Pharmacy Students’ Knowledge and Attitude toward Registration Trials and Clinical Research: A Survey in a Japanese University Hospital
Abstract
:1. Introduction
2. Methods
2.1. Setting and Participants
2.2. Questionnaire to Assess Pharmacy Students’ Knowledge of and Attitudes toward Registration Trials and Clinical Research
2.3. Statystical Analysis
3. Results
3.1. Respondent Characteristics
3.2. General Awareness of Registration Trials, Clinical Research, and the Role of the CRC
3.3. Awareness of Issues Related to Registration Trials and Clinical Research
3.4. General Perception of Registration Trials
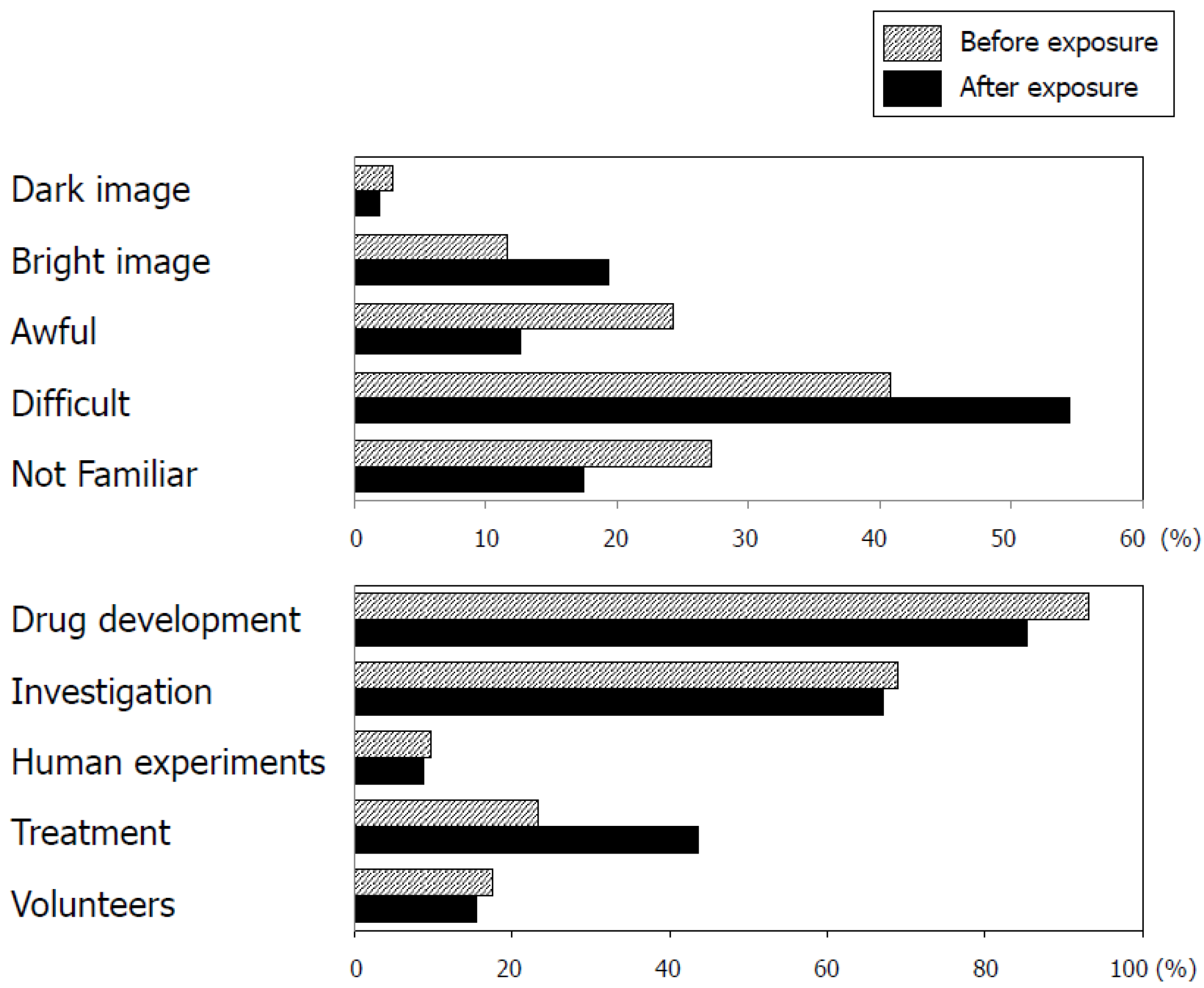
3.5. Awareness of Research-Related Terminology
3.6. Views on the Need to Learn More about Registration Trials and Clinical Research and Willingness to Act as Study Participants
3.7. Willingness to Act as Investigators and/or CRC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Yanagawa, H. Current regulatory systems for clinical trials in Japan: Still room for improvement. Clin. Res. Regul. Aff. 2014, 31, 25–28. [Google Scholar] [CrossRef]
- Tsuji, K.; Tsutani, K. Approval of new drugs 1999–2007: Comparison of the US, the EU and Japan situations. J. Clin. Pharm. Ther. 2010, 35, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Asahina, Y.; Tanaka, A.; Yamada, H.; Nakamura, M.; Uyama, Y. Significant differences in drug lag in clinical development among various strategies used for regulatory submissions in Japan. Clin. Pharmacol. Ther. 2014, 95, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Honig, P.K. Recent trends and success factors in reducing the lag time to approval of new drugs in Japan. Clin. Pharmacol. Ther. 2014, 95, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, H.; Nishiya, M.; Miyamoto, T.; Shikishima, M.; Imura, M.; Nakanishi, R.; Ariuchi, N.; Akaishi, A.; Takai, S.; Abe, S.; et al. Clinical trials for drug approval: A pilot study of the view of doctors at Tokushima University Hospital. J. Med. Investig. 2006, 53, 292–296. [Google Scholar] [CrossRef]
- Yanagawa, H.; Takai, S.; Yoshimaru, M.; Miyamoto, T.; Katashima, R.; Kida, K. Nurse awareness of clinical research: A survey in a Japanese University Hospital. BMC Med. Res. Methodol. 2014, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Practice Room for Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Tokushima University. Available online: http://150.59.84.2/?&rf=115 (accessed on 4 December 2017). (In Japanese).
- Ebihara, A.; Takahashi, K.; Ikemoto, F.; Yamamoto, K. Clinical pharmacology and clinical trials in Japan. J. Mol. Med. 1996, 74, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Normile, D. Tampered data cast shadow on drug trial. Science 2013, 341, 223. [Google Scholar] [CrossRef] [PubMed]
- Blair, G. Novartis faces charges in Japan over promotion of valsartan to doctors. Br. Med. J. 2014, 348, g287. [Google Scholar] [CrossRef]
- Koyanagi, T.; Mizota, S.; Nishikawa, F.; Terasaimoto, N.; Kamoharai, K.; lmakyure, O.; Shuto, H.; Kataoka, Y.; Hirakawai, M. Development of practical clinical trials education program for pharmacy students. Jpn. J. Pharm. Health Care Sci. 2009, 35, 636–643. (In Japanese) [Google Scholar] [CrossRef]
- Yoshida, Y.; Yamashita, R.; Tanaka, A.; Sagara, H.; Suemaru, K.; Tanaka, M.; Araki, H. Evaluation of an experience-based type of practical training in a clinical therapeutic trial center. J. Jpn. Soc. Hosp. Pharm. 2015, 51, 301–304. (In Japanese) [Google Scholar]
- Arita, E.; Ujihara, A.; Omori, R.; Koshiba, S.; Kamata, A.; Azuma, K.; Nishijima, K.; Taga, M.; Okamoto, M.; Astuda, K. Evaluation of program to develop communication skills for health care professionals—Role-playing exercises introduced for training in informed consent process in clinical trials—1. Jpn. J. Pharm. Health Care Sci. 2008, 34, 727–735. (In Japanese) [Google Scholar] [CrossRef]
- Imakyure, O.; Shuto, H.; Nishikawa, F.; Hagiwara, Y.; Inoue, S.; Koyanagi, T.; Hirakawa, M.; Kataoka, Y. Development of clinical trial education program for pharmaceutical science students through small group discussion and role-playing using protocol. Yakugaku Zasshi 2010, 130, 1075–1084. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- American College of Clinical Pharmacy. The Clinical Pharmacist as Principal Investigator: A Commentary from the American College of Clinical Pharmacy. Pharmacotherapy 2000, 20, 599–608. [Google Scholar]
- Marinac, J.S.; Gerkovich, M.M. Outcomes from a mentored research boot camp: Focused Investigator Training (FIT) Program. Pharmacotherapy 2012, 32, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Overholser, B.R.; Foster, D.R.; Henry, J.R.; Plake, K.S.; Sowinski, K.M. The influence of an elective introductory clinical research course on pharmacy student interest in pursuing research-based careers. Am. J. Pharm. Educ. 2010, 74, 165. [Google Scholar] [CrossRef] [PubMed]

| Confident | Quite Aware | Aware | Less Aware | Not Aware | |
|---|---|---|---|---|---|
| Registration trials | 25 (24.3%) | 69 (67.0%) | 9 (8.7%) | 0 (0.0%) | 0 (0.0%) |
| Clinical research | 15 (14.6%) | 70 (68.0%) | 17 (16.5%) | 1 (1.0%) | 0 (0.0%) |
| Difference between registration trials and clinical research | 3 (2.9%) | 23 (22.3%) | 40 (38.8%) | 34 (33.0%) | 3 (2.9%) |
| Presence of CRC | 9 (8.7%) | 49 (47.6%) | 37 (35.9%) | 84 (7.8%) | 0 (0.0%) |
| Role of CRC | 1 (1.0%) | 11 (10.7%) | 38 (36.9%) | 51 (49.5%) | 2 (1.9%) |
| Aware | Not Aware | |
|---|---|---|
| 1. Awareness of issues related to registration trials | ||
| Registration trials are necessary for drug registration | 101 (98.1%) | 2 (1.9%) |
| Review by institutional review board is mandatory | 81 (78.6%) | 22 (21.4%) |
| CRC support registration trials | 70 (68.0%) | 33 (32.0%) |
| Informed consent is essential for a registration trial | 102 (99.0%) | 1 (1.0%) |
| Refusal of a registration trial causes no disadvantage | 101 (98.1%) | 2 (1.9%) |
| Some registration trials use placebo | 103 (100.0%) | 0 (0%) |
| Participants can withdraw anytime | 100 (97.1%) | 3 (2.9%) |
| Participants need not to pay for investigational drugs and tests related to registration trials | 76 (73.8%) | 27 (26.2%) |
| Reward for participants is prepared in registration trials | 57 (55.3%) | 46 (44.7%) |
| 2. Awareness of issues related to clinical research | ||
| Clinical research includes research using labeled drugs | 63 (61.2%) | 40 (38.8%) |
| Review by ethics committee is mandatory | 63 (61.2%) | 40 (38.8%) |
| Governmental ethical guidelines are applied to clinical research | 69 (67.0%) | 34 (33.0%) |
| 1. Before Exposure | |||||
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | |
| It is necessary for pharmacy students to know more about registration trials and clinical research. | 59 (57.3%) | 41 (39.8%) | 1 (1.0%) | 1 (1.0%) | 0 (0%) |
| If it was suggested I participate in some registration trial if eligible for the trial, I would participate. | 13 (12.6%) | 31 (30.1%) | 26 (25.2%) | 29 (28.2%) | 4 (3.9%) |
| 2. After Exposure | |||||
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | |
| It is necessary for pharmacy students to know more about registration trials and clinical research. | 59 (57.3%) | 43 (41.7%) | 0 (0%) | 1 (1.0%) | 0 (0%) |
| If it was suggested I participate in some registration trial if eligible for the trial, I would participate. | 15 (14.6%) | 35 (34.0%) | 24 (23.3%) | 22 (21.4%) | 7 (6.8%) |
| I think it is effective for my career to learn about registration trials by more practical method, such as role playing. | 27 (26.5%) | 42 (41.2%) | 22 (21.6%) | 10 (9.8%) | 2 (1.9%) |
| Investigator | CRC | None | |
|---|---|---|---|
| pre-exposure | 30 (29.1%) | 42 (40.8%) | 43 (41.7%) |
| post-exposure | 32 (31.1%) | 43 (41.8%) | 42 (40.8%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ise, N.; Takechi, K.; Miyamoto, T.; Ishizawa, K.; Yanagawa, H. Pharmacy Students’ Knowledge and Attitude toward Registration Trials and Clinical Research: A Survey in a Japanese University Hospital. Pharmacy 2017, 5, 67. https://doi.org/10.3390/pharmacy5040067
Ise N, Takechi K, Miyamoto T, Ishizawa K, Yanagawa H. Pharmacy Students’ Knowledge and Attitude toward Registration Trials and Clinical Research: A Survey in a Japanese University Hospital. Pharmacy. 2017; 5(4):67. https://doi.org/10.3390/pharmacy5040067
Chicago/Turabian StyleIse, Natsuko, Kenshi Takechi, Toshiko Miyamoto, Keisuke Ishizawa, and Hiroaki Yanagawa. 2017. "Pharmacy Students’ Knowledge and Attitude toward Registration Trials and Clinical Research: A Survey in a Japanese University Hospital" Pharmacy 5, no. 4: 67. https://doi.org/10.3390/pharmacy5040067
APA StyleIse, N., Takechi, K., Miyamoto, T., Ishizawa, K., & Yanagawa, H. (2017). Pharmacy Students’ Knowledge and Attitude toward Registration Trials and Clinical Research: A Survey in a Japanese University Hospital. Pharmacy, 5(4), 67. https://doi.org/10.3390/pharmacy5040067




