Assessment of General Public’s Knowledge and Opinions towards Antibiotic Use and Bacterial Resistance: A Cross-Sectional Study in an Urban Setting, Rufisque, Senegal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Type and Period of Study
2.3. Study Population
2.4. Sample Size
- (i)
- response distribution: 50%;
- (ii)
- source population size: 20,000;
- (iii)
- desired level of confidence: 95%;
- (iv)
- margin of error: 5%.
2.5. Sampling Procedure
2.6. Data Collection
2.6.1. Data Collection Instrument
- “less than 25% of correct answers” = bad
- “more than or equal to 25% of correct answers and less than 50% of correct answers” = insufficient
- “more than or equal to 50% of correct answers and less than 70% of correct answers” = average
- “more than or equal to 70% of correct answers” = good
2.6.2. Data Collection Method
2.6.3. Data Collected
2.7. Data Analysis
2.8. Ethical Considerations
3. Results
3.1. Socio-Demographic Characteristics
3.2. Filter Question
3.3. Knowledge
3.4. Knowledge Level Classification
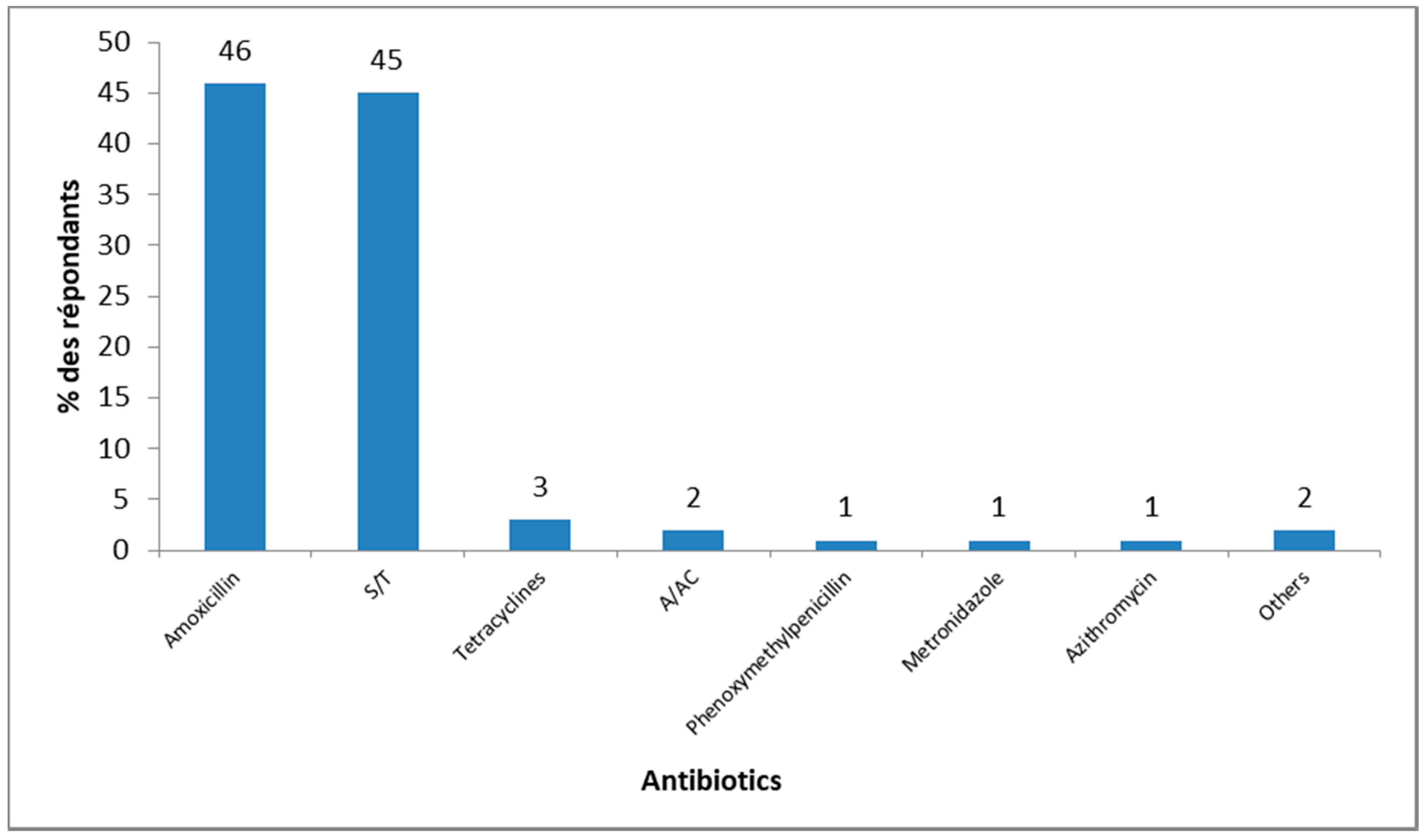
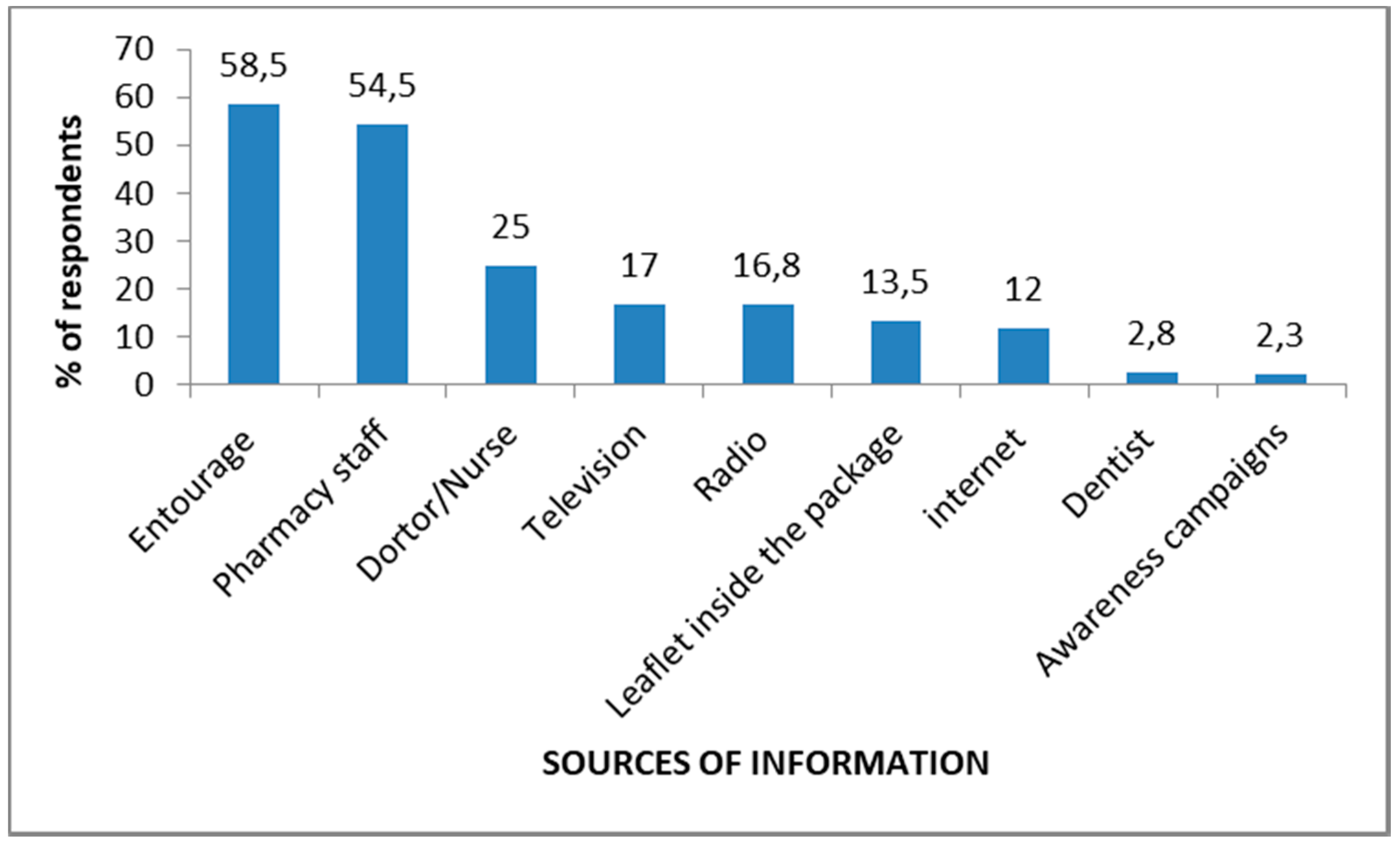
3.5. Sources of Knowledge
3.6. Opinions
4. Discussion
- ‘Only use antibiotics when prescribed by a certified health professional’
- ‘Always take the full prescription, even if you feel better’
- ‘Never use left-over antibiotics’
- ‘Never share antibiotics with others’
- ‘Prevent infections by regularly washing your hands, avoiding close contact with sick people and keeping your vaccinations up to date’
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. Wellcome Trust and HM Government 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 10-May 2018).
- Martínez, J.L.; Baquero, F. Emergence and spread of antibiotic resistance: Setting a parameter space. Upsal. J. Med. Sci. 2014, 119, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014; Available online: http://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf;jsessionid=5290CC8F73C7B46FDD0DD9FB65AC549F?sequence=1 (accessed on 10 May 2018).
- Organisation Mondiale de la Santé. Plus Sain, Plus Juste, Plus sûr: L’itinéraire de la Santé Dans le Monde, 2007–2017; OMS: Geneve, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/handle/10665/259203/9789242512366-fre.pdf?sequence=1&isAllowed=y (accessed on 3 July 2018).
- Australian Government. Australia’s First National Antimicrobial Resistance Strategy 2015–2019; Progress Report; Australian Government: Canberra, Australia, 2017. Available online: https://www.amr.gov.au/resources/australias-first-national-antimicrobial-resistance-strategy-2015-2019-progress-report (accessed on 3 July 2018).
- Centers for Diseases Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 25 June 2018).
- Tadesse, B.T.; Ashley, E.A.; Ongarello, S.; Havumaki, J.; Wijegoonewardena, M.; González, I.J.; Dittrich, S. Antimicrobial resistance in Africa: A systematic review. BMC Infect. Dis. 2017, 17, 616. [Google Scholar] [CrossRef] [PubMed]
- Ampaire, L.; Muhindo, A.; Orikiriza, P.; Mwanga-Amumpaire, J.; Bebell, L.; Boum, Y. A review of antimicrobial resistance in East Africa. Afr. J. Lab. Med. 2016, 5, 432. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, K.J.; Langendorf, C.; Ford, N.; Ronat, J.B.; Murphy, R.A. Antimicrobial resistance in West Africa: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2017, 50, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Sambe-Ba, B.; Seck, A.; Wane, A.A.; Fall-Niang, N.K.; Gassama-Sow, A. Sensibilité aux antibiotiques et supports génétiques de la résistance des souches de Shigella flexneri isolées à Dakar de 2001 à 2010. Bull. Soc. Pathol. Exot. 2013, 106, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Breurec, S.; Bouchiat, C.; Sire, J.; Moquet, O.; Bercion, R.; Cisse, M.F.; Seck, A. High third-generationcephalosporin resistant Enterobacteriaceae prevalence rate among neonatal infections in Dakar, Senegal. BMC Infect. Dis. 2016, 16, 587. [Google Scholar] [CrossRef] [PubMed]
- A Global Declaration on Appropriate Use of Antimicrobial Agents across the Surgical Pathway. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29173054 (accessed on 9 August 2018).
- Salm, F.; Ernsting, C.; Kuhlmey, A.; Kanzler, M.; Gastmeier, P.; Gellert, P. Antibiotic use, knowledge and health literacy among the general population in Berlin, Germany and its surrounding rural areas. PLoS ONE 2018, 13, e0193336. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.M.; Dolk, F.C.K.; Pouwels, K.B.; Christie, M.; Robotham, J.V.; Smieszek, T. Defining the appropriateness and inappropriateness of antibiotic prescribing in primary care. J. Antimicrob. Chemother. 2018, 73, ii11–ii18. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to minimize antibiotic resistance. Int. J. Environ. Res. Public Health 2013, 10, 4274–4305. [Google Scholar] [CrossRef] [PubMed]
- Kotwani, A.; Holloway, K. Trends in antibiotic use among outpatients in New Delhi, India. BMC Infect. Dis. 2011, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Chem, E.D.; Anong, D.N.; Akoachere, J.K.T. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. PLoS ONE 2018, 13, e0193353. [Google Scholar] [CrossRef] [PubMed]
- Organisation Mondiale de la Santé. Rapport Sur la Santé Dans le Monde. Plus de Santé Pour Son Argent; OMS: Généve, Switzerland, 2010; Available online: http://apps.who.int/iris/bitstream/handle/10665/44372/9789242564020_fre.pdf?sequence=1 (accessed on 10 October 2017).
- Massele, A.; Tiroyakgosi, C.; Matome, M.; Desta, A.; Muller, A.; Paramadhas, B.D.A.; Godman, B. Research activities to improve the utilization of antibiotics in Africa. Expert Rev. Pharmacoecon. Outcomes Res. 2017, 17, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Donyai, P.; Okafor, S.; Virgo, R.; Amin, K.; Nasr, M. Messages about Antibiotic Resistance in Different Newspaper Genres. Pharmacy 2013, 1, 181–192. [Google Scholar] [CrossRef]
- Bonten, M.J.; Austin, D.J.; Lipsitch, M. Understanding the spread of antibiotic resistant pathogens in hospitals: Mathematical models as tools for control. Clin. Infect. Dis. 2001, 33, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- World Bank Group. Drug-Resistant Infections: A Threat to Our Economic Future; Final Report; WBG: Washington, DC, USA, 2017; Available online: http://documents.worldbank.org/curated/en/323311493396993758/pdf/114679-REVISED-v2-Drug-Resistant-Infections-Final-Report.pdf (accessed on 10 October 2017).
- World Bank Group. Communiqués de Presse; WBG: Washington, DC, USA, 2016; Available online: http://www.banquemondiale.org/fr/news/press-release/2016/09/18/by-2050-drug-resistant-infections-could-cause-global-economic-damage-on-par-with-2008-financial-crisis (accessed on 10 October 2017).
- Organisation Mondiale de la Santé. Plan D’action Mondial Pour Combattre la Résistance Aux Antimicrobiens; OMS: Généve, Switzerland, 2016; Available online: http://apps.who.int/iris/bitstream/handle/10665/249548/9789242509762 fre.pdf; jsessionid=6BA7B094CA69BC758FC52F00A7E179E0?sequence=1 (accessed on 20 January 2018).
- Coll-Seck, A.M.; Seck, I.; Sow, A.I.; Ndoye, B.; Ngom, B.; Diack, P.A. La gouvernance contre la résistance aux antimicrobiens en Afrique: Faire face quand les ressources sont limitées—L’exemple du sénégal. In AMR Control. Surmonter La Résistance Aux Antimicrobiens, 3rd ed.; WAAAR; Global Health Dynamics: Suffolk, UK, 2017; Volume 1, pp. 10–15. ISBN 978-0-9576072-7-9. Available online: http://view.pagetiger.com/AMR/AMR2018/PDF.pdf (accessed on 10 January 2018).
- Ministère du commerce. Du sectuer informel. De la consommation, de la promotion des produits locaux et des petites et moyennes entreprises. In Santé et Sécurité Sanitaire des Aliments; Ministère du commerce: Dakar, Senegal, 2018. Available online: http://www.commerce.gouv.sn/article.php3?id_article=503#sthash.JHrOiFPf.BuhJMRZj.dpbs (accessed on 16 May 2018).
- Organisation Mondiale de la santé. Évaluation Externe Conjointe des Principales Capacités RSI de la Republique du Senegal; Rapport de Mission: 28 Novembre–2 Décembre 2016; OMS: Généve, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/handle/10665/255765/WHO-WHE-CPI-2017.31-fre.pdf?sequence=1 (accessed on 20 January 2018).
- World Health Organization. Antibiotic Resistance: Multi-Country Public Awareness Survey; WHO: Généve, Switzerland, 2015; Available online: http://apps.who.int/iris/bitstream/handle/10665/194460/9789241509817_eng.pdf?sequence=1&isAllowed=y (accessed on 20 January 2018).
- Gualano, M.R.; Gili, R.; Scaioli, G.; Bert, F.; Siliquini, R. General population’s knowledge and attitudes about antibiotics: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2015, 24, 2–10. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.R.; Parekh, S.; Rathbone, J.; Del Mar, C.B.; Hoffmann, T.C. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 2016, 71, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Agence Nationale de la Statistique et de la Démographie. Projection de la Population de la Region de Dakar, 2013–2025; ANSD: Dakar, Senegal, 2013; Available online: http://www.ansd.sn/ressources/publications/indicateurs/Projections-demographiques-2013-2025+.htm (accessed on 16 May 2018).
- Agence Nationale de la Statistique et de la Démographie. Recensement Général de la Population et de l’Habitat, de l’Agriculture et de l’Elevage; Rapport Régional Définitif; ANSD: Dakar, Senegal, 2017; Available online: http://www.ansd.sn/ressources/RGPHAE-2013/ressources/doc/pdf/RGPHAE-Rapport-regional_DAKAR_vf.pdf (accessed on 17 May 2018).
- Agence Nationale de la Statistique et de la Démographie et Fonds des Nations Unies pour l’enfance. MICS V Dakar Enquête par Grappes à Indicateurs Multiples 2015–2016; Rapport Final; ANSD: Dakar, Sénégal, 2016; Available online: http://www.ansd.sn/ressources/publications/RAPPORT%20MICS%20Urbaine%20Dakar%202015-2016_FINAL%20novembre_version%20finale%20.pdf (accessed on 17 May 2018).
- Office National de L’assainissement du; ONAS: Dakar, Sénégal, 2018; Available online: https://www.onas.sn/onas/actualites/actualites-onas/probleme-dassainissement-a-rufisque-le-ministere-de-lhydraulique-et-de (accessed on 16 May 2018).
- Raosoft, Inc. Raosoft Sample Size Calculator. 2004. Available online: http://www.raosoft.com/samplesize.html (accessed on 30 October 2017).
- José, E.M.; Oudou, N. L’enquête CAP (Connaissances, Attitudes, Pratiques) en recherche médicale. Health Sci. Dis. 2013, 14, 1–3. [Google Scholar]
- Hosmer, D.W.J.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; Wiley-Interscience Publication: New York, NY, USA, 2000; p. 392. [Google Scholar]
- Omulo, S.; Thumbi, S.M.; Lockwood, S.; Verani, J.R.; Bigogo, G.; Masyongo, G.; Call, D.R. Evidence of superficial knowledge regarding antibiotics and their use: Results of two cross-sectional surveys in an urban informal settlement in Kenya. PLoS ONE 2017, 12, e0185827. [Google Scholar] [CrossRef] [PubMed]
- Padget, M.; Tamarelle, J.; Herindrainy, P.; Ndir, A.; Diene Sarr, F.; Richard, V.; BIRDY Study Group. A community survey of antibiotic consumption among children in Madagascar and Senegal: The importance of healthcare access and care quality. J. Antimicrob. Chemother. 2017, 72, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Ocan, M.; Bbosa, G.S.; Waako, P.; Ogwal-Okeng, J.; Obua, C. Factors predicting home storage of medicines in Northern Uganda. BMC Public Health. 2014, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, C.N.; Klein, R.D.; Schreiber, H.L.; Janetka, J.W.; Hultgren, S.J. Precision antimicrobial therapeutics: The path of least resistance? NPJ Biofilms Microbiomes 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.S.; Diallo, B.A.; Kanté, D.; Diallo, I.S. Antimicrobial susceptibility profile of community-acquired urinary tract infection in adults: A seven months prospective cross-sectional study in Dakar Town, Senegal. Afr. J. Urol. 2017, 23, 166–171. [Google Scholar] [CrossRef]
- Abujheisha, K.Y.; Al-Shdefat, R.; Ahmed, N.; Fouda, M.I. Public Knowledge and Behaviours Regarding Antibiotics Use: A Survey among the General Public. Int. J. Med. Res. Health Sci. 2017, 6, 82–88. [Google Scholar]
- ESCMID Sore Throat Guideline Group; Pelucchi, C.; Grigoryan, L.; Galeone, C.; Esposito, S.; Huovinen, P.; Little, P.; Verheij, T. Guideline for the management of acute sore throat. Clin. Microbiol. Infect. 2012, 18, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.L.; Jackson, M.A.; Hicks, L.A.; American Academy of Pediatrics Committee on Infectious Diseases. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics 2013, 132, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.S.; DuPont, H.L.; Connor, B.A. ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults. Am. J. Gastroenterol. 2016, 111, 602–622. [Google Scholar] [CrossRef] [PubMed]
- Eilat-Tsanani, S.; Tabenkin, H.; Chazan, B.; Lavi, I.; Cwikel-Hamzany, S. Acute cough: The use of antibiotics and health care services in an urban health centre in Israel. Eur. J. Gen. Pract. 2013, 19, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hassali, M.A.; Arief, M.; Saleem, F.; Khan, M.U.; Ahmad, A.; Mariam, W.; Bheemavarapu, H.; Syed, I.A. Assessment of attitudes and practices of young Malaysian adults about antibiotics use: A cross-sectional study. Pharm. Pract. (Granada) 2017, 15, 929. [Google Scholar] [CrossRef] [PubMed]
- Cortez, J.; Rosário, E.; Pires, J.E.; Taborda Lopes, J.; Francisco, M.; Vlieghe, E.; Brito, M. Antimicrobial storage and antibiotic knowledge in the community: A cross-sectional pilot study in north-western Angola. Int. J. Infect. Dis. 2017, 60, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.K.; Teh, C.C. A Cross Sectional Study of Public Knowledge and Attitude towards Antibiotics in Putrajaya, Malaysia. South. Med. Rev. 2012, 5, 26–33. [Google Scholar] [PubMed]
- Chandrakanth, P.; Mohamed Saleem, T.S.; Madhan Mohan, R.; Gopinath, C.; Madhan Mohan, R. Assessment of public knowledge and attitude regarding antibiotic use in a tertiary care hospital. Asian J. Pharm. Clin. Res. 2016, 9, 118–122. [Google Scholar]
- Gebeyehu, E.; Bantie, L.; Azage, M. Inappropriate Use of Antibiotics and Its Associated Factors among Urban and Rural Communities of Bahir Dar City Administration, Northwest Ethiopia. PLoS ONE 2015, 10, e0138179. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.C.; Schwabe-Warf, D.; Goldman, R. Reducing inappropriate antibiotic use among children with influenza infection. Can. Fam. Phys. 2011, 57, 42–44. [Google Scholar]
- Truter, I. Antimicrobial prescribing in South Africa using a large pharmacy database: A drug utilisation study. South Afr. J. Infect. Dis. 2015, 30, 52–56. [Google Scholar] [CrossRef]
- Topor, G.; Grosu, I.A.; Ghiciuc, C.M.; Strat, A.L.; Lupuşoru, C.E. Awareness about antibiotic resistance in a self-medication user group from Eastern Romania: A pilot study. PeerJ 2017, 5, e3803. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.L.; Hassali, M.A.; Al-haddad, M.S.; Azhar, S.; Sulaiman, S. Original Article Public knowledge and attitudes towards antibiotic usage: A cross-sectional study among the general public in the state of Penang, Malaysia. J. Infect. Dev. Ctries. 2010, 5, 338–347. [Google Scholar]
- Awadh, A.M.; Raja, A.K.; Mahdi, A.I.; Khalid, A.A. Assessment of Knowledge, Attitude and Practice Regarding Antibiotics Misuse among the Public in Saudi Arabia. Egypt. J. Hosp. Med. 2017, 69, 2405–2411. [Google Scholar] [CrossRef]
- Pereko, D.D.; Lubbe, M.S.; Essack, S.Y. Public knowledge, attitudes and behaviour towards antibiotic usage in Windhoek, Namibia Public knowledge, attitudes and behaviour towards antibiotic usage in Windhoek, Namibia. S. Afr. J. Infect. Dis. 2015, 30, 134–137. [Google Scholar] [CrossRef]
- Holloway, K.A.; Ivanovska, V.; Wagner, A.K.; Vialle-Valentin, C.; Ross-Degnan, D. Have we improved use of medicines in developing and transitional countries and do we know how to? Two decades of evidence. Trop. Med. Int. Health 2013, 18, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, M.B. Self-medication practice in Ethiopia: A systematic review. Patient Prefer Adherence 2017, 11, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, A.S.; Jean Pierre, H.; Bañuls, A.L.; Ouédraogo, R.; Godreuil, S. Émergence et diffusion de la résistance aux antibiotiques en Afrique de l’Ouest: Facteurs favorisants et évaluation de la menace. Med. Sante Trop. 2017, 27, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Labi, A.K.; Obeng-Nkrumah, N.; Bjerrum, S.; Aryee, N.A.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Physicians’ knowledge, attitudes, and perceptions concerning antibiotic resistance: A survey in a Ghanaian tertiary care hospital. BMC Health Serv. Res. 2018, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Ahiabu, M.A.; Magnussen, P.; Bygbjerg, I.C.; Tersbøl, B.P. Treatment practices of households and antibiotic dispensing in medicine outlets in developing countries: The case of Ghana. Res. Social Adm. Pharm. 2018, 7, 29428578. [Google Scholar] [CrossRef] [PubMed]
- Komolafe, O.O. Antibiotic resistance in bacteria—An emerging public health problem. Malawi Med. J. 2003, 15, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Mutua, J.M.; Gitao, C.G.; Bebora, L.C.; Mutua, F.K. Antimicrobial Resistance Profiles of Bacteria Isolated from the Nasal Cavity of Camels in Samburu, Nakuru, and Isiolo Counties of Kenya. J. Vet. Med. 2017, 2017, 1216283. [Google Scholar] [CrossRef] [PubMed]
- Nadimpalli, M.; Delarocque-Astagneau, E.; Love, D.C.; Price, L.B.; Huynh, B.T.; Collard, J.M.; Guillemot, D. Bacterial Infections and antibiotic-Resistant Diseases among Young children in low-income countries (BIRDY) Study Group. Combating Global Antibiotic Resistance: Emerging One Health Concerns in Lower- and Middle-Income Countries. Clin. Infect. Dis. 2018, 66, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Hiki, M.; Ozawa, M.; Koike, R.; Eguchi, K.; Kawanishi, M.; Kojima, A.; Endoh, Y.S.; Hamamoto, S.; Sakai, M.; et al. Control of the development and prevalence of antimicrobial resistance in bacteria of food animal origin in Japan: A new approach for risk management of antimicrobial veterinary medicinal products in Japan. Foodborne Pathog. Dis. 2014, 11, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, M.H.; Lynfield, R.; Schaffner, W.; Craig, A.S.; Hadler, J.; Reingold, A.; Facklam, R.R.; Active Bacterial Core Surveillance of the Emerging Infections Program Network. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. New. Engl. J. Med. 2006, 354, 1455–1463. [Google Scholar]
- Cafiero-Fonseca, E.T.; Stawasz, A.; Johnson, S.T.; Sato, R.; Bloom, D.E. The full benefits of adult pneumococcal vaccination: A. systematic review. PLoS ONE 2017, 12, e0186903. [Google Scholar] [CrossRef] [PubMed]
- Golding, G.R.; Quinn, B.; Bergstrom, K.; Stockdale, D.; Woods, S.; Nsungu, M.; Brooke, B.; Levett, P.N.; Horsman, G.; McDonald, R.; et al. Community-based educational intervention to limit the dissemination of community-associated methicillin-resistant Staphylococcus aureus in Northern Saskatchewan, Canada. BMC Public Health 2012, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.I.; Aboud, E.A. Knowledge, attitude and practice towards antibiotic use among the public in Kuwait. PLoS ONE 2015, 10, e0117910. [Google Scholar] [CrossRef] [PubMed]
- Pavydė, E.; Veikutis, V.; Mačiulienė, A.; Mačiulis, V.; Petrikonis, K.; Stankevičius, E. Public Knowledge, Beliefs and Behavior on Antibiotic Use and Self-Medication in Lithuania. Int. J. Environ. Res. Public Health 2015, 12, 7002–7016. [Google Scholar] [CrossRef] [PubMed]
- El Zowalaty, M.E.; Belkina, T.; Bahashwan, S.A.; El Zowalaty, A.E.; Tebbens, J.D.; Abdel-Salam, H.A.; Nohi, N.I. Knowledge, awareness, and attitudes toward antibiotic use and antimicrobial resistance among Saudi population. Int. J. Clin. Pharm. 2016, 38, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Hawking, M.K.; Lecky, D.M.; Touboul Lundgren, P.; Aldigs, E.; Abdulmajed, H.; Ioannidou, E.; Mappouras, D. Attitudes and behaviours of adolescents towards antibiotics and self-care for respiratory tract infections: A qualitative study. BMJ Open 2017, 7, e015308. [Google Scholar] [CrossRef] [PubMed]
- Organisation Mondiale de la Santé. Campagnes Mondiales de Santé Publique de l’OMS. Available online: http://www.who.int/mediacentre/events/2015/world-antibiotic-awareness-week/antibioresistance-agir-affiche.pdf?ua=1 (accessed on 18 May 2018).
- Asante, K.P.; Boamah, E.A.; Abdulai, M.A.; Buabeng, K.O.; Mahama, E.; Dzabeng, F.; Gyansa-Lutterodt, M.; Ghana Antimicrobial Resistance Working Group. Knowledge of antibiotic resistance and antibiotic prescription practices among prescribers in the Brong Ahafo Region of Ghana; a cross-sectional study. BMC Health Serv. Res. 2017, 17, 422. [Google Scholar] [CrossRef] [PubMed]
- Organisation Mondiale de la Santé. Fédération internationale pharmaceutique. In Elargir la Pratique Pharmaceutique. Recentrer les Soins sur les Patients; OMS: Généve, Switzerland, 2006; Available online: http://apps.who.int/medicinedocs/documents/s16219f/s16219f.pdf (accessed on 18 May 2018).
- Bornstein, M.H.; Jager, J.; Putnick, D.L. Sampling in Developmental Science: Situations, Shortcomings, Solutions, and Standards. Dev. Rev. 2013, 33, 357–370. [Google Scholar] [CrossRef] [PubMed]
| Socio-Demographic Characteristics | n | % |
|---|---|---|
| Age (years) | ||
| [18–30] | 114 | 28.5 |
| [31–40] | 130 | 32.5 |
| [41–50] | 87 | 21.8 |
| [51–60] | 43 | 10.8 |
| >60 | 26 | 6.5 |
| Sex | ||
| Male | 220 | 55 |
| Female | 180 | 45 |
| Marital status | ||
| Married | 222 | 55.5 |
| Unmarried | 178 | 44.5 |
| Professional status | ||
| Employed | 248 | 62 |
| Unemployed | 152 | 38 |
| Education | ||
| Yes | 195 | 48.75 |
| No | 205 | 51.25 |
| Close proximity to a health facility | ||
| Yes (<300 m) | 197 | 49.25 |
| No (>300 m) | 203 | 50.75 |
| Close proximity to a community pharmacy | ||
| Yes (<300 m) | 274 | 68.5 |
| No (>300 m) | 126 | 31.5 |
| Statements | True n (%) | False n (%) | Don’t Know n (%) |
|---|---|---|---|
| Antibiotics work against: | |||
| 1. Cold/Flu | 279 (69.8) | 97 (24.3) | 24 (6.0) |
| 2. Cough | 289 (72.3) | 85 (21.3) | 26 (6.5) |
| 3. Sore throat | 257 (64.3) | 84 (21.0) | 59 (14.8) |
| 4. Diarrhea | 141 (35.3) | 184 (46.0) | 75 (18.8) |
| 5. Fever | 167 (41.8) | 205 (51.3) | 28 (7.0) |
| 6. Fatigue | 203 (50.8) | 153 (38.3) | 44 (11.0) |
| 7. One should stop taking an antibiotic treatment as soon as one feels better | 171 (42.8) | 227 (56.8) | 2 (0.5) |
| 8. High antibiotic consumption can lead to bacterial resistance | 335 (83.8) | 54(13.5) | 11(2.8) |
| 9. Poor patient compliance with antibiotic treatment may be harmful to people | 102 (25.5) | 267 (66.8) | 31 (7.8) |
| 10. Handwashing can prevent bacterial resistance | 35 (8.8) | 228 (57) | 137 (34.3) |
| 11. Vaccination can prevent bacterial resistance | 167 (41.8) | 205 (51.3) | 28 (7) |
| 12. Inappropriate antibiotic use in animals can result in negative impact on human health | 270 (67.5) | 117 (29.3) | 13 (3.3) |
| SDC | GLN | BA | MA | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | OR | IC 95% | p-Value | OR | IC 95% | p-Value | |
| Age | ||||||||
| ≤40 | 22 | 222 | 2.4 | [1.0–6.2] | 0.048 | 1.3 | [0.5–3.8] | 0.566 |
| >40 | 6 | 150 | 1 | |||||
| Sex | ||||||||
| Male | 13 | 207 | 1 | |||||
| Female | 15 | 165 | 1.45 | [0.67–3.13] | 0.344 | |||
| MS | ||||||||
| Married | 8 | 214 | 1 | |||||
| Unmarried | 20 | 158 | 3.39 | [1.46–7.89] | 0.003 | 2.5 | [0.9–6.6] | 0.056 |
| Education | ||||||||
| Yes | 20 | 175 | 2.81 | [1.21–6.54] | 0.012 | 6.4 | [0.8–48.1] | 0.073 |
| No | 8 | 197 | 1 | |||||
| PS | ||||||||
| Employed | 13 | 235 | 1 | |||||
| Unemployed | 15 | 137 | 1.98 | [0.91–4.28] | 0.078 | 1.3 | [0.6–2.9] | 0.563 |
| CP to HF | ||||||||
| Yes | 14 | 183 | 1.03 | [0.48–2.22] | 0.934 | |||
| No | 14 | 189 | 1 | |||||
| CP to CP | ||||||||
| Yes | 22 | 252 | 1.75 | [0.69–4.43] | 0.234 | 1.3 | [0.5–3.4] | 0.591 |
| No | 6 | 120 | 1 | |||||
| Statements | Yes, n (%) | No, n (%) | No Opinion, n (%) |
|---|---|---|---|
| Do you think that the population overuses antibiotics? | 313 (78.3) | 26 (6,5) | 61 (15.3) |
| Do you think we give you enough information, during the consultations, about antibiotics regarding their ineffectiveness if they are misused? | 112 (28) | 278 (69.5) | 10 (2.5) |
| Do you think we give you enough information, during the dispensation, about antibiotics regarding their ineffectiveness if they are misused? | 214 (53.5) | 181 (45.3) | 5 (1.3) |
| Do you think you can play a big role in fighting bacterial resistance? | 183 (45.8) | 112 (28) | 105 (26.3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassoum, O.; Sougou, N.M.; Diongue, M.; Lèye, M.M.M.; Mbodji, M.; Fall, D.; Seck, I.; Faye, A.; Tal-Dia, A. Assessment of General Public’s Knowledge and Opinions towards Antibiotic Use and Bacterial Resistance: A Cross-Sectional Study in an Urban Setting, Rufisque, Senegal. Pharmacy 2018, 6, 103. https://doi.org/10.3390/pharmacy6040103
Bassoum O, Sougou NM, Diongue M, Lèye MMM, Mbodji M, Fall D, Seck I, Faye A, Tal-Dia A. Assessment of General Public’s Knowledge and Opinions towards Antibiotic Use and Bacterial Resistance: A Cross-Sectional Study in an Urban Setting, Rufisque, Senegal. Pharmacy. 2018; 6(4):103. https://doi.org/10.3390/pharmacy6040103
Chicago/Turabian StyleBassoum, Oumar, Ndèye Marème Sougou, Mayassine Diongue, Mamadou Makhtar Mbacke Lèye, Mouhamad Mbodji, Djibril Fall, Ibrahima Seck, Adama Faye, and Anta Tal-Dia. 2018. "Assessment of General Public’s Knowledge and Opinions towards Antibiotic Use and Bacterial Resistance: A Cross-Sectional Study in an Urban Setting, Rufisque, Senegal" Pharmacy 6, no. 4: 103. https://doi.org/10.3390/pharmacy6040103
APA StyleBassoum, O., Sougou, N. M., Diongue, M., Lèye, M. M. M., Mbodji, M., Fall, D., Seck, I., Faye, A., & Tal-Dia, A. (2018). Assessment of General Public’s Knowledge and Opinions towards Antibiotic Use and Bacterial Resistance: A Cross-Sectional Study in an Urban Setting, Rufisque, Senegal. Pharmacy, 6(4), 103. https://doi.org/10.3390/pharmacy6040103





