Responsible Prescribing of Opioids for Chronic Non-Cancer Pain: A Scoping Review
Abstract
1. Introduction
- How should clinicians select CNCP patients who are suitable for long-term opioid therapy?
- What opioids should be prescribed, and how?
- What are the best monitoring strategies to assess effectiveness, safety, and misuse for patients receiving long-term opioid therapy?
- What system-level policies or regulations enable or assist responsible prescribing?
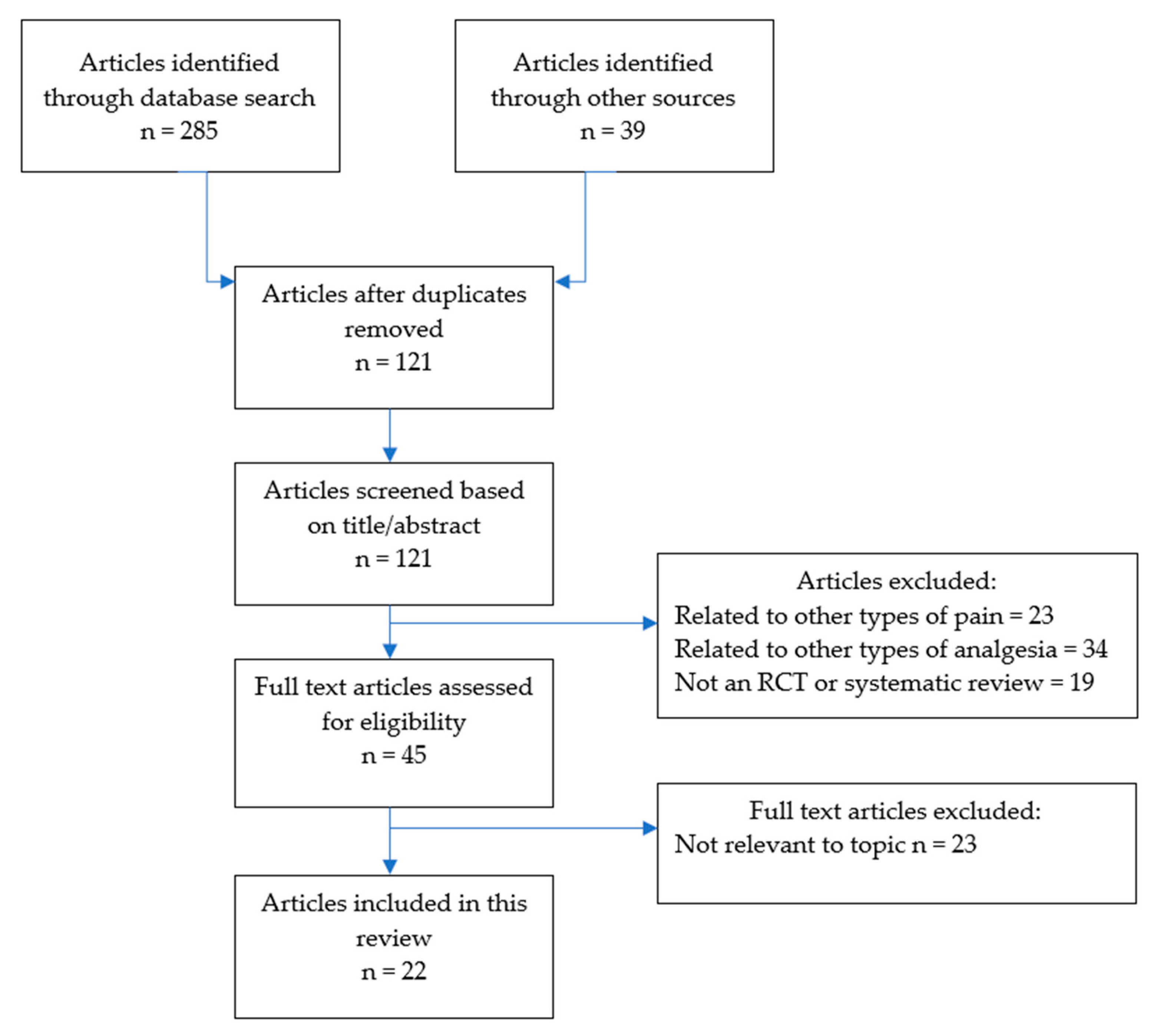
2. Methodology
- Faculty of Pain Medicine, Australian and New Zealand College of Anaesthetists [27],
- American Pain Society—American Academy of Pain Medicine [28],
- American Society of Interventional Pain Physicians (ASIPP) [29],
- DeGroote National Pain Centre, Canada [30],
- Pain Association of Singapore [19],
- Faculty of Pain Medicine, Royal College of Anaesthetists [31],
- Centers for Disease Control and Prevention [32].
3. Results
3.1. Patient-Related Factors
3.1.1. Assessment of the Patient and Their Pain
3.1.2. Predicting Risk for Opioid Misuse
3.1.3. Informed Consent
3.2. Prescriber-Related Factors
3.2.1. Initiating and Titrating Opioid Therapy
3.2.2. Opioid Formulation
3.2.3. Opioid Rotation
3.2.4. Monitoring for Effects and Misuse
3.2.5. Opioid Tapering
3.2.6. Managing CNCP Patients with Opioid Use Disorder
3.3. System-Level Factors
3.3.1. Policy Approaches
3.3.2. Prescription Monitoring Programs
3.3.3. Health Care Provider Training
3.3.4. Model of Healthcare Delivery
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global regional and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Rice, A.S.C.; Smith, B.H.; Blyth, F.M. Pain and the global burden of disease. Pain 2016, 157, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, A.J.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 21163–21196. [Google Scholar] [CrossRef]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Dahlhamer, J.M.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR 2018, 67, 1001. [Google Scholar] [CrossRef]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 2016, 6. [Google Scholar] [CrossRef]
- International Association for the Study of Pain. Classification of chronic pain. Pain 1986, 26 (Suppl. S3), S1–S8. [Google Scholar]
- Currow, D.; Phillips, J.; Clark, K. Using opioids in general practice for chronic non-cancer pain: An overview of current evidence. MJA 2016, 204, 305–309. [Google Scholar]
- Courtney, C.A.; Fernández-de-las-Peñas, C.; Bond, S. Mechanisms of chronic pain–key considerations for appropriate physical therapy management. J. Man. Manip. Ther. 2017, 25, 118–127. [Google Scholar] [CrossRef]
- Sullivan, M.; Howe, C. Opioid therapy for chronic pain in the US: Promises and perils. Pain 2013, 154, S94–S100. [Google Scholar] [CrossRef]
- Campbell, G.; Nielsen, S.; Larance, B.; Bruno, R.; Mattick, R.; Hall, W.; Lintzeris, N.; Cohen, M.; Smith, K.; Degenhardt, L. Pharmaceutical opioid use and dependence among people living with chronic pain: Associations observed within the Pain and Opioids in Treatment (POINT) Cohort. Pain Med. 2015, 16, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- James, J.R.; Scott, J.M.; Klein, J.W.; Jackson, S.; McKinney, C.M.; Novack, M.; Chew, L.; Merrill, J.O. Mortality After Discontinuation of Primary Care—Based Chronic Opioid Therapy for Pain: A Retrospective Cohort Study. J. Gen. Intern. Med. 2019, 34, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.D.; Sandoval, J.A.; Mailis-Gagnon, A.; Tunks, E. Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects. CMAJ 2006, 174, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Kalso, E.; Edwards, J.E.; Moore, R.A.; McQuay, H.J. Opioids in chronic non-cancer pain: Systematic review of efficacy and safety. Pain 2004, 112, 372–380. [Google Scholar] [CrossRef]
- Noble, M.; Tregear, S.J.; Treadwell, J.R.; Schoelles, K. Long-term opioid therapy for chronic noncancer pain: A systematic review and meta-analysis of efficacy and safety. J. Pain Symptom Manag. 2008, 35, 214–228. [Google Scholar] [CrossRef]
- Busse, J.W.; Wang, L.; Kamaleldin, M.; Craigie, S.; Riva, J.J.; Montoya, L.; Mulla, S.M.; Lopes, L.C.; Vogel, N.; Chen, E.; et al. Opioids for chronic noncancer pain: A systematic review and meta-analysis. JAMA 2018, 320, 2448–2460. [Google Scholar] [CrossRef]
- Chou, R.; A Turner, J.; Devine, E.B.; Hansen, R.N.; Sullivan, S.D.; Blazina, I.; Dana, T.; Bougatsos, C.; Deyo, R.A. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review of a national institutes of health pathways to prevention workshop. Ann. Int. Med. 2015, 162, 276–286. [Google Scholar] [CrossRef]
- Ballantyne, J.C. Opioids for the treatment of chronic pain: Mistakes made, lessons learned, and future directions. Anesthesia Analg. 2017, 125, 1769–1778. [Google Scholar] [CrossRef]
- Ho, K.Y.; Chua, N.H.; George, J.M.; Pain Association of Singapore Task Force. Evidence-based guidelines on the use of opioids in chronic non-cancer pain-a consensus statement by the Pain Association of Singapore Task Force. Ann. Acad. Med. Singap. 2013, 42, 138–152. [Google Scholar]
- Edlund, M.; Martin, B.; Russo, J.; Devries, A.; Braden, J.; Sullivan, M. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic non-cancer pain: The role of opioid prescription. Clin. J. Pain 2014, 30, 557–564. [Google Scholar]
- Whetton, S.; Tait, R.J.; Chrzanowska, A.; Donnelly, N.; McEntee, A.; Muhktar, A.; Zahra, E.; Campbell, G.; Degenhardt, L.; Dey, T.; et al. Quantifying the Social Costs of Pharmaceutical Opioid Misuse and Illicit Opioid Use to Australia in 2015/16; National Drug Research Institute, Curtin University: Perth, WA, USA, 2020; ISBN 978-0-6487367-0-7. [Google Scholar]
- Dowell, D.; Haegerich, T.; Chou, R. No shortcuts to safer opioid prescribing. NEJM 2019, 380, 2285–2287. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, N.; Manchikanti, L.; Smith, H.S. Prescription opioid abuse in chronic pain: A review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician 2012, 15 (Suppl. S3), ES67–ES92. [Google Scholar] [PubMed]
- Coyle, D.T.; Pratt, C.Y.; Ocran-Appiah, J.; Secora, A.; Kornegay, C.; Staffa, J. Opioid analgesic dose and the risk of misuse, overdose, and death: A narrative review. Pharmacoepidemiol. Drug Saf. 2018, 27, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B. Responsible prescribing of opioids for the management of chronic pain. Drugs 2003, 63, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 151, 264–269. [Google Scholar]
- Australian and New Zealand College of Anaesthetists, Faculty of Pain Medicine. Statement Regarding the Use of Opioid Analgesics in Patients with Chronic Non-Cancer Pain. 2020. Available online: http://fpm.anzca.edu.au/FPM/media/FPM-Images/PS01(PM)-Foreground-paper-FINAL-20200511.pdf (accessed on 18 August 2020).
- Chou, R.; Fanciullo, G.J.; Fine, P.G.; Adler, J.A.; Ballantyne, J.C.; Davies, P.; Donovan, M.I.; Fishbain, D.A.; Foley, K.M.; Fudin, J.; et al. American Pain Society—American Academy of Pain Medicine Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic Pain. J. Pain 2009, 10, 113–130. [Google Scholar] [CrossRef]
- Manchikanti, L.; Abdi, S.; Atluri, S.; Balog, C.C.; Benyamin, R.; Boswell, M.V.; Brown, K.R.; Bruel, B.M.; A Bryce, D.; A Burks, P.; et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2—Guidance. Pain Physician 2012, 15 (Suppl. S3), S67–S116. [Google Scholar]
- Busse, J. (Ed.) The 2017 Canadian Guideline for Opioids for Chronic Non-Cancer Pain. 2017. Available online: http://nationalpaincentre.mcmaster.ca/documents/Opioid%20GL%20for%20CMAJ_01may2017.pdf (accessed on 18 August 2020).
- Faculty of Pain Medicine, Royal College of Anaesthetists. Opioids Aware. Available online: fpm.ac.uk/opioids-aware (accessed on 15 May 2020).
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef]
- Sullivan, M.; Turner, J.A.; DiLodovico, C.; D’Appollonio, A.; Stephens, K.; Chan, Y.F. Prescription opioid taper support for outpatients with chronic pain: A randomized controlled trial. J. Pain 2017, 18, 308–318. [Google Scholar] [CrossRef]
- Webster, L.; Gruener, D.; Kirby, T.; Xiang, Q.; Tzanis, E.; Finn, A. Evaluation of the tolerability of switching patients on chronic full μ-opioid agonist therapy to buccal buprenorphine. Pain Med. 2016, 17, 899–907. [Google Scholar]
- Blondell, R.D.; Ashrafioun, L.; Dambra, C.M.; Foschio, E.M.; Zielinski, A.L.; Salcedo, D.M. A clinical trial comparing tapering doses of buprenorphine with steady doses for chronic pain and co-existent opioid addiction. J. Addict. Med. 2010, 4, 140. [Google Scholar] [CrossRef] [PubMed]
- Naliboff, B.D.; Wu, S.M.; Schieffer, B.; Bolus, R.; Pham, Q.; Baria, A.; Aragaki, D.; Van Vort, W.; Davis, F.; Shekelle, P. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. J. Pain 2011, 12, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Jamison, R.N.; Ross, E.L.; Michna, E.; Chen, L.Q.; Holcomb, C.; Wasan, A.D. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. Pain 2010, 150, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Liebschutz, J.M.; Xuan, Z.; Shanahan, C.; LaRochelle, M.; Keosaian, J.; Beers, N.; Guara, G.; O’Connor, K.; Alford, D.P.; Parker, V.; et al. Improving Adherence to Long-term Opioid Therapy Guidelines to Reduce Opioid Misuse in Primary Care: A Cluster-Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 1265–1272. [Google Scholar] [CrossRef]
- Kroenke, K.; Krebs, E.E.; Wu, J.; Yu, Z.; Chumbler, N.R.; Bair, M.J. Telecare Collaborative Management of Chronic Pain in Primary Care: A randomized clinical trial. JAMA 2014, 312, 240–248. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Kolbasovsky, A. Impact of a managed controlled-opioid prescription monitoring program on care coordination. Am. J. Manag. Care 2012, 18, 516–524. [Google Scholar]
- Sullivan, M.D.; Gaster, B.; Russo, J.; Bowlby, L.; Rocco, N.; Sinex, N.; Livovich, J.; Jasti, H.; Arnold, R. Randomized Trial of Web-based Training About Opioid Therapy for Chronic Pain. Clin. J. Pain 2010, 26, 512–517. [Google Scholar]
- Turk, D.C.; Swanson, K.S.; Garchel, R.J. Predicting opioid misuse by chronic pain patients: A systematic review and literature synthesis. Clin. J. Pain 2008, 24, 497–508. [Google Scholar] [CrossRef]
- Gomes, T.; Mamdani, M.; Dhalla, I.; Paterson, J.; Juurlink, D. Opioid dose and drug-related mortality in patients with non malignant pain. Arch. Intern. Med. 2011, 171, 686–691. [Google Scholar] [CrossRef]
- Bohnert, A.S.; Valenstein, M.; Bair, M.J.; Ganoczy, D.; McCarthy, J.F.; Ilgen, M.A.; Blow, F.C. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011, 305, 1315–1321. [Google Scholar] [CrossRef]
- Bohnert, A.S.; Logan, J.E.; Ganoczy, D.; Dowell, D. A detailed exploration into the association of prescribed opioid dosage and overdose deaths among patients with chronic pain. Med. Care 2016, 54, 435. [Google Scholar] [PubMed]
- Pedersen, L.; Borchgrevink, P.C.; Riphagen, I.I.; Fredheim, O.M.S. Long- or short-acting opioids for chronic non-malignant pain? A qualitative systematic review. Acta Anaesth. Scand. 2014, 58, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.C.; Fraenkel, L.; Edelman, E.J.; Holt, S.R.; Glover, J.; Kerns, R.D.; Fiellin, D.A. Instruments to assess patient-reported safety, efficacy, or misuse of current opioid therapy for chronic pain: A systematic review. Pain 2013, 154, 905–916. [Google Scholar] [CrossRef]
- Starrels, J.L.; Becker, W.C.; Alford, D.P.; Kapoor, A.; Williams, A.R.; Turner, B.J. Systematic review: Treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann. Int. Med. 2010, 152, 712–720. [Google Scholar] [PubMed]
- Timmerman, L.; Stronks, D.L.; Groeneweg, J.G.; Huygen, F.J. Prevalence and determinants of medication non-adherence in chronic pain patients: A systematic review. Act Anaesth. Scand. 2016, 60, 416–431. [Google Scholar]
- Eccleston, C.; Fisher, E.; Thomas, K.H.; Hearn, L.; Derry, S.; Stannard, C.; Knaggs, R.; Moore, R.A. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst. Rev. 2017, 2017, CD010323. [Google Scholar] [CrossRef]
- Frank, J.W.; Lovejoy, T.I.; Becker, W.C.; Morasco, B.J.; Koenig, C.J.; Hoffecker, L.; Dischinger, H.R.; Dobscha, S.K.; Krebs, E.E. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: A systematic review. Ann. Intern. Med. 2017, 167, 181–191. [Google Scholar] [CrossRef]
- Beaudoin, F.L.; Banerjee, G.N.; Mello, M.J. State-level and system level opioid prescribing policies: The impact on provider practices and overdose deaths, a systematic review. J. Opioid Manag. 2016, 12, 109–118. [Google Scholar]
- Hossain, M.; Asamoah-Boaheng, M.; Badejo, M.; Bell, O.; Buckley, L.; Busse, N. Prescriber adherence to guidelines for chronic noncancer pain management with opioids: Systematic review and meta-analysis. Health Psychol. 2020, 39, 430–451. [Google Scholar]
- Tournebize, J.; Gibaja, V.; Muszczak, A.; Kahn, J. Are physicians safely prescribing opioids for chronic noncancer pain? A systematic review of current evidence. Pain Pr. 2016, 16, 370–383. [Google Scholar]
- Fink, D.S.; Schleimer, J.P.; Sarvet, A.; Grover, K.K.; Delcher, C.; Castillo-Carniglia, Á.; Kim, J.H.; Rivera-Aguirre, A.; Henry, S.G.; Martins, S.S.; et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: A systematic review. Ann. Intern. Med. 2018, 168, 783–790. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.S.; Eldrige, J.S. Prescription Drug Monitoring Program. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ghodke, A.; Barquero, S.; Chelminski, P.R.; Ives, T.J. Short-Acting Opioids Are Associated with Comparable Analgesia to Long-Acting Opioids in Patients with Chronic Osteoarthritis with a Reduced Opioid Equivalence Dosing. Pain Med. 2017, 19, 2191–2195. [Google Scholar] [CrossRef] [PubMed]
| Reference | Participants | Aim | Intervention | Outcome Measures | Findings | Conclusions |
|---|---|---|---|---|---|---|
| Prescriber-related factors | ||||||
| [33] | 35 CNCP patients receiving long-term opioids, recruited from pain clinics and primary care clinics | To evaluate the feasibility and effectiveness of a prescription opioid taper support intervention | 22 weeks of opioid taper support, consisting of: psychiatric consultation, opioid dose tapering, and meetings with a physician assistant to learn pain self-management skills (compared with usual care for control group) |
| At 22 weeks:
| An opioid taper support intervention was feasible and enabled reductions in prescribed opioid dose without increasing pain intensity or interference |
| [34] | 39 CNCP patients receiving full opioid agonist therapy and confirmed to be opioid dependent by naloxone challenge | To determine whether CNCP patients receiving high-dose full agonist opioid treatment could be safely converted to SL BPN without inducing precipitated withdrawal or resulting in worsening pain | Double-blind, active-controlled crossover RCT: each group randomised to a different order of treatment. Group one received SL BPN 12 h after last dose of full agonist; and then resumed normal dosing of full agonist. One week later they received half dose of full agonist 12 h after last dose full agonist. Group two received these in the reverse order. |
|
| CNCP patients treated with full opioid agonists can be switched to SL BPN at 50% of the full opioid agonist dose without an increased risk of opioid withdrawal or loss of pain control |
| [35] | 12 CNCP patients receiving opioid therapy, with concurrent opioid use disorder and recruited via a pain management program | To compare a BPN tapering /discontinuation protocol with an opioid replacement protocol using steady BPN doses in CNCP patients with opioid use disorder | Participants in the active comparator arm were started on tapering doses of BPN with gradual reductions over 4 months and discontinuation by 4 months; participants in the experimental arm were continued on a steady dose for 6 months |
|
| CNCP patients with opioid use disorder are more likely to adhere to an opioid replacement protocol than a weaning protocol; steady doses of BPN are associated with improved pain control and functioning compared with tapered dosing |
| [36] | 135 CNCP patients recruited from a chronic pain clinic | To compare the effectiveness of a liberal versus conservative approach to dose escalation among CNCP patients receiving opioid therapy | Participants in escalating dose group who reported inadequate pain relief were given moderate opioid dose increase; participants in the stable dose group had increases kept to a minimum, and only when medically necessary |
| At 12 months:
| The escalating dose strategy led to small improvements in self-reported pain relief without an increase in opioid misuse; no differences between groups for other measures |
| [37] | 42 CNCP patients (back or neck pain) meeting criteria for high-risk for opioid misuse | To determine whether cognitive behavioural counselling improves treatment compliance among CNCP patients at higher risk for prescription opioid misuse | Intervention group participated in a structured experimental compliance treatment consisting of monthly UDT, compliance checklists, and motivational counselling (compared with usual treatment protocols for control group) |
| At 6 months:
| Compliance training and close monitoring may improve treatment compliance among CNCP patients at high risk for prescription opioid misuse |
| System-level factors | ||||||
| [38] | Cluster-randomised trial among 53 primary care clinicians and their 985 CNCP patients receiving long-term opioid therapy | To determine whether a multicomponent intervention improves guideline adherence and/or reduces opioid misuse risk | 12 months multicomponent intervention consisting of nurse care management, an electronic registry, and electronic decision tools for safe opioid prescribing (compared with electronic decision tool only for control group) |
| At 12 months:
| A multicomponent intervention led to improved provider adherence to guidelines, and patients were more likely to have a reduction in opioid prescription dose |
| [39] | 250 CNCP patients enrolled from primary care clinics with MSK pain of at least moderate intensity | To determine the effectiveness of a telecare intervention for CNCP patients | Participants in the intervention group received telecare management (automated symptom monitoring coupled with an algorithm-guided stepped care approach to optimising analgesia). This was compared to usual care from the primary care physician. |
| At 12 months:
| Telecare collaborative management increased the proportion of primary care patients with improved chronic MSK pain |
| [40] | 754 patients recruited from a care organisation who had filled opioid prescriptions by 3 or more prescribers, at 3 or more pharmacies, within a 3-month period | To evaluate the impact on prescribing practices of providing prescription opioid claims information to prescribers | Prescribers in intervention group received a letter and medication report detailing the multiple prescriptions and suggestions to limit number of dispensing pharmacies, as well as a clinical pharmacist contact. Prescribers in control received a letter detailing national trends in prescription misuse. | Change in:
| At 12 months:
| Enhancing prescriber access to opioid prescription claims information can facilitate informed treatment decisions and improve patient safety |
| [41] | 213 internal medicine residents from 5 medicine residencies | To determine whether an interactive web-based training improves knowledge and competence around opioid prescribing for CNCP | Intervention group completed an interactive, web-based training (‘COPE’—collaborative opioid prescribing education) with a focus on shared decision-making, collaborative goal setting and careful outcome assessment (compared with exposure to clinical guidelines alone for control group) |
| At 60 days post-training:
| Exposure to an interactive web-based training was more effective than exposure to practice guidelines for knowledge and competence in prescribing opioids for CNCP |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, E.; Khor, K.E.; Demirkol, A. Responsible Prescribing of Opioids for Chronic Non-Cancer Pain: A Scoping Review. Pharmacy 2020, 8, 150. https://doi.org/10.3390/pharmacy8030150
Black E, Khor KE, Demirkol A. Responsible Prescribing of Opioids for Chronic Non-Cancer Pain: A Scoping Review. Pharmacy. 2020; 8(3):150. https://doi.org/10.3390/pharmacy8030150
Chicago/Turabian StyleBlack, Eleanor, Kok Eng Khor, and Apo Demirkol. 2020. "Responsible Prescribing of Opioids for Chronic Non-Cancer Pain: A Scoping Review" Pharmacy 8, no. 3: 150. https://doi.org/10.3390/pharmacy8030150
APA StyleBlack, E., Khor, K. E., & Demirkol, A. (2020). Responsible Prescribing of Opioids for Chronic Non-Cancer Pain: A Scoping Review. Pharmacy, 8(3), 150. https://doi.org/10.3390/pharmacy8030150





