Outcomes When Using Adjunct Dexmedetomidine with Propofol Sedation in Mechanically Ventilated Surgical Intensive Care Patients
Abstract
1. Introduction
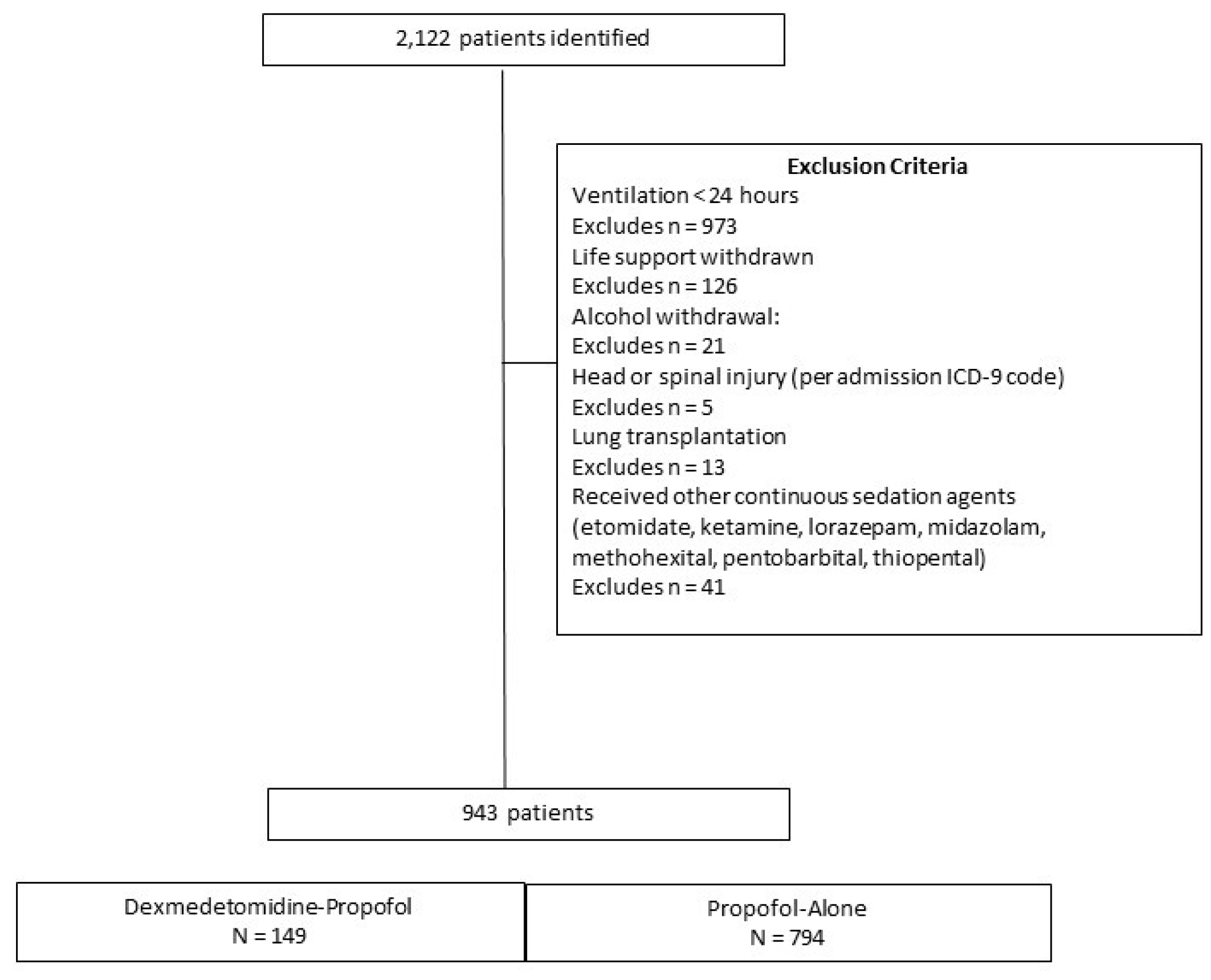
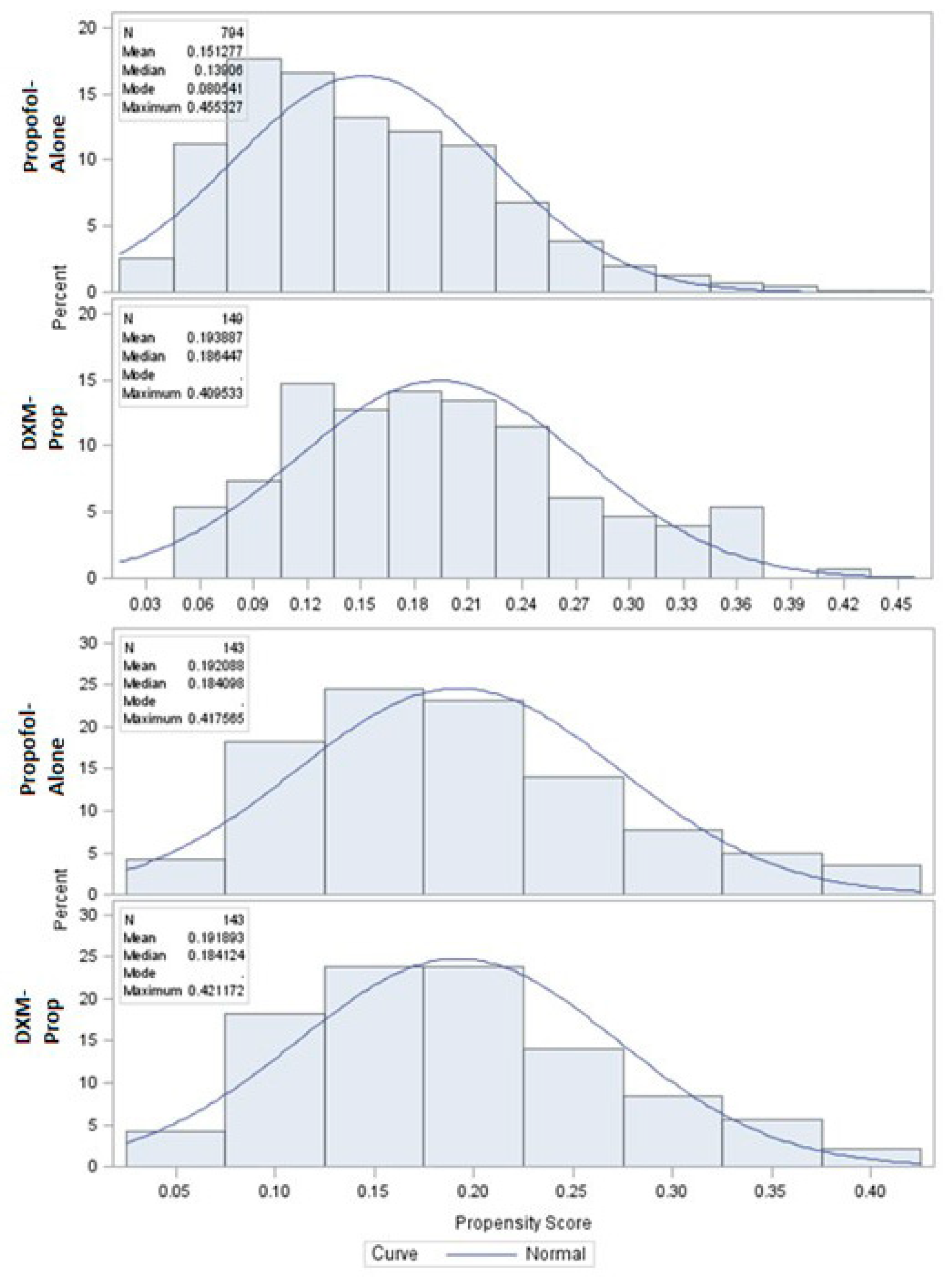
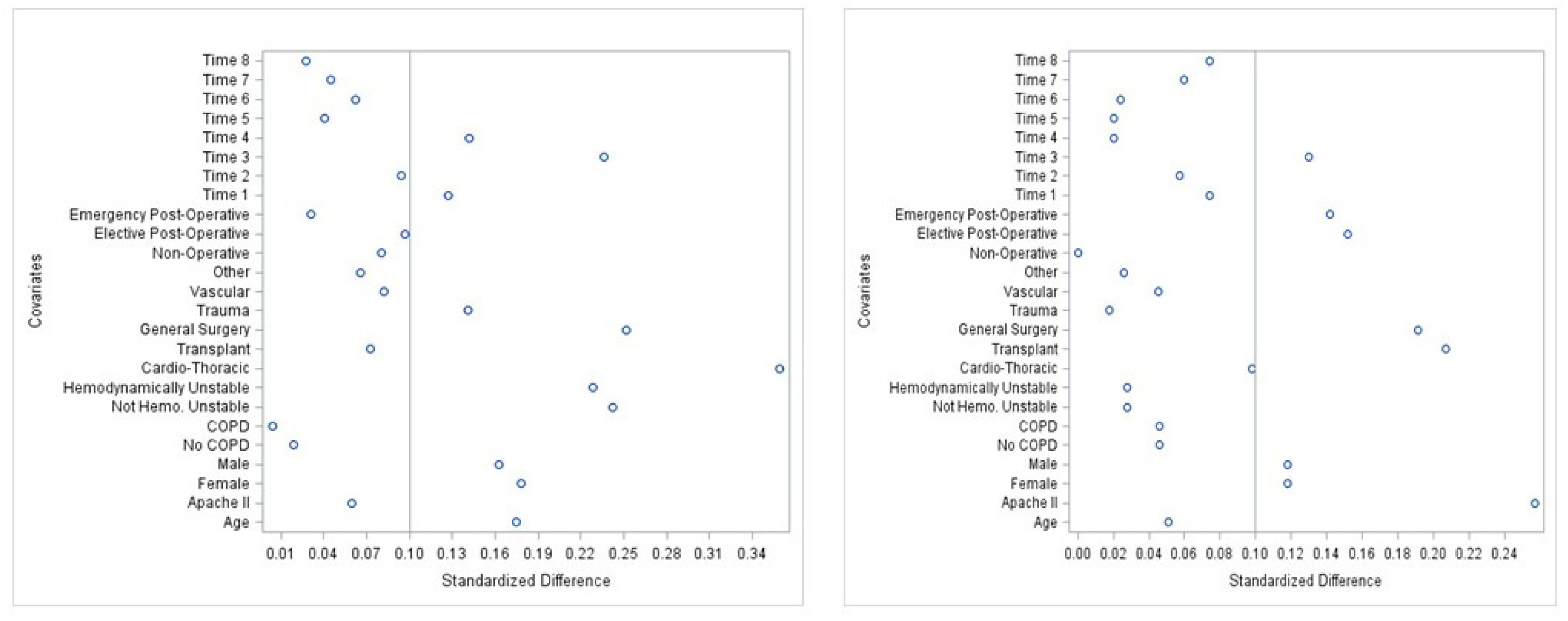
2. Materials and Methods
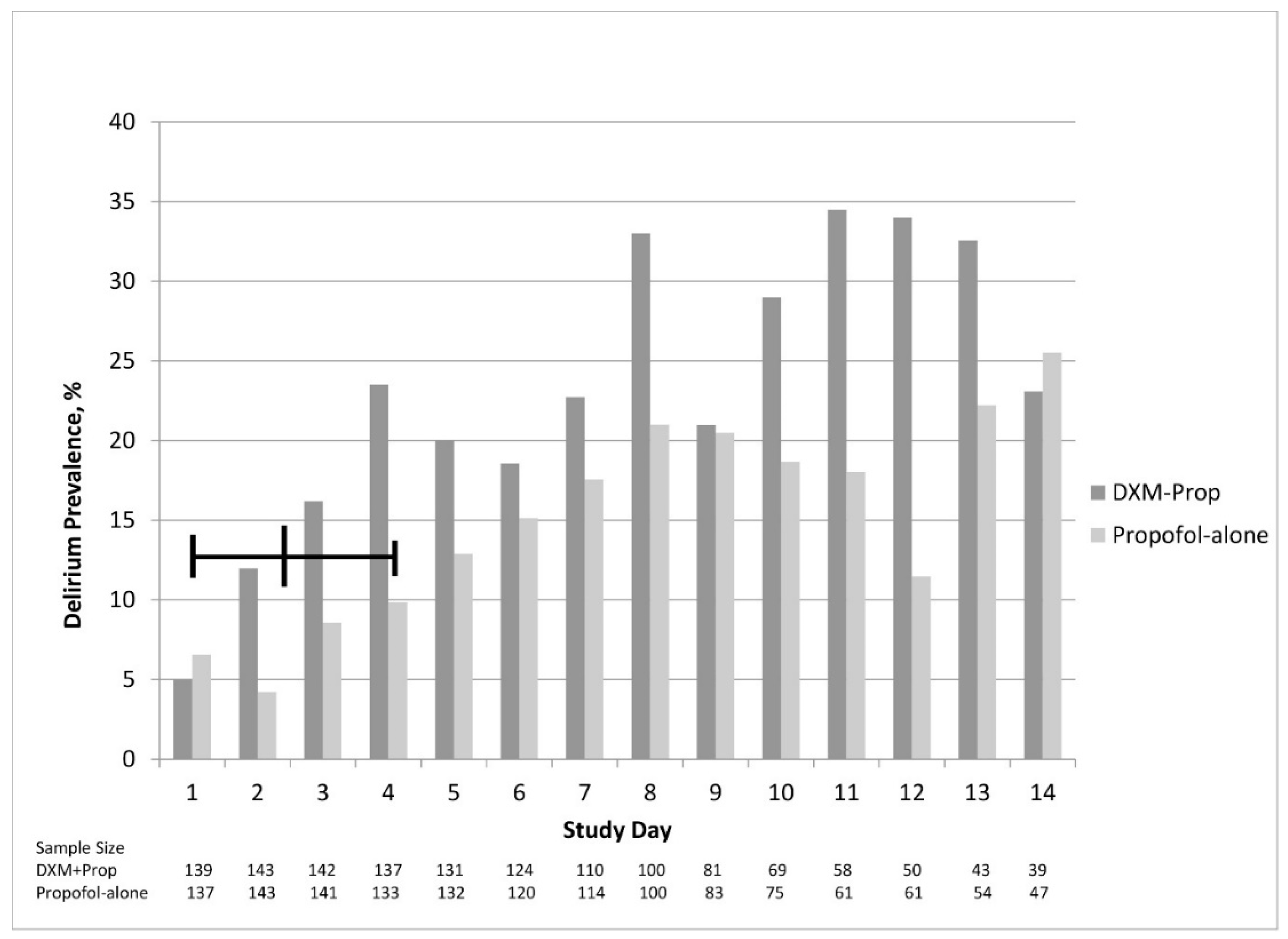
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wunsch, H.; Wagner, J.; Herlim, M.; Chong, D.; Kramer, A.; Halpern, S.D. ICU occupancy and mechanical ventilator use in the United States. Crit. Care Med. 2013, 41, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, H.; Linde-Zwirble, W.T.; Angus, D.C.; Hartman, M.E.; Milbrandt, E.B.; Kahn, J.M. The epidemiology of mechanical ventilation use in the United States. Crit. Care Med. 2010, 38, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Levy, N.T.; Ahrens, T.S.; Schaiff, R.; Prentice, D.; Sherman, G. The use of continuous iv sedation is associated with prolongation of mechanical ventilation. Chest 1998, 114, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Pandaripande, P.P.; Pun, B.T.; Herr, D.L.; Maze, M.; Girard, T.D.; Miller, R.R.; Shintani, A.K.; Thompson, J.L.; Jackson, J.C.; Deppen, S.A.; et al. Effect of sedation with dexmedetomidine vs. lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA 2007, 298, 2644–2653. [Google Scholar] [CrossRef] [PubMed]
- Reade, M.C.; Finfer, S. Sedation and delirium in the intensive care unit. N. Engl. J. Med. 2014, 370, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Hospira, Inc. Precedex [Package Insert]; Hospira, Inc.: Lake Forest, IL, USA, 2015. [Google Scholar]
- Venn, M.; Newman, J.; Grounds, M. A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensiv. Care Med. 2003, 29, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Siobal, M.S.; Kallet, R.H.; Kivett, V.A.; Tang, J.F. Use of dexmedetomidine to facilitate extubation in surgical intensive-care-unit patients who failed previous weaning attempts following prolonged mechanical ventilation: A pilot study. Respir. Care 2006, 51, 492–496. [Google Scholar] [PubMed]
- Shehabi, Y.; Ruettimann, U.; Adamson, H.; Innes, R.; Ickeringill, M. Dexmedetomidine infusion for more than 24 hours in critically ill patients: Sedative and cardiovascular effects. Intensiv. Care Med. 2004, 30, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, R.; Forrest, L.K.; Kiser, T.H. Adjunctive dexmedetomidine therapy in the intensive care unit: A retrospective assessment of impact on sedative and analgesic requirements, levels of sedation and analgesia, and ventilator and hemodynamic parameters. Pharmacotherapy 2007, 27, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Arpino, P.A.; Kalafatas, K.; Thompson, B.T. Feasibility of dexmedetomidine in facilitating extubation in the intensive care unit. J. Clin. Pharm. Ther. 2008, 33, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Shehabi, Y.; Riker, R.R.; Bokesch, P.M.; Wisemandle, W.; Shintani, A.; Ely, E.W. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit. Care Med. 2010, 38, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Reade, M.C.; Eastwood, G.M.; Bellomo, R.; Bailey, M.; Bersten, A.; Cheung, B.; Davies, A.; Delaney, A.; Ghosh, A.; van Haren, F.; et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: A randomized clinical trial. JAMA 2016, 315, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.M.; Ruokonen, E.; Grounds, R.M.; Sarapohja, T.; Garratt, C.; Pocock, S.J.; Bratty, J.R.; Takala, J.; Dexmedetomidine for Long-Term Sedation Investigators. Dexmedetomidine vs. midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA 2012, 30, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Riker, R.; Shehabi, Y.; Bokesch, P.M.; Ceraso, D.; Wisemandle, W.; Koura, F.; Whitten, P.; Margolis, B.D.; Byrne, D.W.; Ely, E.W.; et al. Dexmedetomidine vs. midazolam for sedation of critically ill patients: A randomized trial. JAMA 2009, 301, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Dasta, J.F.; Jacobi, J.; Sesti, A.M.; McLaughlin, T.P. Addition of dexmedetomidine to standard sedation regimens after cardiac surgery: An outcomes analysis. Pharmacotherapy 2006, 26, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, N.W.; Mone, M.C.; Nirula, R.; Kimball, E.J.; Ludwig, K.; Zhou, X.; Sauer, B.C.; Nechodom, K.; Teng, C.; Barton, R.G. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am. J. Respir. Crit. Care Med. 2014, 189, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Li, L.; Szumita, P.; Kleinman, K.; Murphy, M.V.; CDC Prevention Epicenters Program. Associations between different sedatives and ventilator-associated events, length of stay, and mortality in patients who were mechanically ventilated. Chest 2016, 149, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.M.; Kelley, A.S.; Paris, J.; Roza, K.; Meier, D.E.; Morrison, R.S.; Aldridge, M.D. Methods for constructing and assessing propensity scores. Health Serv. Res. 2014, 49, 1701–1720. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. A tutorial and case study in propensity score analysis: An application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivar. Behav. Res. 2011, 46, 119–151. [Google Scholar] [CrossRef] [PubMed]
- Brookhart Ma Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stürmer, T. Variable selection for propensity score models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Sauer, B.C.; Brookhart, M.A.; Roy, J.; VanderWeele, T. A review of covariate selection for non-experimental comparative effectiveness research. Pharmacoepidemiol. Drug Saf. 2013, 22, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Lu, B. Propensity score matching with time-dependent covariates. Biometrics 2005, 61, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Chelluri, L.; Im, K.A.; Belle, S.H.; Schulz, R.; Rotondi, A.J.; Donahoe, M.P.; Sirio, C.A.; Mendelsohn, A.B.; Pinsky, M.R. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit. Care Med. 2004, 32, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Costa, M.A.; Trouillet, J.L.; Baudot, J.; Mokhtari, M.; Gibert, C.; Chastre, J. Morbidity, mortality, and quality of life outcomes of patients requiring ≥ 14 days of mechanical ventilation. Crit. Care Med. 2003, 31, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Nabozny, M.J.; Barnato, A.E.; Rathouz, P.J.; Havlena, J.A.; Kind, A.J.; Ehlenbach, W.J.; Zhao, Q.; Ronk, K.; Smith, M.A.; Greenberg, C.C.; et al. Trajectories and prognosis of older patients who have prolonged mechanical ventilation after high-risk surgery. Crit. Care Med. 2016, 44, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Herr, D.L.; Sum-Ping, S.T.J.; England, M. Setting: ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine-based vs. propofol-based sedation regimens. J. Cardiothorac. Vasc. Anesth. 2003, 17, 576–584. [Google Scholar] [CrossRef]
- Carson, S.S.; Kress, J.P.; Rodgers, J.E.; Vinayak, A.; Campbell-Bright, S.; Levitt, J.; Bourdet, S.; Ivanova, A.; Henderson, A.G.; Pohlman, A.; et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit. Care Med. 2006, 34, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Girard, T.D.; Pandaripande, P.P.; Ely, E.W. Delirium in the intensive care unit. Crit. Care 2008, 12 (Suppl. S3), S3. [Google Scholar] [CrossRef] [PubMed]
- Hospira, Inc. Propofol [Package Insert]; Hospira, Inc.: Lake Forest, IL, USA, 2015. [Google Scholar]
- Terry, K.J.; Anger, K.E.; Szumita, P.M. Prospective evaluation of inappropriate unable-to-assess CAM-ICU documentations of critically ill patients. J. Intensiv. Care 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed]
| Unmatched Cohort | Matched Cohort | |||||
|---|---|---|---|---|---|---|
| Variable | DXM-Propofol (n = 149) | Propofol (n = 794) | p-Value | DXM-Propofol (n = 143) | Propofol (n = 143) | p-Value |
| (SD or IQR, N (%)) | (SD or IQR, N (%)) | |||||
| Age (mean) | 53.5 (17.4) | 56.5 (17.2) | 0.05 a | 53.6 (17.4) | 52.7 (16.9) | 0.67 a |
| APACHE II Score (median) | 16.0 (6.0) | 16.0 (9.0) | 0.74 a | 16.0 (6.0) | 17.0 (8.0) | 0.03 a |
| Female | 45 (30.2) | 305 (38.4) | 0.06 b | 45 (31.5) | 53 (37.1) | 0.32 b |
| Male | 104 (69.8) | 489 (61.6) | 98 (68.5) | 90 (62.9) | ||
| COPD Diagnosis c | ||||||
| No | 135 (90.6) | 719 (90.6) | 0.98 b | 129 (90.2) | 127 (88.8) | 0.70 b |
| Yes | 14 (9.4) | 75 (9.4) | 14 (9.8) | 16 (11.2) | ||
| Hemodynamic Instability c | ||||||
| No | 71 (47.6) | 468 (59.3) | 0.008 b | 74 (51.8) | 71 (49.7) | 0.81 b |
| Yes | 78 (52.4) | 318 (40.7) | 69 (48.2) | 72 (50.4) | ||
| Admitting Service | ||||||
| Cardio-Thoracic | 77 (51.7) | 269 (34.3) | 0.001 b | 72 (50.4) | 65 (45.4) | 0.35 b |
| Transplant | 3 (2.0) | 25 (3.2) | 3 (2.1) | 0 (0.0) | ||
| General urgery | 24 (16.1) | 208 (26.5) | 24 (16.8) | 35 (24.5) | ||
| Trauma | 29 (19.5) | 200 (25.5) | 29 (20.3) | 28 (19.6) | ||
| Vascular | 4 (2.7) | 33 (4.2) | 4 (2.8) | 3 (2.1) | ||
| Other | 12 (8.0) | 50 (6.4) | 11 (7.7) | 12 (8.4) | ||
| Admission Type | ||||||
| Non-Operative | 50 (33.6) | 229 (28.8) | 0.48 b | 49 (34.3) | 39 (27.3) | 0.37 b |
| Elective Postoperative | 58 (38.9) | 319 (40.2) | 54 (37.8) | 64 (44.8) | ||
| Emergency Postoperative | 41 (27.5) | 246 (30.9) | 40 (27.9) | 40 (27.9) | ||
| Time Period c | ||||||
| 1 | 14 (9.4) | 106 (13.4) | 0.13 b | 14 (9.8) | 11 (7.6) | 0.94 b |
| 2 | 24 (16.1) | 101 (12.7) | 22 (15.4) | 25 (17.5) | ||
| 3 | 9 (6.0) | 102 (12.8) | 9 (6.3) | 5 (3.505) | ||
| 4 | 25 (16.8) | 93 (11.7) | 22 (15.4) | 21 (14.7) | ||
| 5 | 20 (13.4) | 95 (11.9) | 20 (13.9) | 19 (13.3) | ||
| 6 | 14 (9.4) | 89 (11.2) | 14 (9.8) | 13 (9.1) | ||
| 7 | 19 (12.8) | 89 (11.2) | 19 (13.3) | 22 (15.4) | ||
| 8 | 24 (16.1) | 119 (14.9) | 23 (16.1) | 27 (18.9) | ||
| Outcome | DXM-Propofol (n = 143) | Propofol (n = 143) | Risk Ratio | 95% Confidence Intervals | p-Value |
|---|---|---|---|---|---|
| Mechanical ventilation duration hours; Median (IQR) | 137.0 (132.3) | 142.8 (153.4) | 1.086 | (0.924, 1.275) | 0.31 |
| SICU length of stay hours; Median (IQR) | 217.9 (178.9) | 212.6 (225.6) | 0.937 | (0.799, 1.100) | 0.43 |
| SICU mortality; N (%) | 5 (3.5) | 3 (2.1) | 1.002 | (0.967, 1.038) | 0.88 |
| Variable | DXM-Propofol | Propofol | p-Value a | Median Difference (IQR) |
|---|---|---|---|---|
| Median (IQR) or N (%) | ||||
| Duration of Propofol Infusion (hours) | 96.0 (97.0) | 118.6 (99.6) | 0.07 | –9.1 (120) |
| Duration of DXM Infusion (hours) | 48.0 (65.0) | |||
| Dexmedetomidine Dose (mcg/kg/hour) | 0.32 (0.37) | |||
| Propofol Dose (mcg/kg/min) | 14.08 (14.4) | 11.03 (10.6) | 0.002 | 3.05 (4.2) |
| Fentanyl Dose (mcg/hour) | 77.6 (71.6) | 52.5 (48) | 0.002 | 30.3 (93.7) |
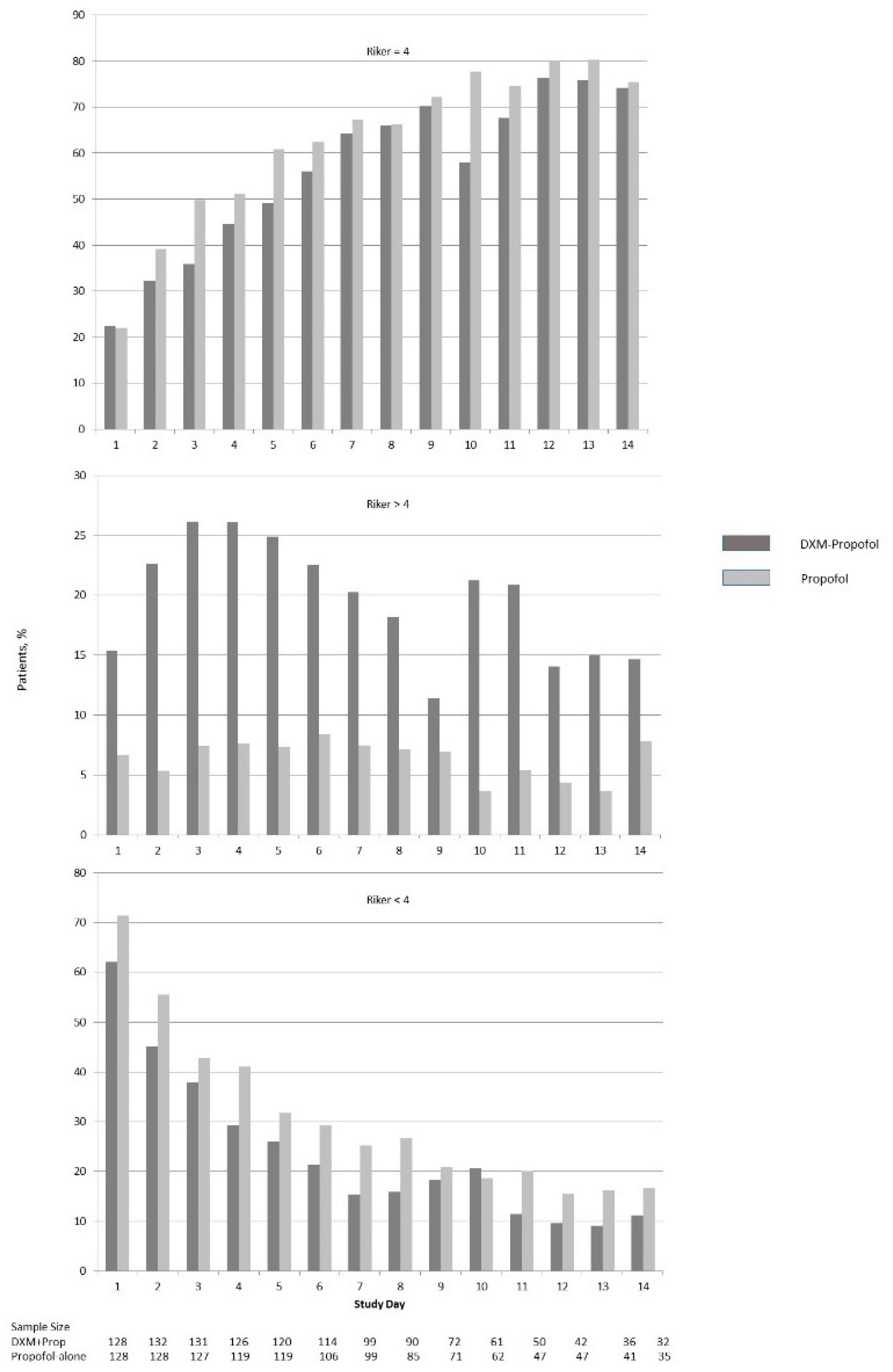
| Percentage at Target Sedation (Riker = 4) While on Sedative Medication | 34 (34) | 36 (47) | 0.32 | –2 (13) |
| Percentage Above Target Sedation (Riker > 4) While on Sedative Medication | 25 (27) | 2 (10) | <0.001 | 23 (17) |
| Percentage Below Target Sedation (Riker < 4) While on Sedative Medication | 37 (29) | 52 (48) | <0.001 | –12 (19) |
| Variable | DXM-Propofol N (%) | Propofol N (%) | p-Value |
|---|---|---|---|
| Tracheostomy | |||
| Yes | 17 (11.9) | 20 (13.9) | 0.60 a |
| No | 126 (88.1) | 123 (86.0) | |
| Fentanyl continuous infusion | |||
| Yes | 142 (99.3) | 138 (96.5) | 0.21 b |
| No | 1 (0.7) | 5 (3.5) | |
| Continuous infusion neuromuscular blocking agent | |||
| Yes | 11 (7.7) | 15 (10.5) | 0.41 a |
| No | 132 (92.3) | 128 (89.5) | |
| Triglyceride level | |||
| ≥200 mg/L | 5 (3.5) | 1 (0.7) | 0.21 b |
| <200 mg/L | 138 (96.5) | 142 (99.3) | |
| Use of any intermittent antipsychotic medication | |||
| Yes | 98 (68.5) | 75 (52.4) | 0.005 a |
| No | 45 (31.4) | 68 (47.5) | |
| Use of any intermittent benzodiazepine medication | |||
| Yes | 52 (36.3) | 50 (34.9) | 0.81 a |
| No | 91 (63.6) | 93 (65.0) | |
| Mean arterial blood pressure < 60 mm Hg | |||
| Yes | 109 (76.2) | 77 (53.8) | < 0.001 a |
| No | 34 (23.8) | 66 (46.1) | |
| Heart rate < 50 beats/minute | |||
| Yes | 8 (5.6) | 28 (19.6) | < 0.001 a |
| No | 135 (94.4) | 115 (80.4) |
| Medication | DXM-Propofol (n = 143) N (%) | Propofol (n = 143) N (%) | p-Value |
|---|---|---|---|
| Haloperidol | 96 (67.1) | 65 (45.5) | < 0.001 a |
| Olanzapine | 7 (4.90) | 4 (2.8) | 0.36 a |
| Quetiapine | 54 (37.7) | 28 (19.6) | < 0.001 a |
| Risperidone | 0 (100) | 0 (100) | NA |
| Fentanyl intermittent | 58 (40.6) | 74 (51.8) | 0.06 a |
| Hydromorphone drip | 3 (2.1) | 0 | 0.25 b |
| Hydromorphone intermittent | 58 (40.6) | 53 (37.0) | 0.54 a |
| Morphine drip | 0 (100) | 0 (100) | NA |
| Morphine intermittent | 25 (17.5) | 29 (20.2) | 0.55 a |
| Oxycodone | 105 (73.4) | 95 (66.4) | 0.20 a |
| Hydrocodone | 28 (19.6) | 20 (13.9) | 0.21 a |
| Patient Controlled Analgesia | 24 (16.8) | 21 (14.7) | 0.63 a |
| Epidural | 5 (3.5) | 5 (3.5) | NA |
| Methadone | 3 (2.1) | 5 (3.5) | 0.72 b |
| Alprazolam | 5 (3.5) | 4 (2.8) | NA |
| Clonazepam | 4 (2.8) | 2 (1.4) | 0.68 b |
| Diazepam | 6 (4.2) | 3 (2.1) | 0.50 b |
| Lorazepam | 30 (20.9) | 20 (13.4) | 0.12 a |
| Midazolam | 21 (14.7) | 27 (18.9) | 0.34 a |
| Temazepam | 2 (1.4) | 0 (0) | 0.50 b |
| First Author | Year | n | Control Group | Primary or Secondary Outcomes |
|---|---|---|---|---|
| Venn 8 | 2003 | 12 | No | Efficacy of sedation |
| Shehabi 10 | 2004 | 20 | No | Sedative and cardiovascular effects |
| Siobal 9 | 2006 | 5 | No | Facilitate withdrawal of mechanical ventilation |
| Dasta 17 | 2006 | 9996 vs. 356 | Yes | Hospital mortality, total hospital LOS, charges, # receiving mechanical ventilation, ventilation duration, ICU LOS |
| MacLaren 11 | 2007 | 40 | No | Discontinuation or dosage reduction of other sedatives or fentanyl from the hour before to 6 h after starting DXM |
| Arpino 12 | 2008 | 20 | No | Rate of extubation at 24 & 48 h post-DXM, mean time to extubation after DXM, mean rate of propofol/midazolam/morphine infusion before & after DXM initiation, heart rate and mean arterial pressure |
| Shehabi 13 | 2010 | 28 | No | Effect of DXM on agitation during weaning of mechanical ventilation in critically ill patients |
| Reade 14 | 2016 | 39 vs. 32 | Yes | Ventilator-free hours in the 7 days following randomization during the incident ICU admission |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louie, J.M.; Lonardo, N.W.; Mone, M.C.; Stevens, V.W.; Deka, R.; Shipley, W.; Barton, R.G. Outcomes When Using Adjunct Dexmedetomidine with Propofol Sedation in Mechanically Ventilated Surgical Intensive Care Patients. Pharmacy 2018, 6, 93. https://doi.org/10.3390/pharmacy6030093
Louie JM, Lonardo NW, Mone MC, Stevens VW, Deka R, Shipley W, Barton RG. Outcomes When Using Adjunct Dexmedetomidine with Propofol Sedation in Mechanically Ventilated Surgical Intensive Care Patients. Pharmacy. 2018; 6(3):93. https://doi.org/10.3390/pharmacy6030093
Chicago/Turabian StyleLouie, Jessica M., Nick W. Lonardo, Mary C. Mone, Vanessa W. Stevens, Rishi Deka, Wayne Shipley, and Richard G. Barton. 2018. "Outcomes When Using Adjunct Dexmedetomidine with Propofol Sedation in Mechanically Ventilated Surgical Intensive Care Patients" Pharmacy 6, no. 3: 93. https://doi.org/10.3390/pharmacy6030093
APA StyleLouie, J. M., Lonardo, N. W., Mone, M. C., Stevens, V. W., Deka, R., Shipley, W., & Barton, R. G. (2018). Outcomes When Using Adjunct Dexmedetomidine with Propofol Sedation in Mechanically Ventilated Surgical Intensive Care Patients. Pharmacy, 6(3), 93. https://doi.org/10.3390/pharmacy6030093





