Opioid Use Disorders in People Living with HIV/AIDS: A Review of Implications for Patient Outcomes, Drug Interactions, and Neurocognitive Disorders
Abstract
1. Introduction
2. Methods
3. Current Landscape of Opioid Use Disorder in PLWHA
4. Disparities in Healthcare and Access to Care in PLWHA
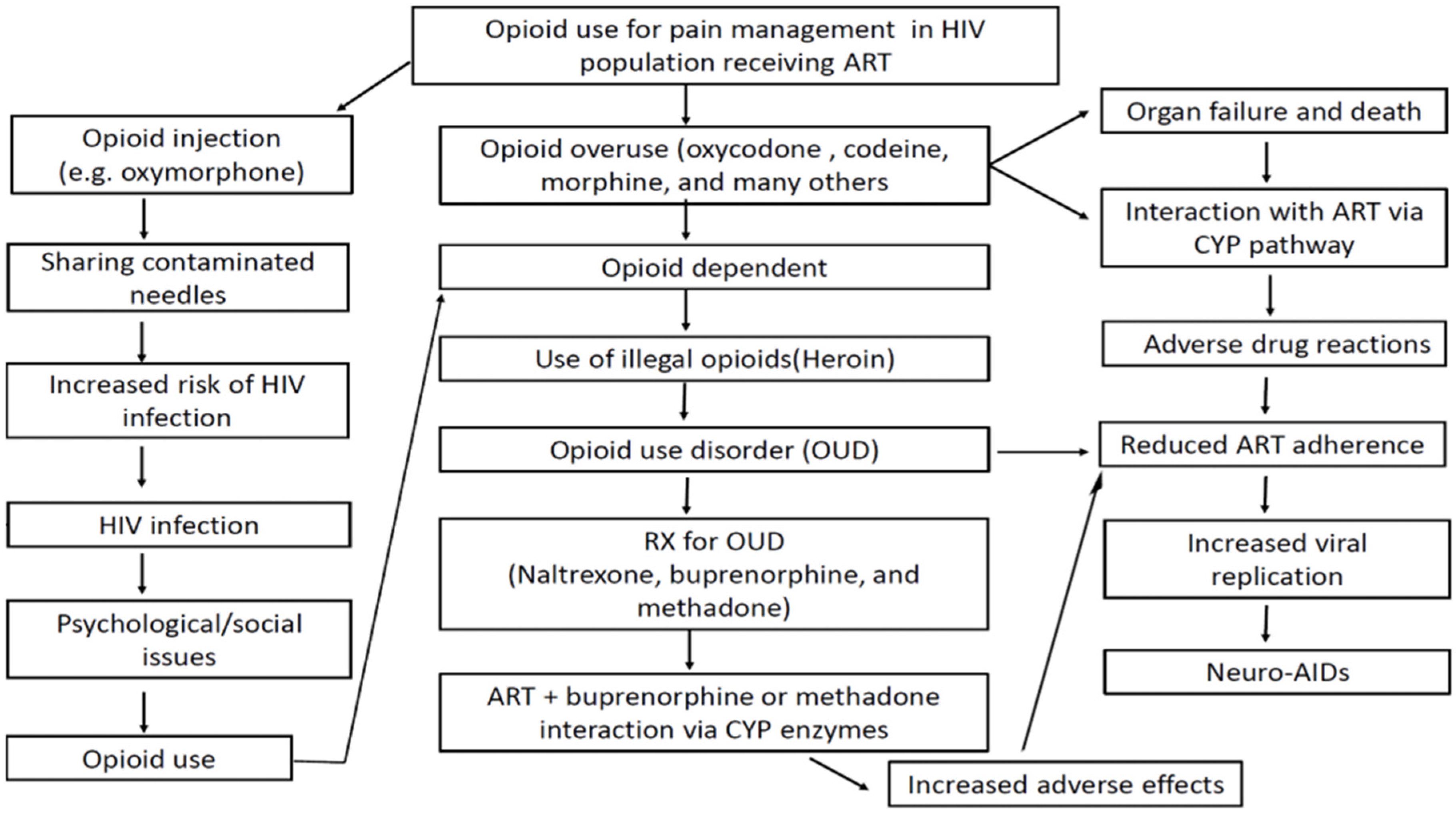
5. The Vicious Cycle of Opioid Use Disorder in the PLWHA Population
6. Drug–Drug Interactions
6.1. Antiretroviral Drug–Drug Interactions with Opioids
6.2. Antiretroviral Drug–Drug Interactions with Medication-Assisted Treatment
7. Opioid-Associated Neurocognitive Disorders in HIV/AIDS
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyer, J.P.; Althoff, A.L.; Altice, F.L. Optimizing Care for HIV-Infected People Who Use Drugs: Evidence-Based Approaches to Overcoming Healthcare Disparities. Clin. Infect. Dis. 2013, 57, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, B.; Sindhu, S.S.; Chemi, C.; Lewis, C.F.; Dickson, V.V.; Lee, J.D. Perspectives on the HIV continuum of care among adult opioid users in New York City: A qualitative study. Harm Reduct. J. 2019, 16, 58–59. [Google Scholar] [CrossRef]
- Burden, M.; Kingston, A.; Wallace, M.A.; Busse, J.W.; Casademont, J.; Chadaga, S.R.; Chandrasekaran, S.; Cicardi, M.; Cunningham, J.M.; Filella, D.; et al. Opioid utilization and perception of pain control in hospitalized patients: A cross-sectional study of 11 sites in 8 countries. J. Hosp. Med. 2019, 14, 737–745. [Google Scholar] [CrossRef] [PubMed]
- The Centers for Disease Control and Prevention (CDC). America’s Drug Overdose Epidemic: Data to Action. 2019. Available online: https://www.cdc.gov/injury/features/prescription-drug-overdose/index.html (accessed on 20 March 2020).
- Florence, C.S.; Zhou, C.; Luo, F.; Xu, L. The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Med Care 2016, 54, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Hauser, K.F.; Fitting, S.; Dever, S.M.; Podhaizer, E.M.; Knapp, P.E. Opiate drug use and the pathophysiology of neuroAIDS. Curr. HIV Res. 2012, 10, 435–452. [Google Scholar] [CrossRef]
- Chastain, D.B.; Veve, M.P.; Wagner, J.L. Abnormal QTc syndrome in HIV-infected patients: A systematic review of prevalence and risk factors. Antivir. Ther. 2019, 24, 459–465. [Google Scholar] [CrossRef]
- Patel, N.; Abdelsayed, S.; Veve, M.P.; Miller, C. Predictors of clinically significant drug-drug interactions among patients treated with nonnucleoside reverse transcriptase inhibitor-, protease inhibitor-, and raltegravir-based antiretroviral regimens. Ann. Pharmacother. 2011, 45, 317–324. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Assessing and Addressing Opioid Use Disorder (OUD). Available online: https://www.cdc.gov/drugoverdose/training/oud/accessible/index.html (accessed on 3 February 2020).
- Centers for Disease Control U.S. Statistics. HIV.gov. Available online: www.hiv.gov/hiv-basics/overview/data-and-trends/statistics (accessed on 13 March 2019).
- Centers for Disease Control and Prevention. HIV/AIDS. Available online: www.cdc.gov/hiv/statistics/overview/ataglance.html (accessed on 29 January 2019).
- Cunningham, C.O. Opioids and HIV infection: From pain management to addiction treatment. Top. Antivir. Med. 2012, 25, 143–146. [Google Scholar]
- National Institute of Mental Health, U.S. Department of Health and Human Services. HIV/AIDS and Mental Health. Available online: www.nimh.nih.gov/health/topics/hiv-aids/index.shtml (accessed on 20 March 2020).
- World Health Organization. HIV/AIDS Treatment and Care for Injecting Drug Users. Available online: www.hareact.eu/sites/default/files/215_0.pdf (accessed on 20 April 2020).
- National Alliance of State. Syringe Services Programs: Effective for HIV Prevention. HHS.gov, US Department of Health and Human Services. Available online: www.hhs.gov/hepatitis/blog/2016/12/6/syringe-services-programs-effective-for-hiv-prevention.html (accessed on 11 March 2018).
- Metzger, D.S.; Zhang, Y. Drug treatment as HIV prevention: Expanding treatment options. Curr. HIV/AIDS Rep. 2010, 7, 220–225. [Google Scholar] [CrossRef]
- Injection Drug Use and HIV Risk. Available online: https://www.cdc.gov/hiv/risk/idu.html (accessed on 2 March 2020).
- Comprehensive, Up-to-Date Information on HIV/AIDS Treatment and Prevention from the University of California San Francisco. Interactions with Methadone. Available online: http://hivinsite.ucsf.edu/insite?page=ar-00-02&post=8¶m=42 (accessed on 20 April 2020).
- Wejnert, C.; Hess, K.L.; Hall, H.I.; Van Handel, M.; Hayes, D.; Fulton, P.; An, Q.; Koenig, L.J.; Prejean, J.; Valleroy, L.A. Vital signs: Trends in HIV diagnoses, risk behaviors, and prevention among persons who inject drugs—United States. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 1336–1342. [Google Scholar] [CrossRef]
- Schafer, K.R.; Albrecht, H.; Dillingham, R.; Hogg, R.S.; Jaworsky, D.; Kasper, K.; Loutfy, M.; MacKenzie, L.J.; McManus, K.A.; Oursler, K.A.K.; et al. The continuum of HIV care in rural communities in the United States and Canada. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 75, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Van Handel, M.M.; Rose, C.E.; Hallisey, E.J.; Kolling, J.L.; Zibbell, J.E.; Lewis, B.; Bohm, M.K.; Jones, C.M.; Flanagan, B.E.; Siddiqi, A.E.A.; et al. County-level vulnerability assessment for rapid dissemination of HIV or HCV infections among persons who inject drugs, United States. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 73, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Weisgrau, S. Issues in rural health: Access, hospitals, and reform. Health Care Financ. Rev. 1995, 17, 1–14. [Google Scholar]
- Van Dis, J. Where we live: Health care in rural vs urban America. JAMA 2002, 287, 1. [Google Scholar] [CrossRef]
- Thakarar, K.; Morgan, J.R.; Gaeta, J.M.; Hohl, C.; Drainoni, M.-L. Homelessness, HIV, and Incomplete Viral Suppression. J. Health Care Poor Underserved 2016, 27, 145–156. [Google Scholar] [CrossRef] [PubMed]
- National Alliance to End Homelessness. Homelessness and HIV/AIDS. Available online: http://www.endhomelessness.org (accessed on 2 February 2020).
- Summers, N.A.; Colasanti, J.; Feaster, D.J.; Armstrong, W.S.; Rodriguez, A.E.; Jain, M.K.; Jacobs, P.; Metsch, L.R.; Del Rio, C. Predictors for Poor Linkage to Care Among Hospitalized Persons Living with HIV and Co-Occurring Substance Use Disorder. AIDS Res. Hum. Retrovir. 2020, 36, 406–414. [Google Scholar] [CrossRef]
- Hoffman, K.A.; Baker, R.; Kunkel, L.E.; Waddell, E.N.; Lum, P.J.; Mccarty, D.; Korthuis, P.T. Barriers and facilitators to recruitment and enrollment of HIV-infected individuals with opioid use disorder in a clinical trial. BMC Health Serv. Res. 2019, 19, 862. [Google Scholar] [CrossRef]
- Storholm, E.D.; Silverberg, M.J.; Satre, D.D. Racial and Ethnic Differences in Substance Use Diagnoses, Comorbid Psychiatric Disorders, and Treatment Initiation among HIV-Positive and HIV-Negative Women in an Integrated Health Plan. J. Psychoact. Drugs 2016, 48, 377–383. [Google Scholar] [CrossRef]
- Brooks, A.J.; Lokhnygina, Y.; Meade, C.S.; Potter, J.; Calsyn, N.A.; Greenfield, S.F. Racial/ethnic differences in the rates and correlates of HIV risk behaviors among drug abusers. Am. J. Addict. 2013, 22, 136–147. [Google Scholar] [CrossRef]
- Acevedo, A.; Garnick, D.W.; Lee, M.T.; Horgan, C.M.; Ritter, G.; Panas, L.; Reynolds, M. Racial and ethnic differences in substance abuse treatment initiation and engagement. J. Ethn. Subst. Abus. 2012, 11, 1–21. [Google Scholar] [CrossRef]
- Sharpe, T.T.; Voûte, C.; Rose, M.A.; Cleveland, J.; Dean, H.D.; Fenton, K. Social Determinants of HIV/AIDS and Sexually Transmitted Diseases Among Black Women: Implications for Health Equity. J. Women’s Health 2012, 21, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Edelman, E.J.; Gordon, K.; Becker, W.C.; Beam-Goulet, J.; Skanderson, M.; Gaither, J.R.; Braden, J.B.; Gordon, A.J.; Kerns, R.D.; Justice, A.C.; et al. Receipt of opioid analgesics by HIV-infected and uninfected patients. J. Gen. Intern. Med. 2012, 28, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Peters, P.J.; Pontones, P.; Hoover, K.W.; Patel, M.R.; Galang, R.R.; Shields, J.; Blosser, S.J.; Spiller, M.; Combs, B.; Switzer, W.M.; et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014–2015. N. Engl. J. Med. 2016, 375, 229–239. [Google Scholar] [CrossRef]
- Dole, V.P. Implications of Methadone Maintenance for Theories of Narcotic Addiction. JAMA 1988, 260, 3025. [Google Scholar] [CrossRef]
- Woods, J.S.; Joseph, H. From Narcotic to Normalizer: The Misperception of Methadone Treatment and the Persistence of Prejudice and Bias. Subst. Use Misuse 2017, 53, 323–329. [Google Scholar] [CrossRef]
- Connery, H.S. Medication-Assisted Treatment of Opioid Use Disorder. Harv. Rev. Psychiatry 2015, 23, 63–75. [Google Scholar] [CrossRef]
- Manchikanti, L.; Giordano, J.; Boswell, M.V.; Fellows, M.B.; Manchukonda, B.R.; Pampati, M.V. Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. J. Opioid Manag. 2007, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Samnaliev, M.; Baxter, J.D.; Leung, G.Y. The Evidence Doesn’t Justify Steps By State Medicaid Programs To Restrict Opioid Addiction Treatment With Buprenorphine. Health Aff. 2011, 30, 1425–1433. [Google Scholar] [CrossRef]
- Policy Impact: Prescription Painkiller Overdoses. Available online: https://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf (accessed on 2 March 2019).
- Bounthavong, M.; Harvey, M.A.; Wells, D.L.; Popish, S.J.; Himstreet, J.; Oliva, E.M.; Kay, C.L.; Lau, M.K.; Randeria-Noor, P.P.; Phillips, A.G.; et al. Trends in naloxone prescriptions prescribed after implementation of a National Academic Detailing Service in the Veterans Health Administration: A preliminary analysis. J. Am. Pharm. Assoc. 2017, 57, S68–S72. [Google Scholar] [CrossRef]
- Sohn, M.; Talbert, J.C.; Huang, Z.; Lofwall, M.R.; Freeman, P.R. Association of Naloxone Coprescription Laws with Naloxone Prescription Dispensing in the United States. JAMA Netw. Open 2019, 2, e196215. [Google Scholar] [CrossRef]
- Green, T.C.; Case, P.; Fiske, H.; Baird, J.; Cabral, S.; Burstein, D.; Schwartz, V.; Potter, N.; Walley, A.Y.; Bratberg, J. Perpetuating stigma or reducing risk? Perspectives from naloxone consumers and pharmacists on pharmacy-based naloxone in 2 states. J. Am. Pharm. Assoc. 2017, 57, S19–S27. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.B.; Pilakka-Kanthikeel, S.; Saxena, S.K.; Saiyed, Z.; Nair, M.N. Interactive Effects of Morphine on HIV Infection: Role in HIV-Associated Neurocognitive Disorder. AIDS Res. Treat. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McCance-Katz, E.F.; Rainey, P.M.; Smith, P.; Morse, G.; Friedland, G.; Gourevitch, M.N.; Jatlow, P. Drug Interactions between Opioids and Antiretroviral Medications: Interaction between Methadone, LAAM, and Nelfinavir. Am. J. Addict. 2004, 13, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S. Opioid Metabolism. Mayo Clin. Proc. 2009, 84, 613–624. [Google Scholar] [CrossRef]
- Gong, Y.; Haque, S.; Chowdhury, P.; Cory, T.J.; Kodidela, S.; Yallapu, M.M.; Norwood, J.M.; Kumar, S. Pharmacokinetics and pharmacodynamics of cytochrome P450 inhibitors for HIV treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 417–427. [Google Scholar] [CrossRef]
- Walubo, A. The role of cytochrome P450 in antiretroviral drug interactions. Expert. Opin. Drug Metab. Toxicol. 2007, 3, 583–598. [Google Scholar] [CrossRef]
- Mercer, S.L.; Coop, A. Opioid analgesics and P-glycoprotein efflux transporters: A potential systems-level contribution to analgesic tolerance. Curr. Top. Med. Chem. 2011, 11, 1157–1164. [Google Scholar] [CrossRef]
- Robillard, K.R.; Chan, G.N.Y.; Zhang, G.; La Porte, C.; Cameron, D.W.; Bendayan, R. Role of P-Glycoprotein in the Distribution of the HIV Protease Inhibitor Atazanavir in the Brain and Male Genital Tract. Antimicrob. Agents Chemother. 2013, 58, 1713–1722. [Google Scholar] [CrossRef]
- Chan, G.N.Y.; Patel, R.; Cummins, C.L.; Bendayan, R. Induction of P-Glycoprotein by Antiretroviral Drugs in Human Brain Microvessel Endothelial Cells. Antimicrob. Agents Chemother. 2013, 57, 4481–4488. [Google Scholar] [CrossRef]
- Tramadol Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020281s041lbl.pdf (accessed on 2 February 2020).
- Codeine Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022402s010s011lbl.pdf (accessed on 2 February 2020).
- HIV-Opioid Drug Interactions. Available online: https://www.hivclinic.ca/main/drugs_interact_files/narc-int.pdf (accessed on 2 February 2020).
- Olkkola, K.T.; Palkama, V.J.; Neuvonen, P.J. Ritonavir’s role in reducing fentanyl clearance and prolonging its half-life. Anesthesiology 1999, 91, 681–685. [Google Scholar] [CrossRef]
- Mukwaya, G.; MacGregor, T.; Hoelscher, D.; Heming, T.; Legg, D.; Kavanaugh, K.; Johnson, P.; Sabo, J.P.; McCallister, S. Interaction of Ritonavir-Boosted Tipranavir with Loperamide Does Not Result in Loperamide-Associated Neurologic Side Effects in Healthy Volunteers. Antimicrob. Agents Chemother. 2005, 49, 4903–4910. [Google Scholar] [CrossRef] [PubMed]
- Demerol FDA Package Insert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/005010s055lbl.pdf (accessed on 2 February 2020).
- Nieminen, T.H.; Hagelberg, N.M.; Saari, T.I.; Neuvonen, M.; Neuvonen, P.J.; Laine, K.; Olkkola, K.T. Oxycodone concentrations are greatly increased by the concomitant use of ritonavir or lopinavir/ritonavir. Eur. J. Clin. Pharmacol. 2010, 66, 977–985. [Google Scholar] [CrossRef]
- Drewe, J.; Gutmann, H.; Fricker, G.; Török, M.; Beglinger, C.; Huwyler, J. HIV protease inhibitor ritonavir: A more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem. Pharmacol. 1999, 57, 1147–1152. [Google Scholar] [CrossRef]
- Gerber, J.G.; Rosenkranz, S.; Segal, Y.; Aberg, J.; D'Amico, R.; Mildvan, D.; Gulick, R.; Hughes, V.; Flexner, C.; Aweeka, F.; et al. Effect of ritonavir/saquinavir on stereoselective pharmacokinetics of methadone: Results of AIDS Clinical Trials Group (ACTG) 401. J. Acquir. Immune. Defic. Syndr. 2001, 27, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gourevitch, M.N.; Friedland, G.H. Interactions between methadone and medications used to treat HIV infection: A review. Mt. Sinai J. Med. A J. Transl. Pers. Med. 2000, 67, 429–436. [Google Scholar]
- Kharasch, E.D.; Walker, A.; Whittington, D.; Hoffer, C.; Bedynek, P.S. Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend. 2009, 101, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Altice, F.L.; Friedland, G.H.; Cooney, E.L. Nevirapine induced opiate withdrawal among injection drug users with HIV infection receiving methadone. AIDS 1999, 13, 957–962. [Google Scholar] [CrossRef]
- Clarke, S.M.; Mulcahy, F.; Tjia, J.; Reynolds, H.E.; Gibbons, S.E.; Barry, M.G.; Back, D.J. Pharmacokinetic Interactions of Nevirapine and Methadone and Guidelines for Use of Nevirapine to Treat Injection Drug Users. Clin. Infect. Dis. 2001, 33, 1595–1597. [Google Scholar] [CrossRef][Green Version]
- McCance-Katz, E.F.; Rainey, P.M.; Jatlow, P.; Friedland, G. Methadone Effects on Zidovudine Disposition (AIDS Clinical Trials Group 262). J. Acquir. Immune Defic. Syndr. Hum. Retrovirology 1998, 18, 435–443. [Google Scholar] [CrossRef]
- McCance-Katz, E.F.; Sullivan, L.E.; Nallani, S. Drug Interactions of Clinical Importance among the Opioids, Methadone and Buprenorphine, and Other Frequently Prescribed Medications: A Review. Am. J. Addict. 2010, 19, 4–16. [Google Scholar] [CrossRef]
- McCance-Katz, E.F.; Moody, D.E.; Smith, P.F.; Morse, G.D.; Friedland, G.; Pade, P.; Baker, J.; Alvanzo, A.; Jatlow, P.; Rainey, P.M. Interactions between Buprenorphine and Antiretrovirals. II. The Protease Inhibitors Nelfinavir, Lopinavir/Ritonavir, and Ritonavir. Clin. Infect. Dis. 2006, 43, S235–S246. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Ma, J.D.; Morello, C.M.; Atayee, R.S.; Best, B.M. Naltrexone Metabolism and Concomitant Drug Concentrations in Chronic Pain Patients. J. Anal. Toxicol. 2014, 38, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.; Winkle, P.; Custodio, J.; Yin, X.; Rhee, M.; Andrews, J. Pharmacokinetics of Cobicistat-Boosted Elvitegravir Administered in Combination with Methadone or Buprenorphine/Naloxone. In Proceedings of the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 9–12 September 2012. [Google Scholar]
- Eggers, C.; Arendt, G.; Hahn, K.; Husstedt, I.W.; Maschke, M.; Neuen-Jacob, E.; Obermann, M.; Rosenkranz, T.; Schielke, E.; Straube, E.; et al. HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Sacktor, N.; Skolasky, R.L.; Seaberg, E.; Munro, C.; Becker, J.T.; Martin, E.; Ragin, A.; Levine, A.; Miller, E.N. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurol. 2015, 86, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Barbaro, J.; Martínez-Aguado, P.; Chilunda, V.; Jaureguiberry-Bravo, M.; Berman, J.W. The Effects of Opioids on HIV Neuropathogenesis. Front. Immunol. 2019, 10, 2445. [Google Scholar] [CrossRef]
- Clayton, K.L.; Garcia, J.V.; Clements, J.E.; Walker, B.D. HIV Infection of Macrophages: Implications for Pathogenesis and Cure. Pathog. Immun. 2017, 2, 179–192. [Google Scholar] [CrossRef]
- Cosenza, M.A.; Zhao, M.; Si, Q.; Lee, S.C. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002, 12, 442–455. [Google Scholar] [CrossRef]
- Joseph, S.B.; Arrildt, K.T.; Sturdevant, C.B.; Swanstrom, R. HIV-1 target cells in the CNS. J. NeuroVirol. 2014, 21, 276–289. [Google Scholar] [CrossRef]
- Gorry, P.; Ong, C.; Thorpe, J.; Bannwarth, S.; Thompson, K.; Gatignol, A.; Wesselingh, S.; Purcell, D. Astrocyte Infection by HIV-1: Mechanisms of Restricted Virus Replication, and Role in the Pathogenesis of HIV-1-Associated Dementia. Curr. HIV Res. 2003, 1, 463–473. [Google Scholar] [CrossRef]
- Vincendeau, M.; Krämer, S.; Hadian, K.; Rothenaigner, I.; Bell, J.; Hauck, S.M.; Bickel, C.; Nagel, D.; Kremmer, E.; Werner, T.; et al. Control of HIV replication in astrocytes by a family of highly conserved host proteins with a common Rev-interacting domain (Risp). AIDS 2010, 24, 2433–2442. [Google Scholar] [CrossRef]
- Chen, N.C.; Partridge, A.T.; Sell, C.; Torres, C.; Martín-García, J. Fate of microglia during HIV-1 infection: From activation to senescence? Glia 2016, 65, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Celis, V.; Valiente-Echeverría, F.; Rifo, R.S.; Toro-Ascuy, D. New Challenges of HIV-1 Infection: How HIV-1 Attacks and Resides in the Central Nervous System. Cells 2019, 8, 1245. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.E.; Jaworowski, A.; Hearps, A.C. The HIV Reservoir in Monocytes and Macrophages. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Edén, A.; Fuchs, D.; Hagberg, L.; Nilsson, S.; Spudich, S.; Svennerholm, B.; Price, R.W.; Gisslén, M. HIV-1 Viral Escape in Cerebrospinal Fluid of Subjects on Suppressive Antiretroviral Treatment. J. Infect. Dis. 2010, 202, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Ferretti, F.; Peterson, J.; Lee, E.; Fuchs, D.; Boschini, A.; Gisslén, M.; Angoff, N.; Price, R.W.; Cinque, P.; et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012, 26, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Strazza, M.; Pirrone, V.; Wigdahl, B.; Dampier, W.; Lin, W.; Feng, R.; Maubert, M.E.; Weksler, B.; Romero, I.A.; Couraud, P.-O.; et al. Prolonged Morphine Exposure Induces Increased Firm Adhesion in an in Vitro Model of the Blood–Brain Barrier. Int. J. Mol. Sci. 2016, 17, 916. [Google Scholar] [CrossRef]
- El-Hage, N.; Bruce-Keller, A.J.; Yakovleva, T.; Bazov, I.; Bakalkin, G.; Knapp, P.E.; Hauser, K.F. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription. PLoS ONE 2008, 3, e4093. [Google Scholar] [CrossRef]
- Clifford, D.B.; Ances, B. HIV-associated neurocognitive disorder. Lancet Infect. Dis. 2013, 13, 976–986. [Google Scholar] [CrossRef]
- Vaux, D.L.; Silke, J. IAPs—The ubiquitin connection. Cell Death Differ. 2005, 12, 1205–1207. [Google Scholar] [CrossRef]
- Althoff, K.N.; Smit, M.; Reiss, P.; Justice, A.C. HIV and ageing. Curr. Opin. HIV AIDS 2016, 11, 527–536. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernasev, A.; Veve, M.P.; Cory, T.J.; Summers, N.A.; Miller, M.; Kodidela, S.; Kumar, S. Opioid Use Disorders in People Living with HIV/AIDS: A Review of Implications for Patient Outcomes, Drug Interactions, and Neurocognitive Disorders. Pharmacy 2020, 8, 168. https://doi.org/10.3390/pharmacy8030168
Cernasev A, Veve MP, Cory TJ, Summers NA, Miller M, Kodidela S, Kumar S. Opioid Use Disorders in People Living with HIV/AIDS: A Review of Implications for Patient Outcomes, Drug Interactions, and Neurocognitive Disorders. Pharmacy. 2020; 8(3):168. https://doi.org/10.3390/pharmacy8030168
Chicago/Turabian StyleCernasev, Alina, Michael P. Veve, Theodore J. Cory, Nathan A. Summers, Madison Miller, Sunitha Kodidela, and Santosh Kumar. 2020. "Opioid Use Disorders in People Living with HIV/AIDS: A Review of Implications for Patient Outcomes, Drug Interactions, and Neurocognitive Disorders" Pharmacy 8, no. 3: 168. https://doi.org/10.3390/pharmacy8030168
APA StyleCernasev, A., Veve, M. P., Cory, T. J., Summers, N. A., Miller, M., Kodidela, S., & Kumar, S. (2020). Opioid Use Disorders in People Living with HIV/AIDS: A Review of Implications for Patient Outcomes, Drug Interactions, and Neurocognitive Disorders. Pharmacy, 8(3), 168. https://doi.org/10.3390/pharmacy8030168






