Clinical Pharmacy Activities Documented (ClinPhADoc): Development, Reliability and Acceptability of a Documentation Tool for Community Pharmacists
Abstract
1. Introduction
2. Materials and Methods
2.1. Update of the ClinPhADoc Tool
2.2. Validation of the ClinPhADoc Tool
2.2.1. Interrater Reliability
2.2.2. Test-Retest Reliability
2.2.3. Appropriateness
2.2.4. Acceptability and Feasibility
3. Results
3.1. Update
3.2. Validation
3.2.1. Interrater Reliability
3.2.2. Test-Retest Reliability
3.2.3. Appropriateness
3.2.4. Acceptability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American College of Clinical Pharmacy. The definition of clinical pharmacy. Pharmacotherapy 2008, 28, 816–817. [CrossRef]
- Slavik, R.S.; LeBras, M.; Gorman, S.K. Clinical Pharmacy Activities: We Know What to Do, but for Whom Should We Do It? Can. J. Hosp. Pharm. 2016, 69, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Viktil, K.K.; Blix, H.S. The Impact of Clinical Pharmacists on Drug-Related Problems and Clinical Outcomes. Basic Clin. Pharmacol. Toxicol. 2008, 102, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical Care Network Europe. Classification of drug related problems. Available online: https://www.pcne.org/upload/files/230_PCNE_classification_V8-02.pdf (accessed on 11 September 2018).
- Stark, R.G.; John, J.; Leidl, R. Health care use and costs of adverse drug events emerging from outpatient treatment in Germany: A modelling approach. BMC Health Serv. Res. 2011, 11, 9. [Google Scholar] [CrossRef]
- Baranski, B.; Bolt, J.; Albers, L.; Siddiqui, R.; Bell, A.; Semchuk, W. Development of a Documentation Rubric and Assessment of Pharmacists’ Competency for Documentation in the Patient Health Record. Can. J. Hosp. Pharm. 2017, 70, 423–429. [Google Scholar] [CrossRef]
- American Society of Health-System Pharmacists. ASHP guidelines on documenting pharmaceutical care in patient medical records. Am. J. Health Syst. Pharm. 2003, 60, 705–707. [Google Scholar] [CrossRef]
- Wright, D.J.; Twigg, M.J. Community pharmacy: an untapped patient data resource. Integr Pharm Res Pract 2016, 5, 19–25. [Google Scholar] [CrossRef][Green Version]
- Williams, M.; Peterson, G.M.; Tenni, P.C.; Bindoff, I.K.; Stafford, A.C. DOCUMENT: a system for classifying drug-related problems in community pharmacy. Int. J. Clin. Pharm. 2012, 34, 43–52. [Google Scholar] [CrossRef]
- Hepler, C.D.; Strand, L.M. Opportunities and responsibilities in pharmaceutical care. Am. J. Hosp. Pharm. 1990, 47, 533–543. [Google Scholar] [CrossRef]
- Westerlund, T.; Almarsdottir, A.B.; Melander, A. Factors influencing the detection rate of drug-related problems in community pharmacy. Pharm. World Sci. 1999, 21, 245–250. [Google Scholar] [CrossRef]
- Westerlund, T.; Marklund, B. Assessment of the clinical and economic outcomes of pharmacy interventions in drug-related problems. J. Clin. Pharm. Ther. 2009, 34, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Watkins, K.; Wood, H.; Schneider, C.R.; Clifford, R. Effectiveness of implementation strategies for clinical guidelines to community pharmacy: a systematic review. Implement. Sci. 2015, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Rieck, A.M. Exploring the nature of power distance on general practitioner and community pharmacist relations in a chronic disease management context. J. Interprof. Care 2014, 28, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bardet, J.D.; Vo, T.H.; Bedouch, P.; Allenet, B. Physicians and community pharmacists collaboration in primary care: A review of specific models. J. Interprof. Care 2015, 11, 602–622. [Google Scholar] [CrossRef]
- Mesnil, M. Rémunération des pharmaciens basée sur la prestation (only in French). Rev Med Suisse 2001, 3, 488. [Google Scholar]
- Convention tarifaire RBP IV/1 (Convention no 20.500.1036Q) du 1er janvier 2016 concernant les prestations du pharmacien(art. 46 LAMal). Available online: https://www.pharmasuisse.org/data/docs/fr/4711/Convention-tarifaire-RBP-IV-1.pdf?v=1.0 (accessed on 15 October 2018).
- Krahenbuhl, J.M.; Kremer, B.; Guignard, B.; Bugnon, O. Practical evaluation of the drug-related problem management process in Swiss community pharmacies. Pharm. World Sci. 2008, 30, 777–786. [Google Scholar] [CrossRef]
- Simi, E.; Berger, J.; Perraudin, C.; Bugnon, O. Activité clinique du pharmacien d’officine associée à la délivrance de médicaments. Master Thesis, University of Geneva, Geneva, Switzerland, 2017. [Google Scholar]
- Maes, K.A.; Studer, H.; Berger, J.; Hersberger, K.E.; Lampert, M.L. Documentation of pharmaceutical care: Validation of an intervention oriented classification system. J. Eval. Clin. Pract. 2017, 23, 1425–1432. [Google Scholar] [CrossRef]
- Basger, B.J.; Moles, R.J.; Chen, T.F. Development of an aggregated system for classifying causes of drug-related problems. Ann. Pharmacother. 2015, 49, 405–418. [Google Scholar] [CrossRef]
- Ganso, M.; Areschin, S.; Lange, P.; Emser, A.; Rossler, J.; Kramer, I. Verlasslichkeit eines Klassifikationssystems fur pharmazeutische Interventionen. Krankenhauspharmazie 2007, 28, 273. [Google Scholar]
- McHugh, M.L. Interrater reliability: the kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, P.M.; Lampert, M.L.; Kahmann, I.V.; van Mil, J.W.F.; Hersberger, K.E. Classification of drug-related problems with new prescriptions using a modified PCNE classification system. Pharm. World Sci. 2010, 32, 362–372. [Google Scholar] [CrossRef] [PubMed]
- AbuRuz, S.M.; Bulatova, N.R.; Yousef, A.M. Validation of a comprehensive classification tool for treatment-related problems. Pharm. World Sci. 2006, 28, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical Care Network Europe. Classification of drug related problems. Available online: https://www.pcne.org/upload/files/11_PCNE_classification_V6-2.pdf (accessed on 11 September 2018).
- Ganso, M.; Kunkel, M.; Kramer, I. UBERSICHTEN-Dokumentation und Klassifikation der pharmazeutischen Betreuung im Krankenhaus--Problem, Intervention, Ergebnis--das PIE-System. Krankenhauspharmazie 2009, 30, 349. [Google Scholar]
- Meyboom, R.H.B.; Lindquist, M.; Egberts, A.C.G. An ABC of Drug-Related Problems. Drug Saf. 2000, 22, 415–423. [Google Scholar] [CrossRef]
- Comité de Consenso; Grupo de Investigacíon en Aténcion Farmacéutica. Segundo consenso de Granada sobre problemas relacionados con medicamentos. Ars Pharm 2002, 43, 175–184. [Google Scholar]
- Krska, J.; Jamieson, D.; Arris, F.; McGuire, A.; Abbott, S.; Hansford, D.; Cromarty, J. A classification system for issues identified in pharmaceutical care practice. Int. J. Pharm. Pract. 2002, 10, 91–100. [Google Scholar] [CrossRef]
- National Coordinating Council on Medication Errors Reporting and Prevention (NCC MERP). NCC MERP Taxonomy of Medication Errors. Available online: https://www.nccmerp.org/taxonomy-medication-errors-now-available (accessed on 23 February 2015).
- Hämmerlein, A.; Griese, N.; Schulz, M. Medication Safety: Survey of Drug-Related Problems Identified by Community Pharmacies. Ann. Pharmacother. 2007, 41, 1825–1832. [Google Scholar] [CrossRef]
- Schaefer, M. Discussing basic principles for a coding system of drug-related problems: the case of PI-Doc. Pharm. World Sci. 2002, 24, 120–127. [Google Scholar] [CrossRef]
- Dutch. Houten. Proposals for adaptation of the SEP-codes. SHB PlusPunten: the Netherlands, 2003, 15. Available online: https://pdfs.semanticscholar.org/0832/dce32472ae4f62d7efc7b78d3357d3a8ebb4.pdf (accessed on 23 February 2015).
- Mackie, C.A. Randomised controlled trial of medication review. In: Repeat Prescribing in General Practice: The Development and Evaluation of Methodologies to Improve the Quality and CostEffectiveness of Repeat Prescribing. Ph.D. Thesis, University of Strathclyde, Glasgow, UK, 2002. [Google Scholar]
- Ruths, S.; Viktil, K.K.; Blix, H.S. [Classification of drug-related problems]. Tidsskr. Den Nor. Laegeforen. Tidsskr. Praktisk Med. Ny Raekke 2007, 127, 3073–3076. [Google Scholar]
- Westerlund, T. Drug-related problems. Identification, characteristics and pharmacy interventions. Ph.D. Thesis, Göteborg University, Göteborg, Sweden, 2002. [Google Scholar]
- Hohmann, C.; Eickhoff, C.; Klotz, J.M.; Schulz, M.; Radziwill, R. Development of a classification system for drug-related problems in the hospital setting (APS-Doc) and assessment of the inter-rater reliability. J. Clin. Pharm. Ther. 2012, 37, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Bedouch, P.; Charpiat, B.; Roubille, R.; Juste, M.; Rose, F.X.; Escofier, L.; Conort, O.; Allenet, B. Site internet de la Société française de pharmacie clinique pour l’analyse des interventions pharmaceutiques: finalité, mode d’emploi et perspectives. Journal de Pharmacie Clinique 2007, 26, 40–44. [Google Scholar] [CrossRef]
- Hanlon, J.T.; Schmader, K.E.; Samsa, G.P.; Weinberger, M.; Uttech, K.M.; Lewis, I.K.; Cohen, H.J.; Feussner, J.R. A method for assessing drug therapy appropriateness. J. Clin. Epidemiol. 1992, 45, 1045–1051. [Google Scholar] [CrossRef]
- Van Mil, J.; Tromp, T. Coding frequently asked questions during the pharmaceutical care process with the Pas System. J. Appl. Ther. 1997, 1, 351–355. [Google Scholar]
- Strand, L.M.; Morley, P.C.; Cipolle, R.J.; Ramsey, R.; Lamsam, G.D. Drug-related problems: their structure and function. Dicp 1990, 24, 1093–1097. [Google Scholar] [CrossRef]
- Maes, K.A.; Bruch, S.; Hersberger, K.E.; Lampert, M.L. Documentation of pharmaceutical care: development of an intervention oriented classification system. Int. J. Clin. Pharm. 2017, 39, 354–363. [Google Scholar] [CrossRef]
- Tagelsir Mohamed, G. Pharmacoinformatics and Drug Discovery Technologies: Theories and Applications; IGI Global: Hershey, PA, USA, 2012; pp. 1–442. [Google Scholar] [CrossRef]
- Laliberte, M.C.; Perreault, S.; Damestoy, N.; Lalonde, L. Ideal and actual involvement of community pharmacists in health promotion and prevention: a cross-sectional study in Quebec, Canada. BMC public health 2012, 12, 192. [Google Scholar] [CrossRef]
- Quintana-Barcena, P.; Lalonde, L.; Lauzier, S. Beliefs influencing community pharmacists’ interventions with chronic kidney disease patients: A theory-based qualitative study. Res. Soc. Adm. Pharm. 2019, 15, 145–153. [Google Scholar] [CrossRef]
- Mickan, S.; Tilson, J.K.; Atherton, H.; Roberts, N.W.; Heneghan, C. Evidence of effectiveness of health care professionals using handheld computers: a scoping review of systematic reviews. J. Med. Internet. Res. 2013, 15, e212. [Google Scholar] [CrossRef]
- Fox, B.I.; Felkey, B.G.; Berger, B.A.; Krueger, K.P.; Rainer, R.K., Jr. Use of personal digital assistants for documentation of pharmacists’ interventions: a literature review. Am. J. Health Syst. Pharm. 2007, 64, 1516–1525. [Google Scholar] [CrossRef]
- Berger, J.; Bourdin, A.; Pires, F.; Backes, C.; Perraudin, C.; Bugnon, O. Improving patient access to hepatitis C antiviral medicines in Switzerland: Understanding the financial risks for community pharmacies. J. Eval. Clin. Pract. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bujang, M.A.; Baharum, N. Guidelines of the minimum sample size requirements for Kappa agreement test. Epidemiol. Biostat. Public Health 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys. Ther. 2005, 85, 257–268. [Google Scholar] [PubMed]
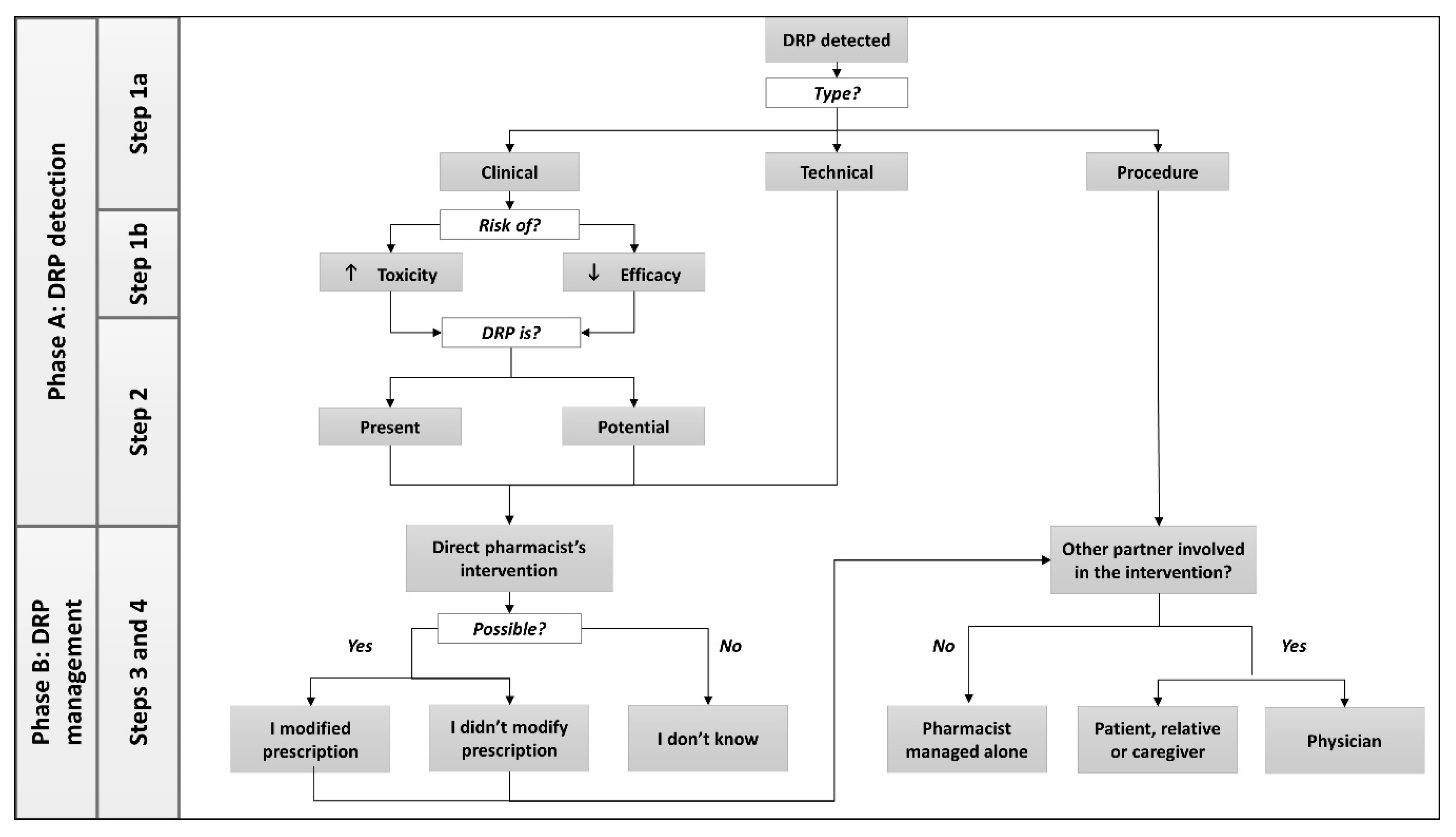


| Step | Modification | Objective |
|---|---|---|
| Step 1 DRP category, type and consequence | Split into Step 1a and Step 1b | To define the category of problem and the possible consequence (risk of inefficacy or safety issue) of clinical DRPs |
| Step 1a DRP type | ||
| Modification of the order of DRP types | Originally considered as a DRP, the risk of inefficiency or safety issue is removed from the list of DRP types and transformed into Step 1b | |
| Addition of type “Complaint of patient, relative, caregiver” Addition of type “Dissatisfaction of patient, relative, caregiver” | To allow documentation of a pharmacist’s intervention related to a complaint and to avoid incorrectly documenting a DRP as “side effect” | |
| The type “Temporary off-trade” replaced by “Problem of procurement” The type “Incomplete prescription” replaced by “Formal or regulatory reason” | To extend the possibilities among technical DRPs | |
| Step 1b DRP consequence | ||
| The type “Indication” deleted The type “Efficacy” reworded to “Loss of efficacy” The type “Toxicity” reworded to “Increased toxicity” | To distinguish the consequences of DRPs between leading to increased toxicity or loss of efficacy | |
| Step 2 DRP status | Step 2 “Clinical result” reworded to step 2 “DRP status” Subcategory “Present” added Subcategory “Potential” added | To determine the status of the DRP at the moment of patient encounter in the community pharmacy: present or potential |
| Step 3 Outcome of pharmacist’s intervention | “Modified prescription” reworded to “I [pharmacist] modified the prescription” “Unmodified prescription” reworded to “I [pharmacist] didn’t modify the prescription” The option “I [pharmacist] don’t know” added | To clarify who is involved in the intervention. Indeed, “modified prescription” could be selected if pharmacists changed the prescription alone, or in collaboration with the physician. The option “I [pharmacist] don’t know” allows pharmacists to document a clinical activity even if the outcome is unknown. |
| Step 4 Involved partners | Wording changed: “Relative”, and “caregiver” were also considered alongside “patient” | If the patient is not directly responsible for treatment (i.e., children or elderly patients), a caregiver or relative can also be considered as involved in the decision making |
| Pharmacist Identification Number (ID) | ID 1 | ID 2 | ID 3 | ID 4 | ID 5 | ID 6 | ID 7 | ID 8 | ID 9 | ID 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Kappa index (95%CI) | 0.78 (0.65; 0.90) | 0.58 (0.50; 0.66) | 0.67 (0.60; 0.74) | 0.68 (0.58; 0.74) | 0.61 (0.54; 0.69) | 0.53 (0.45; 0.61) | 0.52 (0.44; 0.60) | 0.59 (0.52; 0.66) | 0.62 (0.54; 0.69) | 0.57 (0.49; 0.65) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, N.; Quintana Bárcena, P.; Maes, K.A.; Bugnon, O.; Berger, J. Clinical Pharmacy Activities Documented (ClinPhADoc): Development, Reliability and Acceptability of a Documentation Tool for Community Pharmacists. Pharmacy 2019, 7, 162. https://doi.org/10.3390/pharmacy7040162
Hamada N, Quintana Bárcena P, Maes KA, Bugnon O, Berger J. Clinical Pharmacy Activities Documented (ClinPhADoc): Development, Reliability and Acceptability of a Documentation Tool for Community Pharmacists. Pharmacy. 2019; 7(4):162. https://doi.org/10.3390/pharmacy7040162
Chicago/Turabian StyleHamada, Nour, Patricia Quintana Bárcena, Karen Alexandra Maes, Olivier Bugnon, and Jérôme Berger. 2019. "Clinical Pharmacy Activities Documented (ClinPhADoc): Development, Reliability and Acceptability of a Documentation Tool for Community Pharmacists" Pharmacy 7, no. 4: 162. https://doi.org/10.3390/pharmacy7040162
APA StyleHamada, N., Quintana Bárcena, P., Maes, K. A., Bugnon, O., & Berger, J. (2019). Clinical Pharmacy Activities Documented (ClinPhADoc): Development, Reliability and Acceptability of a Documentation Tool for Community Pharmacists. Pharmacy, 7(4), 162. https://doi.org/10.3390/pharmacy7040162





