Cytochrome P450 (CYP450) Interactions Involving Atypical Antipsychotics Are Common in Community-Dwelling Older Adults Treated for Behavioral and Psychological Symptoms of Dementia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Practice Description
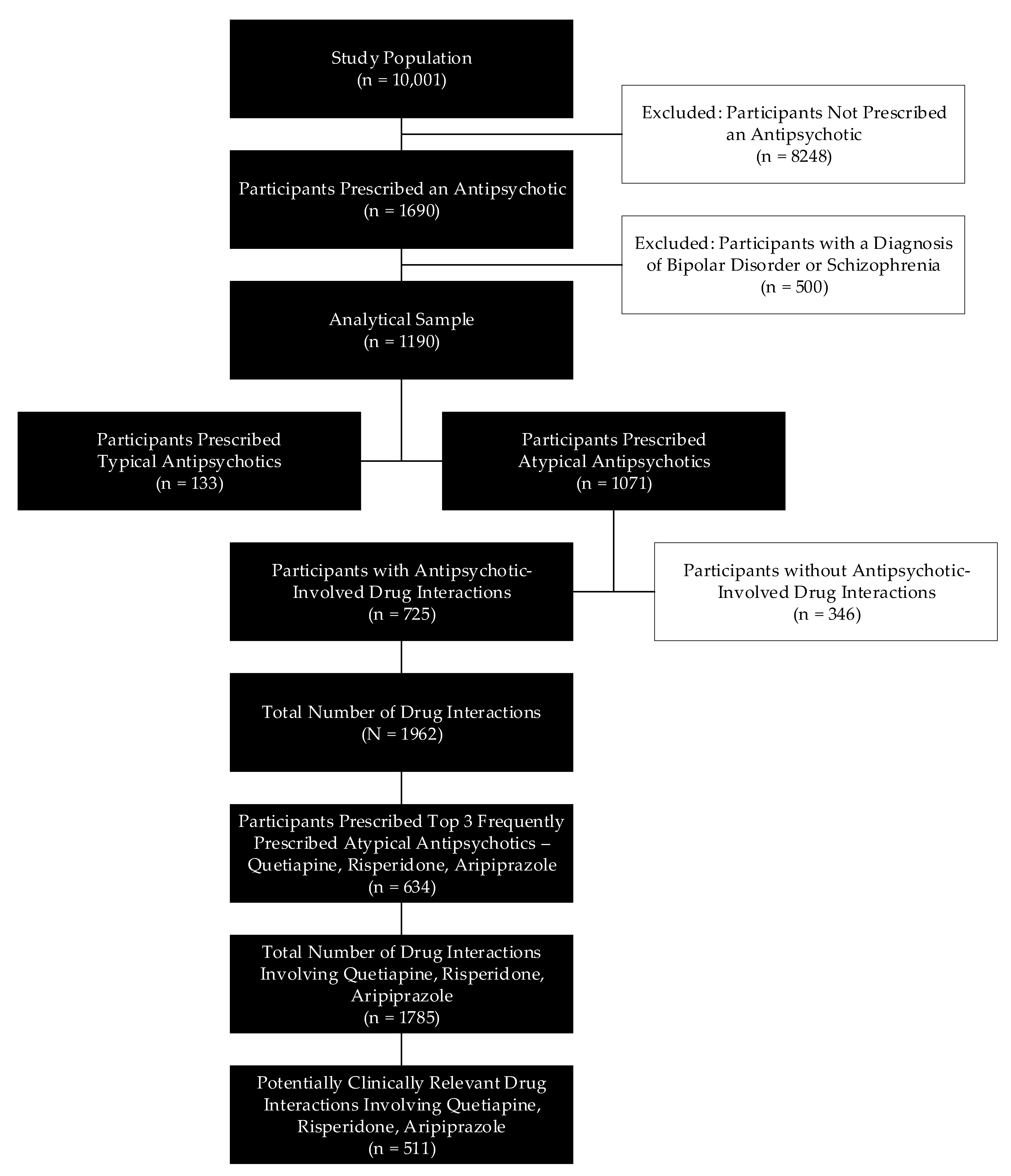
2.2. Study Population
2.3. Analytical Sample
2.4. Definining Potentially Clinically Signifcant DDIs
2.5. Assessing Potentially Clinically Signifcant DDIs
3. Results
4. Discussion
4.1. Quetiapine: Interactions and Implications
4.2. Risperidone: Interactions and Implications
4.3. Aripiprazole: Interactions and Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Level | Property | Value | Outcome |
|---|---|---|---|
| Level 2 | Affinity | Greater | Perpetrator a |
| Weaker | Victim b | ||
| Similar | Not Concerned c | ||
| Level 3 | Metabolic Pathway ≥30% | Yes | Confirm Outcome of Level 2 d |
| No | Not Concerned e |
References
- Bain, K.T.; Schwartz, E.J.; Chan-Ting, R. Reducing off-label antipsychotic use in older community-dwelling adults with dementia: A narrative review. J. Am. Osteopath Assoc. 2017, 117, 441–450. [Google Scholar] [CrossRef] [PubMed]
- US Government Accountability Office. Antipsychotic Drug Use: HHS has Initiatives to Reduce Use Among Older Adults in Nursing Homes, but Should Expand Efforts to other Settings; US Government Accountability Office: Washington, DC, USA, 2015. Available online: https://www.gao.gov/products/GAO-15-211 (accessed on 15 March 2020).
- Liperoti, R.; Pedone, C.; Corsonello, A. Antipsychotics for the treatment of behavioral and psychological symptoms of dementia (BPSD). Curr. Neuropharmacol. 2008, 6, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Salvo, F.; Pariente, A.; Shakir, S.; Robinson, P.; Arnaud, M.; Thomas, S.; Raschi, E.; Fourrier-Reglat, A.; Moore, N.; Sturkenboom, M.; et al. Sudden cardiac and sudden unexpected death related to antipsychotics: A meta-analysis of observational studies. Clin. Pharmacol Ther. 2016, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Gareri, P.; De Fazio, P.; Manfredi, V.G.; De Sarro, G. Use and safety of antipsychotics in behavioral disorders in elderly people with dementia. J. Clin. Psychopharmacol. 2014, 34, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Madhusoodanan, S.; Velama, U.; Parmar, J.; Goia, D.; Brenner, R. A current review of cytochrome P450 interactions of psychotropic drugs. Ann. Clin. Psychiatry 2014, 26, 120–138. [Google Scholar]
- Davies, S.J.; Eayrs, S.; Pratt, P.; Lennard, M.S. Potential for drug interactions involving cytochromes P450 2D6 and 3A4 on general adult psychiatric and functional elderly psychiatric wards. Br. J. Clin. Pharmacol. 2004, 57, 464–472. [Google Scholar] [CrossRef]
- Dechanont, S.; Maphanta, S.; Butthum, B.; Kongkaew, C. Hospital admissions/ visits associated with drug-drug interactions: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2014, 23, 489e497. [Google Scholar] [CrossRef]
- Bankes, D.L.; Amin, N.S.; Bardolia, C.; Awadalla, M.S.; Knowlton, C.H.; Bain, K.T. Medication-related problems encountered in the Program of All-Inclusive Care for the Elderly: An observational study. J. Am. Pharm. Assoc. 2019, 60, 319–327. [Google Scholar] [CrossRef]
- Bouwmeester, C. The PACE program: Home-based long-term care. Consult. Pharm. 2012, 27, 24e30. [Google Scholar] [CrossRef]
- National PACE Association. PACE Fact Sheet. Available online: https://www.npaonline.org/start-pace-program/enrollment-operations/fact-sheet. (accessed on 16 March 2020).
- Vouri, S.M.; Tiemeier, A. The ins and outs of pharmacy services at a Program of All-Inclusive Care for the Elderly. Consult. Pharm. 2012, 27, 803–807. [Google Scholar] [CrossRef]
- National PACE Association. PACE by the Numbers. Available online: https://www.npaonline.org/sites/default/files/PDFs/pace_infographic_update_final_0719.pdf (accessed on 16 March 2020).
- Bain, K.T.; Knowlton, C.H.; Turgeon, J. Medication risk mitigation: Coordinating and collaborating with health care systems, universities, and researchers to facilitate the design and execution of practice-based research. Clin. Geriatr. Med. 2017, 33, 257–281. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.J.; Turgeon, J.; Patel, J.; Patel, P.; Shah, H.; Issa, A.M.; Knowlton, O.V.; Knowlton, C.H.; Bain, K.T. Implementation of a standardized medication therapy management plus approach within primary care. J. Am. Board Fam. Med. 2017, 30, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Doan, J.; Zakrzewski-Jakubiak, H.; Roy, J.; Turgeon, J.; Tannenbaum, C. Prevalence and risk of potential cytochrome P450-mediated drug-drug interactions in older hospitalized patients with polypharmacy. Ann. Pharmacother. 2013, 47, 324–332. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research. Clinical Drug Interaction Studies—Study Design, Data Analysis, and Clinical Implications Guidance for Industry Draft Guidance. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm292362.pdf (accessed on 13 June 2018).
- US Food and Drug Administration. Center for Drug Evaluation and Research. Drug Approvals and Databases—National Drug Code Directory. Available online: https://www.fda.gov/drugs/informationondrugs/ucm142438.htm. (accessed on 13 June 2018).
- Pond, S.M.; Tozer, T.N. First-pass elimination. Basic concepts and clinical consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Sullivan, T. CMS Releases Data on Antipsychotic Drugs Used in Nursing Homes. Available online: https://www.policymed.com/2018/03/cms-releases-data-on-antipsychotic-drugs-used-in-nursing-homes.html (accessed on 13 June 2018).
- Reus, V.I.; Fochtmann, L.J.; Eyler, A.E.; Hilty, D.M.; Horvitz-Lennon, M.; Jibson, M.D.; Lopez, O.L.; Mahoney, J.; Pasic, J.; Tan, Z.S.; et al. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients with Dementia. Am. J. Psychiatry 2016, 173, 543–546. [Google Scholar] [CrossRef]
- Aronow, W.S.; Shamliyan, T.A. Effects of atypical antipsychotic drugs on QT interval in patients with mental disorders. Ann. Transl. Med. 2018, 6, 147. [Google Scholar] [CrossRef]
- Wenzel-Seifert, K.; Wittmann, M.; Haen, E. QTc prolongation by psychotropic drugs and the risk of Torsade de Pointes. Dtsch. Arztebl. Int. 2011, 108, 687–693. [Google Scholar] [CrossRef]
- Hasnain, M.; Vieweg, W.V.; Howland, R.H.; Kogut, C.; Breden Crouse, E.L.; Koneru, J.N.; Hancox, J.C.; Digby, G.C.; Baranchuk, A.; Deshmukh, A.; et al. Quetiapine, QTc interval prolongation, and torsade de pointes: A review of case reports. Ther. Adv. Psychopharmacol. 2014, 4, 130–138. [Google Scholar] [CrossRef]
- Furst, B.A.; Champion, K.M.; Pierre, J.M.; Wirshing, D.A.; Wirshing, W.C. Possible association of QTc interval prolongation with co-administration of quetiapine and lovastatin. Biol. Psychiatry 2002, 51, 264–265. [Google Scholar] [CrossRef]
- Cummings, J.; Ballard, C.; Tariot, P.; Owen, R.; Foff, E.; Youakim, J.; Norton, J.; Stankovic, S. Pimavanserin: Potential Treatment for Dementia-Related Psychosis. J. Prevent. Alzheimer’s Dis. 2018, 5, 253–258. [Google Scholar]
- Ballard, C.; Banister, C.; Khan, Z.; Cummings, J.; Demos, G.; Coate, B.; Youakim, J.M.; Owen, R.; Stankovic, S. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: A phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018, 17, 213–222. [Google Scholar] [CrossRef]
- Ballard, C.; Youakim, J.M.; Coate, B.; Stankovic, S. Pimavanserin in Alzheimer’s Disease Psychosis: Efficacy in Patients with More Pronounced Psychotic Symptoms. J. Prevent. Alzheimer’s Dis. 2019, 6, 27–33. [Google Scholar]
- ACADIA Pharmaceuticals. Nuplazid (Pimavanserin). U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/207318lbl.pdf (accessed on 17 February 2020).
- Kales, H.C.; Lyketsos, C.G.; Miller, E.M.; Ballard, C. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: An international Delphi consensus. Int. Psychogeriatr. 2019, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Scordo, M.G.; Spina, E.; Facciola, G.; Avenoso, A.; Johansson, I.; Dahl, M.L. Cytochrome P450 2D6 genotype and steady state plasma levels of risperidone and 9-hydroxyrisperidone. Psychopharmacology (Berl.) 1999, 147, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Mahatthanatrakul, W.; Nontaput, T.; Ridtitid, W.; Wongnawa, M.; Sunbhanich, M. Rifampin, a cytochrome P450 3A inducer, decreases plasma concentrations of antipsychotic risperidone in healthy volunteers. J. Clin. Pharm. Ther. 2007, 32, 161–167. [Google Scholar] [CrossRef]
- Tiseo, P.J.; Perdomo, C.A.; Friedhoff, L.T. Concurrent administration of donepezil HCl and cimetidine: Assessment of pharmacokinetic changes following single and multiple doses. Br. J. Clin. Pharmacol. 1998, 46, 25–29. [Google Scholar] [CrossRef]
- Yasui-Furukori, N.; Hidestrand, M.; Spina, E.; Facciola, G.; Scordo, M.G.; Tybring, G. Different enantioselective 9-hydroxylation of risperidone by the two human CYP2D6 and CYP3A4 enzymes. Drug Metab. Dispos. 2001, 29, 1263–1268. [Google Scholar]
- Tiseo, P.J.; Perdomo, C.A.; Friedhoff, L.T. Concurrent administration of donepezil HCl and ketoconazole: Assessment of pharmacokinetic changes following single and multiple doses. Br. J. Clin. Pharmacol. 1998, 46, 30–34. [Google Scholar] [CrossRef]
- McEneny-King, A.; Edginton, A.N.; Rao, P.P. Investigating the binding interactions of the anti-Alzheimer’s drug donepezil with CYP3A4 and P-glycoprotein. Bioorg. Med. Chem. Lett. 2015, 25, 297–301. [Google Scholar] [CrossRef]
- Maglione, M.; Ruelaz, M.A.; Hu, J.; Wang, Z.; Shanman, R.; Shekelle, P.G.; Roth, B.; Hilton, L.; Suttorp, M.J.; Ewing, B.A.; et al. Off-label Use of Atypical Antipsychotics: An Update; Sep. Report No.:11-EHC087-EF; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2011.
- Tahami Monfared, A.A.; Meier, G.; Perry, R.; Joe, D. Burden of Disease and Current Management of Dementia with Lewy Bodies: A Literature Review. Neurolog. Ther. 2019, 8, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Feldman, H.; Hecker, J.; Vellas, B.; Ames, D.; Subbiah, P.; Whalen, E.; Emir, B.; Donepezil MSAD Study Investigators Group. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer’s disease. Int. Psychogeriatr. 2002, 14, 389–404. [Google Scholar] [PubMed]
- Cacabelos, R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr Dis. Treat. 2007, 3, 303–333. [Google Scholar] [PubMed]
- Bergman, J.; Brettholz, I.; Shneidman, M.; Lerner, V. Donepezil as add-on treatment of psychotic symptoms in patients with dementia of the Alzheimer’s type. Clin. Neuropharmacol. 2003, 26, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Samer, C.F.; Daali, Y.; Wagner, M.; Hopfgartner, G.; Eap, C.B.; Rebsamen, M.C.; Rossier, M.F.; Hochstrasser, D.; Dayer, P.; Desmeules, J.A. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br. J. Pharmacol. 2010, 160, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.H.; Kane, C.P.; Lobello, K.; Nichols, A.I.; Fayyad, R.; Buckley, G.; Focht, K.; Guico-Pabia, C.J. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: Implications for personalized medicine. J. Clin. Psychiatry 2013, 74, 614–621. [Google Scholar] [CrossRef]
- Seripa, D.; Bizzarro, A.; Pilotto, A.; D’Onofrio, G.; Vecchione, G.; Gallo, A.P.; Cascavilla, L.; Paris, F.; Grandone, E.; Mecocci, P.; et al. Role of cytochrome P4502D6 functional polymorphisms in the efficacy of donepezil in patients with Alzheimer’s disease. Pharmacogenet. Genom. 2011, 21, 225–230. [Google Scholar] [CrossRef]
- Zhong, Y.; Zheng, X.; Miao, Y.; Wan, L.; Yan, H.; Wang, B. Effect of CYP2D6*10 and APOE polymorphisms on the efficacy of donepezil in patients with Alzheimer’s disease. Am. J. Med. Sci. 2013, 345, 222–226. [Google Scholar] [CrossRef]
- Varsaldi, F.; Miglio, G.; Scordo, M.G.; Dahl, M.L.; Villa, L.M.; Biolcati, A.; Lombardi, G. Impact of the CYP2D6 polymorphism on steady-state plasma concentrations and clinical outcome of donepezil in Alzheimer’s disease patients. Eur. J. Clin. Pharmacol. 2006, 62, 721–726. [Google Scholar] [CrossRef]
- Chong, J.W.X.; Tan, E.H.-J.; Chong, C.E.; Ng, Y.; Wijesinghe, R. Atypical antipsychotics: A review on the prevalence, monitoring, and management of their metabolic and cardiovascular side effects. Ment. Health. Clin. 2016, 6, 178–184. [Google Scholar] [CrossRef]
- Peuskens, J.; Pani, L.; Detraux, J.; De Hert, M. The Effects of Novel and Newly Approved Antipsychotics on Serum Prolactin Levels: A Comprehensive Review. CNS Drugs. 2014, 28, 421–453. [Google Scholar] [CrossRef] [PubMed]
- Spina, E.; Avenoso, A.; Scordo, M.G.; Ancione, M.; Madia, A.; Gatti, G.; Perucca, E. Inhibition of risperidone metabolism by fluoxetine in patients with schizophrenia: A clinically relevant pharmacokinetic drug interaction. J. Clin. Psychopharmacol. 2002, 22, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Bondolfi, G.; Eap, C.B.; Bertschy, G.; Zullino, D.; Vermeulen, A.; Baumann, P. The effect of fluoxetine on the pharmacokinetics and safety of risperidone in psychiatric patients. Pharmacopsychiatry 2002, 35, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, C.L.; Lam, Y.W.; Simpson, J.; Ereshefsky, L. CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: Intraindividual variability and plasma concentration correlations. J. Clin. Pharmacol. 2000, 40, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sager, J.E.; Lutz, J.D.; Foti, R.S.; Davis, C.; Kunze, K.L.; Isoherranen, N. Fluoxetine- and norfluoxetine-mediated complex drug-drug interactions: In vitro to in vivo correlation of effects on CYP2D6, CYP2C19, and CYP3A4. Clin. Pharmacol. Ther. 2014, 95, 653–662. [Google Scholar] [CrossRef]
- Zhou, S.F. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin. Pharmacokinet. 2009, 48, 689–723. [Google Scholar] [CrossRef]
- Spina, E.; de Leon, J. Clinically relevant interactions between newer antidepressants and second-generation antipsychotics. Expert Opin. Drug Metab. Toxicol. 2014, 10, 721–746. [Google Scholar] [CrossRef]
- Yeung, E.Y.; Chun, S.; Douglass, A.; Lau, T.E. Effect of atypical antipsychotics on body weight in geriatric psychiatric inpatients. SAGE Open Med. 2017, 5, 2050312117708711. [Google Scholar] [CrossRef]
- Citrome, L. Activating and Sedating Adverse Effects of Second-Generation Antipsychotics in the Treatment of Schizophrenia and Major Depressive Disorder: Absolute Risk Increase and Number Needed to Harm. J. Clin. Psychopharmacol. 2017, 37, 138–147. [Google Scholar] [CrossRef]
- Shuman, M.; Chukwu, A.; Van Veldhuizen, N.; Miller, S.A. Relationship between mirtazapine dose and incidence of adrenergic side effects: An exploratory analysis. Ment. Health Clin. 2019, 9, 41–47. [Google Scholar] [CrossRef]
- Fawcett, J.; Barkin, R.L. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J. Affect. Disord. 1998, 51, 267–285. [Google Scholar] [CrossRef]
| Characteristic | Study Population (N = 10,001) | Analytical Sample (N = 1190) 2 | p-Value 3 | ||
|---|---|---|---|---|---|
| All Drug Users (n = 10,001) | Non-Antipsychotic Users (n = 8248) | Typical Antipsychotic Users (n = 133) | Atypical Antipsychotic Users (n = 1071) | ||
| Age (years) | 76.6 ± 10.4 | 77.1 ± 10.3 | 75.8 ± 10.5 | 75.5 ± 10.4 | <0.01 |
| Gender | 0.10 | ||||
| Male | 3240 (32.4) | 2702 (32.8) | 49 (36.8) | 314 (29.3) | |
| Female | 6771 (67.7) | 5546 (67.2) | 84 (63.2) | 757 (70.7) | |
| Antipsychotic Involved DDIs 2,3 | Top 3 Prescribed Atypical Antipsychotics | ||
|---|---|---|---|
| Quetiapine | Risperidone | Aripiprazole | |
| Total Number of Patients with an AP Rx | 530 | 257 | 157 |
| Total Number of Patients with AP Rx with at least 1 DDI 4 | 334 (63.0) | 174 (67.7) | 130 (82.8) |
| Total Number of DDIs Identified 5 | 694 | 547 | 544 |
| Total Number of Potentially Clinically Relevant DDIs 6 | 112 (16.1) | 252 (46.1) | 147 (27.0) |
| CYP2D6 | |||
| AP as Victim | 18 (21.2) | 8 (5.4) | |
| AP as Perpetrator | 67 (78.8) | 139 (94.6) | |
| CYP3A4 | |||
| AP as Victim | 112 (100.0) | 18 (10.8) | |
| AP as Perpetrator | 149 (89.2) | ||
| Atypical Antipsychotic | Co-Prescribed Medications 1 | CYP450 Isoenzyme |
| Perpetrator 2 | Victim 3 | |
| Aripiprazole | Duloxetine Hydrocodone Metoprolol Mirtazapine Oxycodone Tramadol Venlafaxine | 2D6 |
| Risperidone | Donepezil Hydrocodone Mirtazapine Oxycodone Tramadol Venlafaxine | 2D6 |
| Alprazolam Clonazepam Donepezil Lamotrigine Trazodone Zolpidem | 3A4 | |
| Co-prescribed Medications | Atypical Antipsychotic | CYP450 Isoenzyme |
| Perpetrator | Victim | |
| Amiodarone Paroxetine | Aripiprazole | 2D6 |
| Bupropion Fluoxetine Paroxetine | Risperidone | 2D6 |
| Carbamazepine Diltiazem Phenobarbital Primidone Topiramate | 3A4 | |
| Amlodipine Buspirone Omeprazole Topiramate | Quetiapine | 3A4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, A.; Bain, K.T.; Bankes, D.L.; Furman, A.; Skalski, B.; Verzicco, J.; Turgeon, J. Cytochrome P450 (CYP450) Interactions Involving Atypical Antipsychotics Are Common in Community-Dwelling Older Adults Treated for Behavioral and Psychological Symptoms of Dementia. Pharmacy 2020, 8, 63. https://doi.org/10.3390/pharmacy8020063
Matos A, Bain KT, Bankes DL, Furman A, Skalski B, Verzicco J, Turgeon J. Cytochrome P450 (CYP450) Interactions Involving Atypical Antipsychotics Are Common in Community-Dwelling Older Adults Treated for Behavioral and Psychological Symptoms of Dementia. Pharmacy. 2020; 8(2):63. https://doi.org/10.3390/pharmacy8020063
Chicago/Turabian StyleMatos, Adriana, Kevin T. Bain, David L. Bankes, Anna Furman, Briana Skalski, James Verzicco, and Jacques Turgeon. 2020. "Cytochrome P450 (CYP450) Interactions Involving Atypical Antipsychotics Are Common in Community-Dwelling Older Adults Treated for Behavioral and Psychological Symptoms of Dementia" Pharmacy 8, no. 2: 63. https://doi.org/10.3390/pharmacy8020063
APA StyleMatos, A., Bain, K. T., Bankes, D. L., Furman, A., Skalski, B., Verzicco, J., & Turgeon, J. (2020). Cytochrome P450 (CYP450) Interactions Involving Atypical Antipsychotics Are Common in Community-Dwelling Older Adults Treated for Behavioral and Psychological Symptoms of Dementia. Pharmacy, 8(2), 63. https://doi.org/10.3390/pharmacy8020063






