Relationship between 2-Hour Tacrolimus Concentrations and Clinical Outcomes in Long Term Kidney Transplantation
Abstract
:1. Introduction
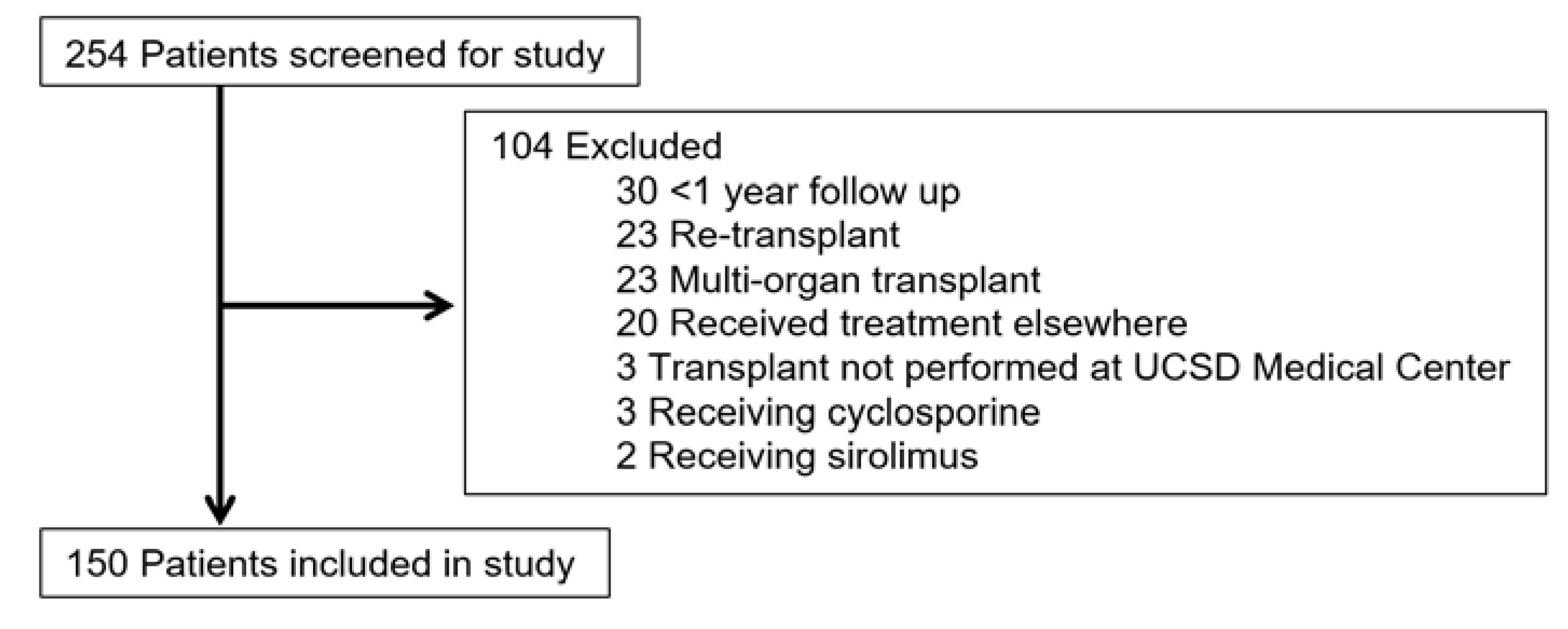
2. Materials and Methods
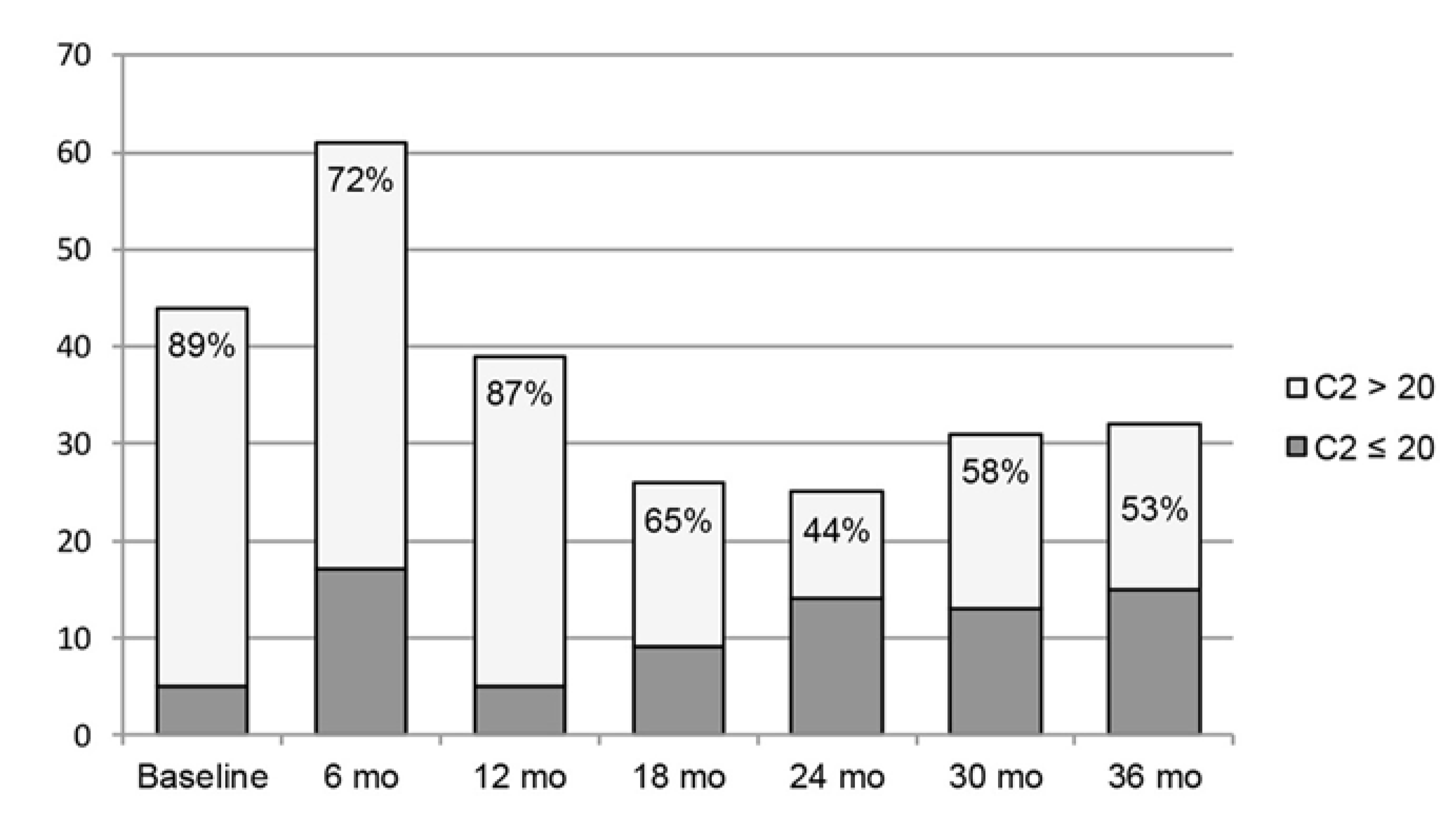
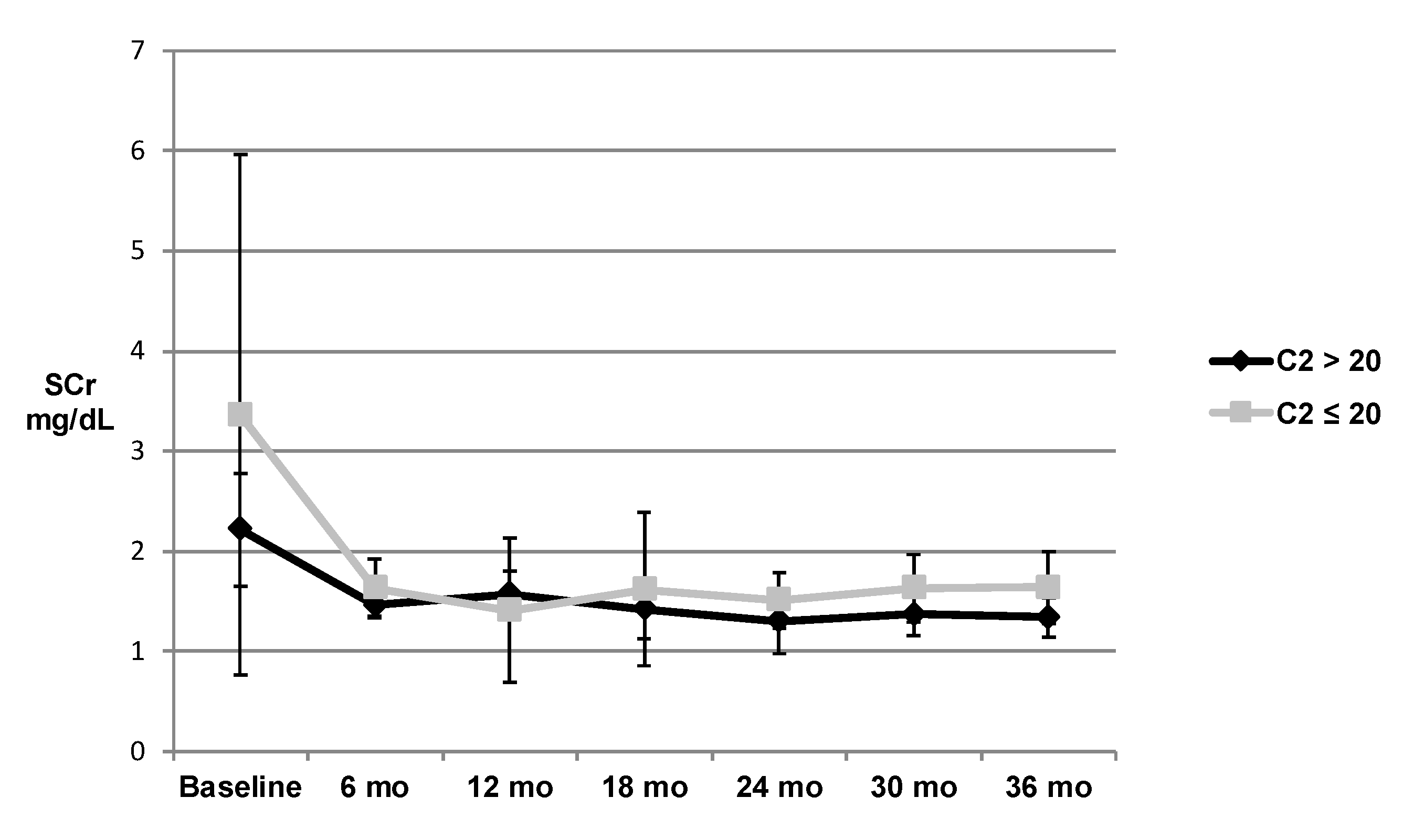
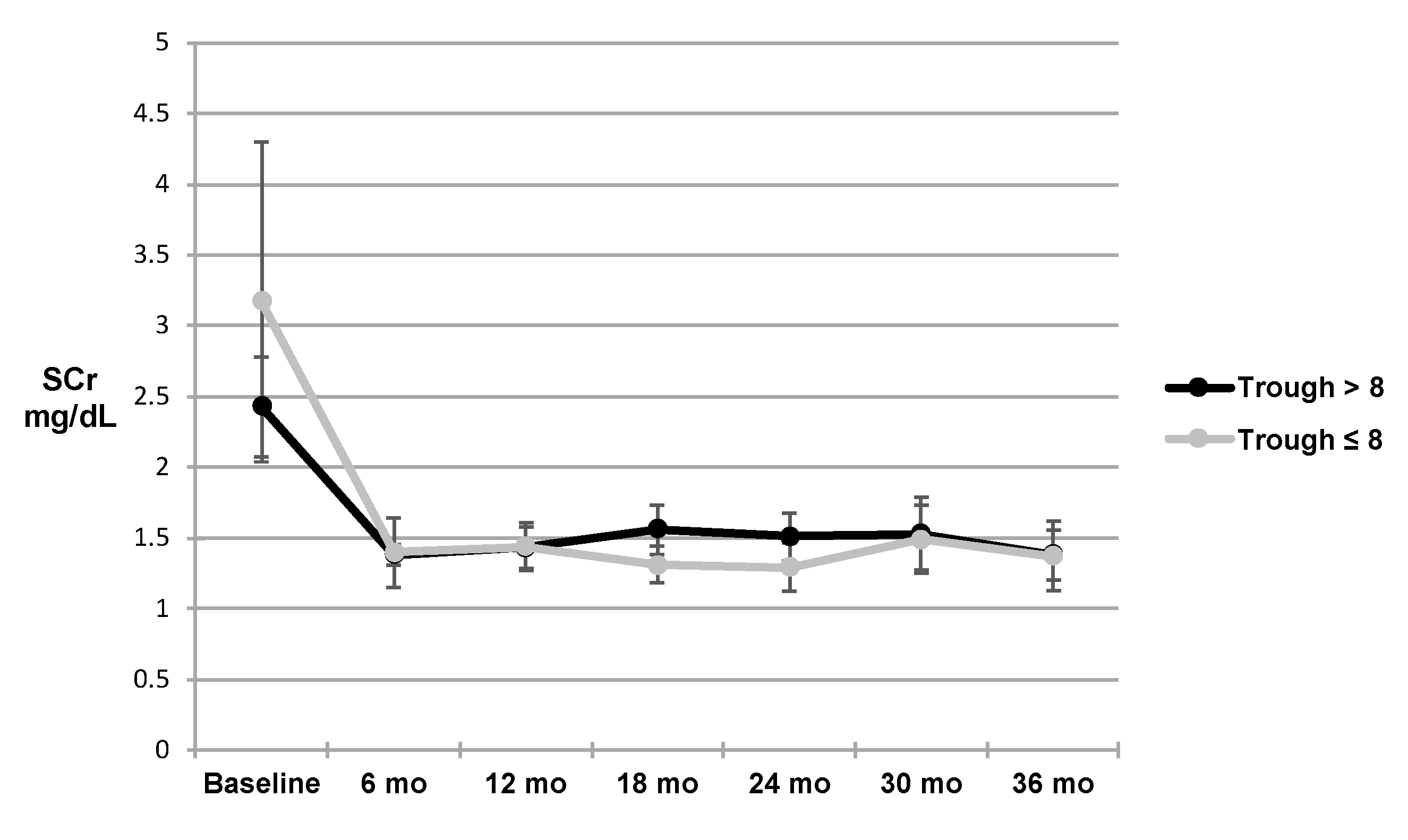
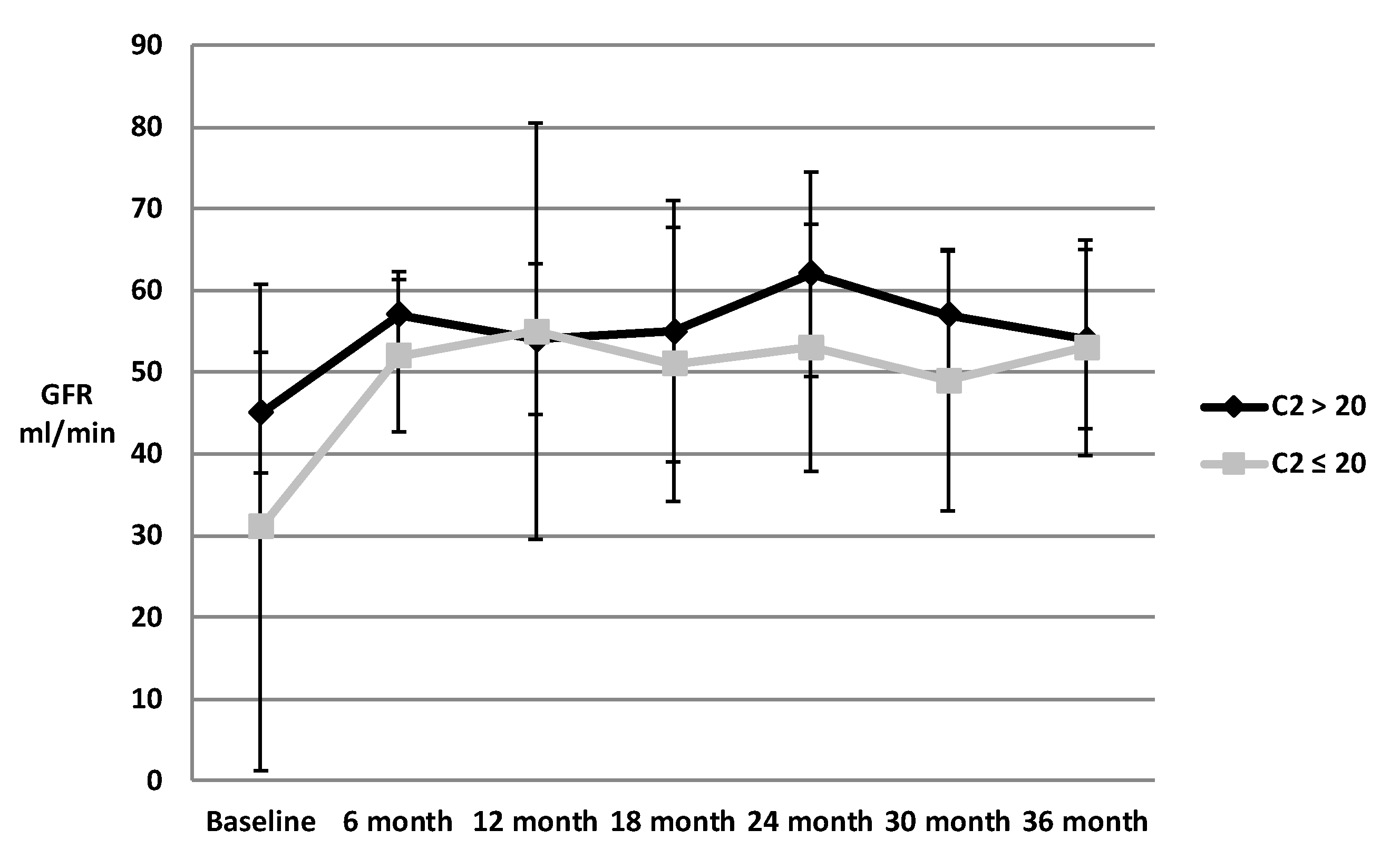
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rath, T. Tacrolimus in transplant rejection. Expert Opin. Pharmacother. 2013, 14, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, R.; Jain, A.; Cadoff, E.; Warty, V.; Iwasaki, K.; Nagase, K.; Krajack, A.; Imventarza, O.; Todo, S.; Fung, J.J.; et al. Pharmacokinetics of FK 506: Preclinical and clinical studies. Transplant. Proc. 1990, 22, 52–56. [Google Scholar] [PubMed]
- Andrews, L.M.; Li, Y.; De Winter, B.C.M.; Shi, Y.Y.; Baan, C.C.; Van Gelder, T.; Hesselink, D.A. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, R.; Jain, A.; Warty, V.S.; Abu-Elmagd, K.; Alessiani, M.; Lever, J.; Fung, J. Pharmacokinetics of FK 506 in transplant patients. Transplant. Proc. 1991, 23, 2736–2740. [Google Scholar] [PubMed]
- Japanese FK 506 Study Group. Japanese study of FK 506 on kidney transplantation: The benefit of monitoring the whole blood FK 506 concentration. Transplant. Proc. 1991, 23, 3085–3088. [Google Scholar]
- Japanese FK506 Study Group. Japanese study of kidney transplantation: 1. Results of early phase II study. Transpl. Int. Off. J. Eur. Soc. Organ. Transplant. 1992, 5, S524–S528. [Google Scholar]
- Yamazaki, R.; Mori, T.; Aisa, Y.; Kato, J.; Nakamura, Y.; Nakazato, T.; Mihara, A.; Ikeda, Y.; Okamoto, S. Interindividual variation of maximal blood levels of tacrolimus after its oral administration in hematopoietic cell transplant recipients. Transplant. Proc. 2009, 41, 1831–1833. [Google Scholar] [CrossRef]
- Kershner, R.P.; Fitzsimmons, W.E. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation 1996, 62, 920–926. [Google Scholar] [CrossRef]
- Wong, K.M.; Shek, C.C.; Chau, K.F.; Li, C.S. Abbreviated tacrolimus area-under-the-curve monitoring for renal transplant recipients. Am. J. Kidney Dis. 2000, 35, 660–666. [Google Scholar] [CrossRef]
- Tsunoda, S.M.; Aweeka, F.T. The use of therapeutic drug monitoring to optimise immunosuppressive therapy. Clin. Pharmacokinet. 1996, 30, 107–140. [Google Scholar] [CrossRef]
- Undre, N.A.; Van Hooff, J.; Christiaans, M.; Vanrenterghem, Y.; Donck, J.; Heeman, U.; Kohnle, M.; Zanker, B.; Land, W.; Morales, J.M.; et al. Low systemic exposure to tacrolimus correlates with acute rejection. Transplant. Proc. 1999, 31, 296–298. [Google Scholar] [CrossRef]
- Hon, Y.; Chamberlain, C.; Kleiner, D.; Ring, M.S.; Hale, D.A.; Kirk, A.D.; Mannon, R.B. Evaluation of tacrolimus abbreviated area-under-the-curve monitoring in renal transplant patients who are potientially at risk for adverse events. Clin. Transpl. 2010, 24, 557–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, K.; Tominaga, Y.; Haba, T.; Katayama, T.; Matsuoka, S. Usefulness of monitoring of AUC (0-4h) during the induction period of immunosuppressive therapy with tacrolimus after renal transplantation. Transplant. Proc. 2002, 34, 1736–1737. [Google Scholar] [CrossRef]
- Scholten, E.M.; Cremers, S.C.L.M.; Schoemaker, R.C.; Rowshani, A.T.; van Kan, E.J.; den Hartigh, J.A.N.; Paul, L.C.; de Fijter, J.W. AUC-guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int. 2005, 67, 2440–2447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbontin, F.G.; Kiberd, B.; Squires, J.; Singh, D.; Fraser, A.; Belitsky, P.; Lawen, J. Tacrolimus monitoring by simplified sparse sampling under the concentration time curve. Transplant. Proc. 2003, 35, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.S.; Fleming, D.H.; Jeyaseelan, V.; Chandy, S.J.; Annapandian, V.M.; Subbanna, P.K.; John, G.T. A limited sampling strategy for tacrolimus in renal transplant patients. Br. J. Clin. Pharmacol. 2008, 66, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Astellas Pharma US, Inc. Prograf(R); Astellas Pharma US, Inc.: Northbrook, IL, USA, 2013. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Kerr, J.; Joliat, J.; Steiner, R.; Shah, M. Low Long-Term Antibody Formation and Preserved Kidney Allograft Function with an “Aggressive” Chronic Immunosuppression Protocol. In Proceedings of the Poster Sess Present Am Soc Nephrol Kidney Week 2012, San Diego, CA, USA, 30 October–4 November 2012; American Society of Nephrology: Washington, DC, USA, 2012. [Google Scholar]
- Christiaans, M.; van Duijnhoven, E.; Beysens, T.; Undre, N.; Schäfer, A. Effect of breakfast on the oral bioavailability of tacrolimus and changes in pharmacokinetics at different times posttransplant in renal transplant recipients. Transplant. Proc. 1998, 30, 1271–1273. [Google Scholar] [CrossRef]
- Kuypers, D.R.J. Immunosuppressive drug monitoring—What to use in clinical practice today to improve renal graft outcome. Transpl Int. 2005, 18, 140–150. [Google Scholar] [CrossRef]
- Wallemacq, P.; Armstrong, V.W.; Brunet, M.; Haufroid, V.; Holt, D.W.; Johnston, A.; Kuypers, D.; Meur, Y.L.; Marquet, P.; Oellerich, M.; et al. Opportunities to Optimize Tacrolimus Therapy in Solid Organ Transplantation: Report of the European Consensus Conference. Ther Drug Monit 2009, 31, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Matas, A.J.; Smith, J.M.; Skeans, M.A.; Thompson, B.; Gustafson, S.K.; Schnitzler, M.A.; Stewart, D.E.; Cherikh, W.S.; Wainright, J.L.; Snyder, J.J.; et al. OPTN/SRTR 2012 Annual Data Report: Kidney. Am. J. Transplant. 2014, 14, 11–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanriover, B.; Jaikaransingh, V.; MacConmara, M.P.; Parekh, J.R.; Levea, S.L.; Ariyamuthu, V.K.; Zhang, S.; Gao, A.; Ayvaci, M.U.S.; Sandikci, B.; et al. Acute Rejection Rates and Graft Outcomes According to Induction Regimen among Recipients of Kidneys from Deceased Donors Treated with Tacrolimus and Mycophenolate. Clin. J. Am. Soc. Nephrol. 2016, 11, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Cordero, E.; Casasola, C.; Ecarma, R.; Danguilan, R. Cytomegalovirus disease in kidney transplant recipients: Incidence, clinical profile, and risk factors. Transplant. Proc. 2012, 44, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.; Bennett, A.; Fergusson, N.; McLean, C.; Mallick, R.; Cameron, D.W.; Knoll, G. Incidence Rate of Post-Kidney Transplant Infection: A Retrospective Cohort Study Examining Infection Rates at a Large Canadian Multicenter Tertiary-Care Facility. Can. J. Kidney Health Dis. 2018, 5, 2054358118799692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, D.C.; Agha, I.; Bohl, D.L.; Schnitzler, M.A.; Hardinger, K.L.; Lockwood, M.; Torrence, S.; Schuessler, R.; Roby, T.; Gaudreault-Keener, M.; et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am. J. Transplant. 2005, 5, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Vincenti, F.; Friman, S.; Tuncer, M.; Citterio, F.; Wiecek, A.; Scheuermann, E.H.; Klinger, M.; Russ, G.; Pescovitz, M.D.; et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: A prospective, randomized, multicenter study. Am. J. Transplant. 2013, 13, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwelack, B.; Malyar, V.; Koch, M.; Sester, M.; Sommerer, C. The influence of immunosuppressive agents on BK virus risk following kidney transplantation, and implications for choice of regimen. Transplant. Rev. 2012, 26, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Astellas Pharma US, Inc. Astagraf XL(R); Astellas Pharma US, Inc.: Northbrook, IL, USA, 2019. [Google Scholar]
- Veloxis Pharmaceuticals, Inc. Envarsus XR(R); Veloxis Pharmaceuticals, Inc.: Cary, NC, USA, 2018. [Google Scholar]
- Osama Gaber, A.; Alloway, R.; Bodziak, K.; Kaplan, B.; Bunnapradist, S. Conversion from Twice-Daily Tacrolimus Capsules to Once-Daily Extended Release Tacrolimus (LCPT): A Phase 2 Trial of Stable Renal Transplant Recipients. Transplantation 2013, 96, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Mean ± SD, N (%) |
|---|---|
| Age (years) | 49.8 ± 13.2 |
| Gender | |
| Male | 90 (60) |
| Weight (kg) | 75.8 ± 17.4 |
| BMI (kg/m2) | 26.9 ± 4.9 |
| CKD Etiology | |
| Glomerulonephritis | 47 (31) |
| Diabetes | 52 (35) |
| Polycystic kidney disease | 15 (10) |
| Other | 36 (24) |
| Pre-transplant Comorbidities | |
| Diabetes | 72 (48) |
| Hypertension | 145 (97) |
| Pre-transplant Scr (mg/dL) | 7.67 ± 3.14 |
| Type of transplant | |
| Deceased | 101 (67) |
| Living Related | 31 (21) |
| Living Unrelated | 18 (12) |
| Induction | |
| Basiliximab | 2 (1) |
| Thymoglobulin | 125 (84) |
| None | 23 (15) |
| Triple Immunosuppression (FK+ MMF+ Pred) | 141 (94) |
| Variable (mean) | Baseline N = 150 | 6 Months N = 150 | 12 Months N = 126 | 18 Months N = 107 | 24 Months N = 83 | 30 Months N = 74 | 36 Months N = 63 |
|---|---|---|---|---|---|---|---|
| SCr (mg/dL) | 2.54 | 1.39 | 1.45 | 1.51 | 1.53 | 1.6 | 1.49 |
| GFR (mL/min) | 43 | 59 | 58 | 57 | 56 | 55 | 59 |
| FK dose (mg/day) | 15.46 | 8.67 | 7.43 | 6.58 | 6.02 | 6.09 | 5.61 |
| FK dose (mg/kg/day) | 0.043 | 0.024 | 0.020 | 0.018 | 0.018 | 0.016 | 0.015 |
| FK C2 (ng/mL) | 34.55 | 27.67 | 30.84 | 26.74 | 19.45 | 23.32 | 21.71 |
| FK trough (ng/mL) | 11.10 | 10.85 | 10.06 | 9.08 | 8.75 | 9.31 | 9.14 |
| FK C2/trough | 3.11 | 2.55 | 3.07 | 2.94 | 2.22 | 2.50 | 2.38 |
| Pred dose (mg/day) | 31.23 | 7.83 | 7.24 | 7.09 | 7.22 | 7.17 | 7.02 |
| Pred dose (mg/kg/day) | 0.41 | 0.1 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 |
| MMF dose (mg/day) | 1385.3 | 1063 | 915.7 | 944.4 | 862.7 | 894.4 | 853.7 |
| MMF dose (mg/kg/day) | 18.27 | 14.09 | 11.94 | 12.19 | 11.22 | 11.36 | 10.68 |
| % Triple Immunosuppression | 94 | 71 | 68 | 67 | 60 | 61 | 65 |
| Average % dose change relative to 12 month | N/A | N/A | N/A | 0.99 | 0.99 | 0.96 | 0.99 |
| # of patients with > 30% dose change | N/A | N/A | N/A | 20 | 21 | 23 | 26 |
| Variable | Mean ± SD, N (%) |
|---|---|
| Biopsy | 26 (17.3) |
| Biopsy Results | |
| Cellular Rejection | 13 |
| Acute tubular necrosis | 4 |
| Calcineurin inhibitor toxicity | 1 |
| Membranoproliferative glomerulonephritis | 1 |
| Donor specific antibody detected | 3 (2) |
| SCr at biopsy (mg/dL) | 2.57 ± 1.48 |
| FK dose (mg/day) | 12.08 ± 6.77 |
| FK C2 (ng/mL) | 26.28 ± 6.73 |
| FK trough (ng/mL) | 10.73 ± 3.76 |
| Median time to first rejection | 1.2 years |
| Variable | 6 Months | 12 Months | 18 Months | 24 Months | 30 Months | 36 Months |
|---|---|---|---|---|---|---|
| CMV, N (%) | 1 (0.7) | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 1 (1.6) |
| BK, N (%) | 14 (10.2) | 3 (3.2) | 2 (3.1) | 0 (0) | 1 (2.2) | 0 (0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Hsu, T.; Kerr, J.S.; Steiner, R.; Awdishu, L. Relationship between 2-Hour Tacrolimus Concentrations and Clinical Outcomes in Long Term Kidney Transplantation. Pharmacy 2020, 8, 60. https://doi.org/10.3390/pharmacy8020060
Yin J, Hsu T, Kerr JS, Steiner R, Awdishu L. Relationship between 2-Hour Tacrolimus Concentrations and Clinical Outcomes in Long Term Kidney Transplantation. Pharmacy. 2020; 8(2):60. https://doi.org/10.3390/pharmacy8020060
Chicago/Turabian StyleYin, Jeffrey, Tammy Hsu, Janice S Kerr, Robert Steiner, and Linda Awdishu. 2020. "Relationship between 2-Hour Tacrolimus Concentrations and Clinical Outcomes in Long Term Kidney Transplantation" Pharmacy 8, no. 2: 60. https://doi.org/10.3390/pharmacy8020060
APA StyleYin, J., Hsu, T., Kerr, J. S., Steiner, R., & Awdishu, L. (2020). Relationship between 2-Hour Tacrolimus Concentrations and Clinical Outcomes in Long Term Kidney Transplantation. Pharmacy, 8(2), 60. https://doi.org/10.3390/pharmacy8020060





