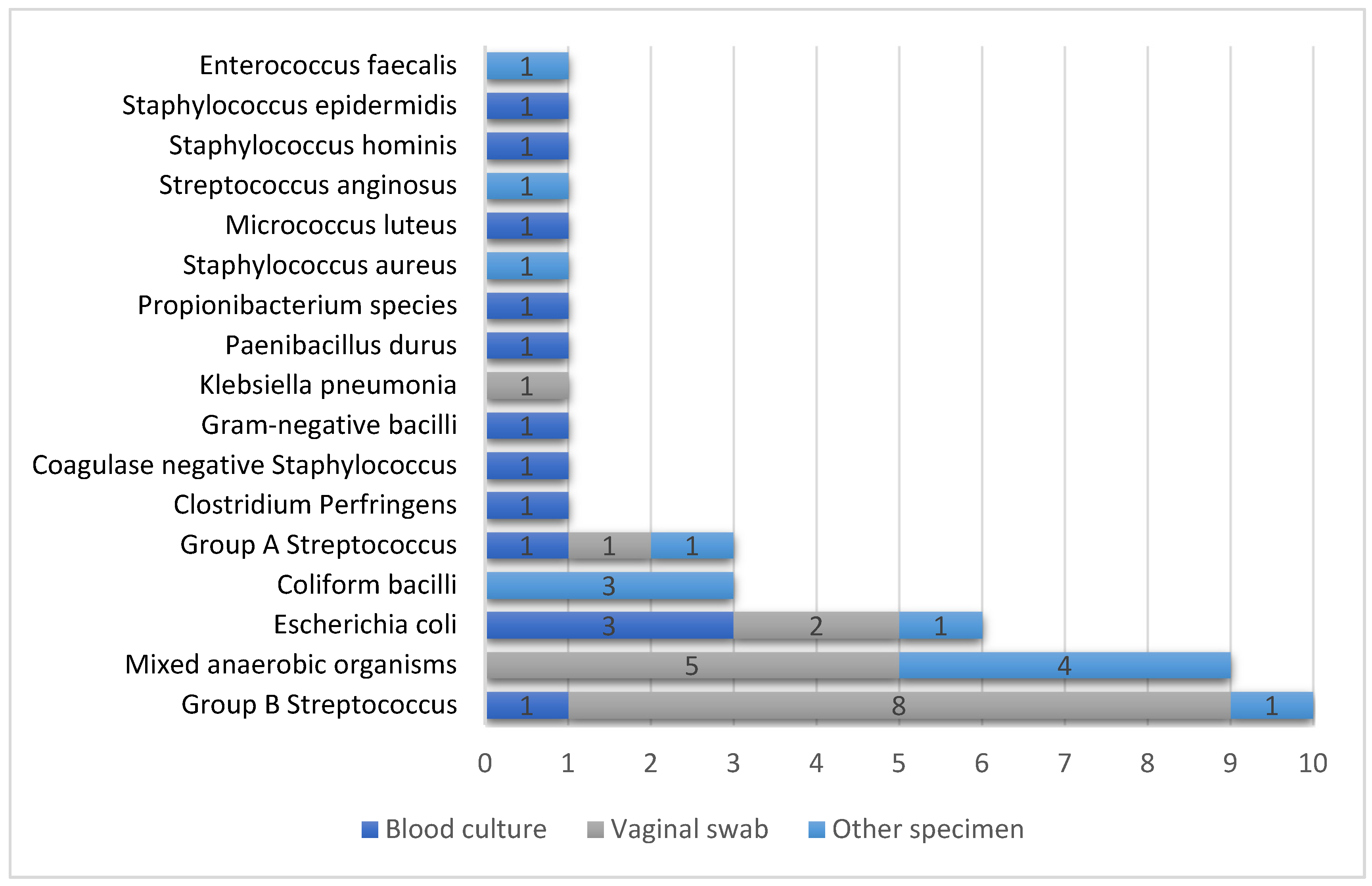
3.2. Microbiology Reporting
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Yealy, D.M.; Huang, D.T.; Delaney, A.; Knight, M.; Randolph, A.G.; Daniels, R.; Nutbeam, T. Recognizing and managing sepsis: What needs to be done? BMC Med. 2015, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Bamfo, J.E. Managing the risks of sepsis in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Kenyon, S.; Brocklehurst, P.; Neilson, J.; Shakespeare, J.; Kurinczuk, J.J. Saving Lives, Improving Mothers’ Care: Lessons Learned to Inform Future Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–2012; National Perinatal Epidemiology Unit: Oxford, UK, 2014. [Google Scholar]
- Mohamed-Ahmed, O.; Nair, M.; Acosta, C.; Kurinczuk, J.J.; Knight, M. Progression from severe sepsis in pregnancy to death: A UK population-based case-control analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Esprit, S.; Pool, A.; Griffiths, S.; Abell, D.; Hopkins, P. Haemorrhage and sepsis: The commonest causes for obstetric related critical care admissions to a central London teaching hospital. In ANAESTHESIA; 111 RIVER ST, HOBOKEN 07030-5774; Wiley: Hoboken, NJ, USA, 2014; Volume 69, p. 73. [Google Scholar]
- Timezguid, N.; Das, V.; Hamdi, A.; Ciroldi, M.; Sfoggia-Besserat, D.; Chelha, R.; Obadia, E.; Pallot, J.L. Maternal sepsis during pregnancy or the postpartum period requiring intensive care admission. Int. J. Obstet. Anesth. 2012, 21, 51–55. [Google Scholar] [CrossRef]
- Knowles, S.J.; O’sullivan, N.P.; Meenan, A.M.; Hanniffy, R.; Robson, M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: A prospective study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 663–671. [Google Scholar] [CrossRef]
- O’Higgins, A.C.; Egan, A.F.; Murphy, O.C.; Fitzpatrick, C.; Sheehan, S.R.; Turner, M.J. A clinical review of maternal bacteremia. Int. J. Gynecol. Obstet. 2014, 124, 226–229. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Singer, M. Puerperal sepsis. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 893–902. [Google Scholar] [CrossRef]
- Greer, O.; Shah, N.M.; Johnson, M.R. Maternal sepsis update: Current management and controversies. Obstet. Gynaecol. 2020, 22, 45–55. [Google Scholar] [CrossRef]
- Royal College of Obstetricians & Gynaecologists. Bacterial Sepsis in Pregnancy. RCOG. 2012. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_64a.pdf (accessed on 20 September 2020).
- National Institute for Health and Care Excellence. Sepsis: Recognition, Diagnosis and Early Management. NICE. 2016. Available online: https://www.nice.org.uk/guidance/ng51/resources/sepsis-recognition-diagnosis-and-early-management-1837508256709 (accessed on 20 September 2020).
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- National Clinical Effectiveness Committee. Sepsis Management; National Clinical Guideline No. 6. NCEC. 2014. Available online: https://www.hse.ie/eng/services/publications/clinical-strategy-and-programmes/guideline-no-6-sepsis-management.pdf (accessed on 20 September 2020).
- Fitzpatrick, F.; Tarrant, C.; Hamilton, V.; Kiernan, F.M.; Jenkins, D.; Krockow, E.M. Sepsis and antimicrobial stewardship: Two sides of the same coin. BMJ Qual. Saf. 2019, 28, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Jeon, J.H.; Park, D.W. Antimicrobial Therapy and Antimicrobial Stewardship in Sepsis. Infect. Chemother. 2020, 52, 19–30. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Committee on Obstetric Practice Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obstet. Gynecol. 2020, 135, e51–e72. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.D.; Knight, M.; Lee, H.C.; Kurinczuk, J.J.; Gould, J.B.; Lyndon, A. The continuum of maternal sepsis severity: Incidence and risk factors in a population-based cohort study. PLoS ONE 2013, 8, e67175. [Google Scholar] [CrossRef]
- Covvey, J.; Grant, J.; Mullen, A. Development of an obstetrics triage tool for clinical pharmacists. J. Clin. Pharm. Ther. 2015, 40, 539–544. [Google Scholar] [CrossRef]
- Information Services Division. Births in Scottish Hospitals- Year ending 31 March 2016. NHS National Service Scotland. ISD. 2016. Available online: https://www.isdscotland.org/Health-Topics/Maternity-and-Births/Publications/2016-11-29/2016-11-29-Births-Report.pdf (accessed on 20 September 2020).
- Segal, S. Labor Epidural Analgesia and Maternal Fever. Anesth Analg 2010, 111, 1467–1475. [Google Scholar] [CrossRef]
- Bebell, L.M.; Ngonzi, J.; Bazira, J.; Fajardo, Y.; Boatin, A.A.; Siedner, M.J.; Bassett, I.V.; Nyehangane, D.; Nanjebe, D.; Jacquemyn, Y.; et al. Antimicrobial-resistant infections among postpartum women at a Ugandan referral hospital. PLoS ONE 2017, 12, e0175456. [Google Scholar] [CrossRef]
- NHS Greater Glasgow & Clyde. Obstetric Guidelines: Maternal Sepsis. NHS Greater Glasgow & Clyde. 2015. Available online: http://www.staffnet.ggc.scot.nhs.uk/Info%20Centre/PoliciesProcedures/GGCClinicalGuidelines/GGC%20Clinical%20Guidelines%20Electronic%20Resource%20Direct/Maternal%20Sepsis,%20Obstetrics.pdf (accessed on 30 January 2020).
- Davies, S.C.; Gibbens, N. UK Five Year Antimicrobial Resistance Strategy 2013 to 2018. London: Department of Health and Department for Environment. Food Rural Aff. 2013. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/662189/UK_AMR_3rd_annual_report.pdf (accessed on 20 September 2020).
- Eppes, C.S.; Clark, S.L. Extended-spectrum β-lactamase infections during pregnancy: A growing threat. Am. J. Obstet. Gynecol. 2015, 213, 650–652. [Google Scholar] [CrossRef]
- Eljaaly, K.; Elarabi, S.; Alshehri, S.; Nix, D.E. Impact of requiring re-authorization of restricted antibiotics on day 3 of therapy. J. Antimicrob. Chemother. 2018, 73, 527–530. [Google Scholar] [CrossRef]
- Leone, M.; Bechis, C.; Baumstarck, K.; Lefrant, J.Y.; Albanèse, J.; Jaber, S.; Lepape, A.; Constantin, J.M.; Papazian, L.; Bruder, N.; et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: A multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014, 40, 1399–1408. [Google Scholar] [CrossRef]
- Rhee, C.; Kadri, S.S.; Dekker, J.P.; Danner, R.L.; Chen, H.C.; Fram, D.; Zhang, F.; Wang, R.; Klompas, M. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Network Open. 2020, 3, e202899. [Google Scholar] [CrossRef] [PubMed]
- Sáez-López, E.; Guiral, E.; Fernández-Orth, D.; Villanueva, S.; Goncé, A.; López, M.; Teixidó, I.; Pericot, A.; Figueras, F.; Palacio, M.; et al. Vaginal versus obstetric infection Escherichia coli isolates among pregnant women: Antimicrobial resistance and genetic virulence profile. PLoS ONE 2016, 11, e0146531. [Google Scholar]
- Lucas, D.; Robinson, P.; Nel, M. Sepsis in obstetrics and the role of the anaesthetist. Int. J. Obstet. Anesth. 2012, 21, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Strich, J.R.; Heil, E.L.; Masur, H. Considerations for Empiric Antimicrobial Therapy in Sepsis and Septic Shock in an Era of Antimicrobial Resistance. J. Infect. Dis. 2020, 222 (Suppl. S2), S119–S131. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.E.; Grobman, W.A.; Lappen, J.R.; Winter, C.; Fox, R.; Lenguerrand, E.; Draycott, T. Modified obstetric early warning scoring systems (MOEWS): Validating the diagnostic performance for severe sepsis in women with chorioamnionitis. Am. J. Obstet. Gynecol. 2015, 212, 536.e1–536.e8. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Antibiotic treatment at delivery shapes the initial oral microbiome in neonates. Sci. Rep. 2017, 7, 43481. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, A.A.; Koren, O. Antibiotic use during pregnancy: How bad is it? BMC Med. 2016, 14, 91. [Google Scholar] [CrossRef]
| Modified SIRS Criteria for Maternity Wards | |
|---|---|
| Temperature | <36 °C or >38 °C |
| HR 1 | >100 beats per minute |
| WCC 2 | <4 or >16 × 109/L |
| RR 3 | >20 breaths per minute |
| SBP 4 | <90 mmHg |
| Mental status | Altered mental status |
| Demographic/Maternal Data | Median (Range) |
|---|---|
| Weight (kg) | 70 (44–154) |
| Body Mass Index (kg/m2) | 25.3 (18.7–55.1) |
| Parity | 0 (0–8) |
| Gravidity | 0 (0–10) |
| Gestation age (week) | 39.5 (13–41) |
| Length of hospital stay (day) | 4 (1–31) |
| Estimated blood loss (ml) | 900 (100–5000) |
| Number of Prescriptions (%) | ||||
|---|---|---|---|---|
| Antibiotic name/class 1 | Total (100%) | SIRS ≥ 2 (59.4%) | SIRS < 2 (28.8%) | Unknown (11.8%) |
| Penicillins | 213 (68.05%) | 124 (66.7%) | 59 (65.5%) | 30 (81.1%) |
| Cephalosporin, Carbapenems and other beta-lactams | 7 (2.24%) | 7 (3.7%) | 0 | 0 |
| Aminoglycosides | 40 (12.78%) | 24 (12.9%) | 12 (13.3%) | 4 (10.8%) |
| Macrolides | 5 (1.60%) | 2 (1.1%) | 3 (3.3%) | 0 |
| Clindamycin | 14 (4.47%) | 10 (5.4%) | 2 (2.2%) | 2 (5.4%) |
| Vancomycin | 5 (1.60%) | 2 (1.1%) | 3 (3.3%) | 0 |
| Trimethoprim | 2 (0.64%) | 2 (1.1%) | 0 | 0 |
| Metronidazole | 25 (7.99%) | 13 (6.9%) | 11 (12.2%) | 1 (2.7%) |
| Quinolones | 2 (0.64%) | 2 (1.1%) | 0 | 0 |
| Total | 313 | 186 | 90 | 37 |
| Antibiotic Name | Number of Prescriptions |
|---|---|
| Vancomycin | 5 |
| Benzylpenicillin | 4 |
| Aztreonam | 3 |
| Clarithromycin | 3 |
| Piperacillin/tazobactam | 3 |
| Ciprofloxacin | 2 |
| Erythromycin | 2 |
| Meropenem | 2 |
| Temocillin | 2 |
| Trimethoprim | 1 |
| Cefalexin | 1 |
| Ceftriaxone | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abutheraa, N.; Grant, J.; Mullen, A.B. An Observational Cohort Study Evaluating Antimicrobial Use in Peripartum Sepsis: A Tendency towards Overdiagnosis? Pharmacy 2020, 8, 211. https://doi.org/10.3390/pharmacy8040211
Abutheraa N, Grant J, Mullen AB. An Observational Cohort Study Evaluating Antimicrobial Use in Peripartum Sepsis: A Tendency towards Overdiagnosis? Pharmacy. 2020; 8(4):211. https://doi.org/10.3390/pharmacy8040211
Chicago/Turabian StyleAbutheraa, Nouf, June Grant, and Alexander B. Mullen. 2020. "An Observational Cohort Study Evaluating Antimicrobial Use in Peripartum Sepsis: A Tendency towards Overdiagnosis?" Pharmacy 8, no. 4: 211. https://doi.org/10.3390/pharmacy8040211
APA StyleAbutheraa, N., Grant, J., & Mullen, A. B. (2020). An Observational Cohort Study Evaluating Antimicrobial Use in Peripartum Sepsis: A Tendency towards Overdiagnosis? Pharmacy, 8(4), 211. https://doi.org/10.3390/pharmacy8040211





