Investigating the Relative Significance of Drug-Related Problem Categories
Abstract
:1. Introduction
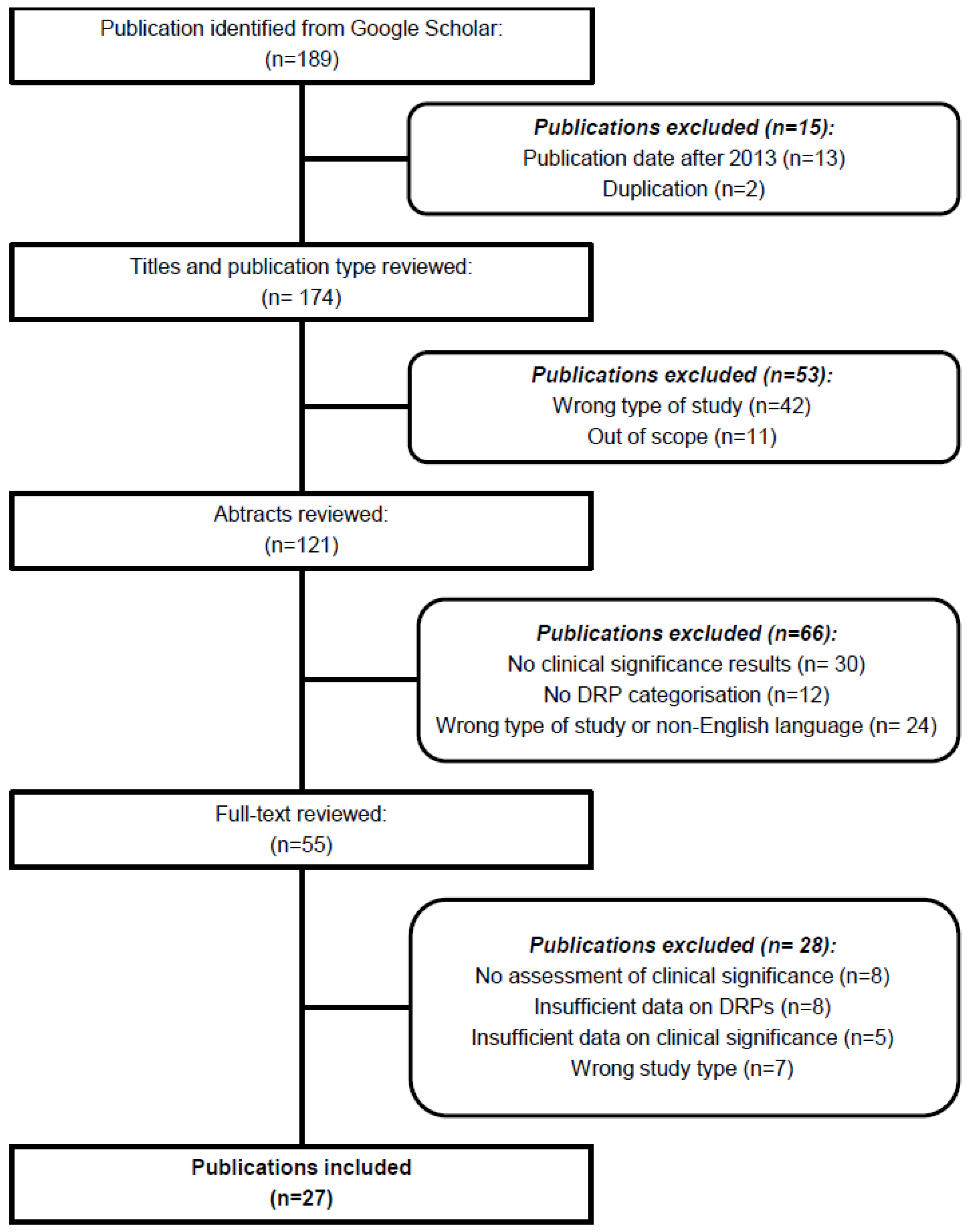
2. Materials and Methods
2.1. Search Strategy
- described primary research
- were published in English
- described interventions delivered by clinical pharmacists
- were not published as a research paper (e.g., reviews, books, congress abstracts, posters, reports, protocols)
- did not present data on DRPs
- described selected DRPs only (e.g., drug-drug interactions)
- did not present a DRP-categorization system
- did not present data on an assessment of clinical significance
- presented data for a sub-study, where the original study had been included
2.2. Assessment
2.3. Study Selection
3. Results
3.1. Description of Studies
3.2. Relations between Clinical Significance and DRP Categorisation
4. Discussion
4.1. Relations between Individual DRP Categories and Clinical Significance
4.2. DRP Categorization Systems
4.3. Clinical Significance Assessment
4.4. Limitations
5. Conclusions
Conflicts of Interest
References
- Van den Bemt, P.M.; Egberts, T.C.; de Jong-van den Berg, L.T.; Brouwers, J.R. Drug-related problems in hospitalised patients. Drug Saf. 2000, 22, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Krähenbühl-Melcher, A.; Schlienger, R.; Lampert, M.; Haschke, M.; Drewe, J.; Krähenbühl, S. Drug-related problems in hospitals: A review of the recent literature. Drug Saf. 2007, 30, 379–407. [Google Scholar] [CrossRef] [PubMed]
- Al Hamid, A.; Ghaleb, M.; Aljadhey, H.; Aslanpour, Z. A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br. J. Clin. Pharmacol. 2014, 78, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Van Mil, F.; Westerlund, T.; Hersberger, K.E.; Schaefer, M.A. Drug related problem classification systems. Ann. Pharmacother. 2004, 38, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Graabaek, T.; Kjeldsen, L.J. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: A systematic review. Basic Clin. Pharmacol. Toxicol. 2013, 112, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.P.; Tremp, R.; Hersberger, K.E.; Lampert, M.L. Inappropriate prescribing: A systematic overview of published assessment tools. Eur. J. Clin. Pharmacol. 2014, 70, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, L.; Grobler, M.P.; Roberts, M.S. Development of a quality use of medicines coding system to rate clinical pharmacists’ medication review recommendations. Pharm. World Sci. 2003, 25, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Kazdin, A.E. The Meanings and Measurement of Clinical Significance. J. Consult. Clin. Consult. 1999, 67, 332–339. [Google Scholar] [CrossRef]
- Overhage, J.M.; Lukes, A. Practical, reliable, comprehensive method for characterizing pharmacists’ clinical activities. Am. J. Health Syst. Pharm. 1999, 56, 2444–2450. [Google Scholar] [PubMed]
- Dean, B.S.; Barber, N.D. A validated, reliable method of scoring the severity of medication errors. Am. J. Health Syst. Pharm. 1999, 56, 57–62. [Google Scholar] [PubMed]
- Hatoum, H.T.; Hutchinson, R.A.; Witte, K.W.; Newby, G.P. Evaluation of the contribution of clinical pharmacists: Inpatient care and cost reduction. Drug Intell. Clin. Pharm. 1988, 22, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Alderman, C.P.; Kong, L.; Kildea, L. Medication-related problems identified in home medicines reviews conducted in an Australian rural setting. Consult. Pharm. 2013, 28, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Basger, B.J.; Chen, T.F.; Moles, R.J. Application of a prescribing indicators tool to assist in identifying drug-related problems in a cohort of older Australians. Int. J. Pharm. Pract. 2012, 20, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.B.; Holmbjer, L.; Midlöv, P.; Höglund, P.; Larsson, L.; Bondesson, A.A.; Eriksson, T. The process of identifying, solving and preventing drug related problems in the LIMM-study. Int. J. Clin. Pharm. 2011, 33, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Lubowski, T.J.; Cronin, L.M.; Pavelka, R.W.; Briscoe-Dwyer, L.A.; Briceland, L.L.; Hamilton, R.A. Effectiveness of a medication reconciliation project conducted by PharmD students. Am. J. Pharm. Educ. 2007, 15, 94. [Google Scholar] [CrossRef]
- Midlöv, P.; Bondesson, A.A.; Eriksson, T.; Petersson, J.; Minthon, L.; Höglund, P. Descriptive study and pharmacotherapeutic intervention in patients with epilepsy or Parkinson’s disease at nursing homes in southern Sweden. Eur. J. Clin. Pharmacol. 2002, 57, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.C.; Stewart, K.; Elliott, R.A.; George, J. Pharmacist consultations in general practice clinics: The Pharmacists in Practice Study (PIPS). Res. Soc. Adm. Pharm. 2014, 10, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Tomás Vecina, S.; Garcia Sanchez, L.; Pascual Arce, E.B.; Riera Paredes, I. Pharmacist intervention program to improve patient safety in an emergency department. Emergencias 2010, 22, 85–90. [Google Scholar]
- Veggeland, T.; Dyb, S. The contribution of a clinical pharmacist to the improvement of medication at a geriatric hospital unit in Norway. Pharm. Pract. (Granada) 2008, 6, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, M.; Ulfvarson, J.; Karlsson, E.A. Nurse-led medication reviews and the quality of drug treatment of elderly hospitalized patients. Eur. J. Clin. Pharmacol. 2009, 65, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Bosma, L.; Jansman, F.G.; Franken, A.M.; Harting, J.W.; Van den Bemt, P.M. Evaluation of pharmacist clinical interventions in a Dutch hospital setting. Pharm. World Sci. 2008, 30, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Easton, K.L.; Chapman, C.B.; Brien, J.A. Frequency and characteristics of hospital admissions associated with drug-related problems in paediatrics. Br. J. Clin. Pharmacol. 2004, 57, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Easton-Carter, K.L.; Chapman, C.B.; Brien, J.E. Emergency department attendances associated with drug-related problems in paediatrics. J. Paediatr. Child Health 2003, 39, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Knez, L.; Laaksonen, R.; Duggan, C. Evaluation of clinical interventions made by pharmacists in chemotherapy preparation. Radiol. Oncol. 2010, 44, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Peterson, G.M.; Tenni, P.C.; Bindoff, I.K.; Curtain, C.; Hughes, J.; Bereznicki, L.R.; Jackson, S.L.; Kong, D.C.; Hughes, J.D. Drug-related problems detected in Australian Community Pharmacies: The PROMISe Trial. Ann. Pharmacother. 2011, 45, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Winterstein, A.G.; Johns, T.E.; Rosenberg, E.I.; Hatton, R.C.; Gonzalez-Rothi, R.; Kanjanarat, P. Nature and causes of clinically significant medication errors in a tertiary care hospital. Am. J. Health Syst. Pharm. 2004, 61, 1908–1916. [Google Scholar] [PubMed]
- Zaal, R.J.; Jansen, M.M.; Duisenberg-van Essenberg, M.; Tijssen, C.C.; Roukema, J.A.; van den Bemt, P.M. Identification of drug-related problems by a clinical pharmacist in addition to computerized alerts. Int. J. Clin. Pharm. 2013, 35, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Akwagyriam, I.; Goodyer, L.I.; Harding, L.; Khakoo, S.; Millington, H. Drug history taking and the identification of drug related problems in an accident and emergency department. J. Accid. Emerg. Med. 1996, 13, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.S.; Erstad, B.L.; Kopp, B.J.; Theodorou, A.A.; Priestley, G. Direct observation approach for detecting medication errors and adverse drug events in a pediatric intensive care unit. Pediatr. Crit. Care Med. 2007, 8, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Desrochers, J.F.; Lemieux, J.P.; Morin-Bélanger, C.; Paradis, F.S.; Lord, A.; Bell, R.; Berbiche, D.; Bárcena, P.Q.; Séguin, N.C.; Desforges, K.; et al. Development and validation of the PAIR (Pharmacotherapy Assessment in Chronic Renal Disease) criteria to assess medication safety and use issues in patients with CKD. Am. J. Kidney Dis. 2011, 58, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.E.; Doucette, W.R.; Dedhiya, S.D.; Osterhaus, M.C.; Kumbera, P.A.; Osterhaus, J.T.; Townsend, R.J. Use of point-of-service health status assessments by community pharmacists to identify and resolve drug-related problems in patients with musculoskeletal disorders. Pharmacotherapy 2001, 21, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Farris, K.B.; Ganther-Urmie, J.M.; Fang, G.; Doucette, W.R.; Brooks, J.M.; Klepser, D.G.; Fries, D.J.; Kuhle, C.L. Population-based medication reviews: A descriptive analysis of the medication issues identified in a medicare not-for-profit prescription discount program. Ann. Pharmacother. 2004, 38, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Blix, H.S.; Viktil, K.K.; Reikvam, A.; Moger, T.A.; Hjemaas, B.J.; Pretsch, P.; Vraalsen, T.F.; Walseth, E.K. The majority of hospitalised patients have drug-related problems: Results from a prospective study in general hospitals. Eur. J. Clin. Pharmacol. 2004, 60, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Draugalis, J.R.; Jones-Grizzle, A.J. Pharmacy education and pharmacoeconomics. J. Pharm. Teach. 1991, 2, 3–10. [Google Scholar] [CrossRef]
- Elliott, R.A.; Woodward, M.C. Assessment of Risk Associated with Medication-Related Problems in Elderly Outpatients. J. Pharm. Pract. Res. 2009, 39, 109–113. [Google Scholar] [CrossRef]
- Fernández-Llamazares, C.M.; Manrique-Rodríguez, S.; Pérez-Sanz, C.; Durán-García, M.E.; Sanjurjo-Sáez, M.; Calleja-Hernández, M.A. Validation of a method for recording pharmaceutical interventions. J. Clin. Pharm. Ther. 2012, 37, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.; Murphy, A.; Willis, D.; Cusack, S.; Bury, G.; O'Sullivan, I.; Deasy, C. Out-of-hospital cardiac arrest in Cork, Ireland. Emerg. Med. J. 2013, 30, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.B.; Amaral, K.M.; Sander, G.B.; Martins, N.L.; Pereira, L.; Picon, P.D. Effectiveness of alpha interferon (+ribavirin) in the treatment of chronic viral hepatitis C genotypes 2 and 3 in a Brazilian sample. Arq. Gastroenterol. 2012, 49, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Reformation of the NHS: Ending professional divisions. Lancet 2007, 370, 1393. [Google Scholar]
- Alagiriswami, B.; Ramesh, M.; Parthasarathi, G.; Basavanagowdappa, H. A Study of Clinical Pharmacist Initiated Changes in Drug Therapy in a Teaching Hospital. Indian J. Pharm. Pract. 2009, 2, 36–45. [Google Scholar]
- Alderman, C.P. A prospective analysis of clinical pharmacy interventions on an acute psychiatric inpatient unit. J. Clin. Pharm. Ther. 1997, 22, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Blix, H.S.; Viktil, K.K.; Moger, T.A.; Reikvam, A. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm. World Sci. 2006, 28, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Bondesson, A.; Eriksson, T.; Kragh, A.; Holmdahl, L.; Midlöv, P.; Höglund, P. In-hospital medication reviews reduce unidentified drug-related problems. Eur. J. Clin. Pharmacol. 2013, 69, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Bondesson, A.; Holmdahl, L.; Midlöv, P.; Höglund, P.; Andersson, E.; Eriksson, T. Acceptance and importance of clinical pharmacists’ LIMM-based recommendations. Int. J. Clin. Pharm. 2012, 34, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Simioni, D.; Brien, J. Implementation of pharmaceutical care plans in a hospital ward. Aust. Hosp. Pharm. 1996, 26, 221–226. [Google Scholar]
- Castelino, R.L.; Sathvik, B.S.; Parthasarathi, G.; Gurudev, K.C.; Shetty, M.S.; Narahari, M.G. Prevalence of medication-related problems among patients with renal compromise in an Indian hospital. J. Clin. Pharm. Ther. 2011, 36, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Celin, A.T.; Seuma, J.; Ramesh, A. Assessment of Drug Related Problems in Stroke Patients Admitted to a South Indian Tertiary Care Teaching Hospital. Indian J. Pharm. Pract. 2012, 5, 28–33. [Google Scholar]
- Chua, S.S.; Kok, L.C.; Yusof, F.A.; Tang, G.H.; Lee, S.W.; Efendie, B.; Paraidathathu, T. Pharmaceutical care issues identified by pharmacists in patients with diabetes, hypertension or hyperlipidaemia in primary care settings. BMC Health Serv. Res. 2012, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.B.P.; Dahal, P.; Venkataraman, R.; Fuloria, P.C. Assessment of clinical pharmacist intervention in tertiary care teaching hospital of southern India. Asian J. Pharm. Clin. Res. 2013, 6 (Suppl. S2), 258–261. [Google Scholar]
- Elliott, R.A.; Woodward, M.C. Medication-related problems in patients referred to aged care and memory clinics at a tertiary care hospital. Australas. J. Ageing 2011, 30, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Granaas, A.G.; Bates, I. The effect of pharmaceutical review of repeat prescriptions in general practice. Int. J. Pharm. Pract. 1999, 7, 264. [Google Scholar] [CrossRef]
- Granas, A.G.; Berg, C.; Hjellvik, V.; Haukereid, C.; Kronstad, A.; Blix, H.S.; Kilhovd, B.; Viktil, K.K.; Horn, A.M. Evaluating categorisation and clinical relevance of drug-related problems in medication reviews. Pharm. World Sci. 2010, 32, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Kassam, R.; Meneilly, G.S. Role of the pharmacist on a multidisciplinary diabetes team. Can. J. Diabaetes 2007, 31, 215–222. [Google Scholar] [CrossRef]
- Kumar, A.Y.; Kumar, R.V.; Ahmad, A.; Mohanta, G.P.; Manna, P.K. Pharmacists interventions and pharmaceutical care in an indian teaching hospital: A prospective study. Int. J. Adv. Res. Pharm. Biol. Sci. 2012, 1, 386–396. [Google Scholar]
- Kwint, H.F.; Faber, A.; Gussekloo, J.; Bouvy, M.L. The contribution of patient interviews to the identification of drug-related problems in home medication review. J. Clin. Pharm. Ther. 2012, 37, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.B.; Yesuf, E.A.; Odegard, P.S.; Wega, S.S. Implementing ward based clinical pharmacy services in an Ethiopian University Hospital. Pharm. Pract. 2013, 11, 51–57. [Google Scholar] [CrossRef]
- Rashed, A.N.; Neubert, A.; Tomlin, S.; Jackman, J.; Alhamdan, H.; AlShaikh, A.; Attar, A.; Aseeri, M.; Wilton, L.; Wong, I.C. Epidemiology and potential associated risk factors of drug-related problems in hospitalised children in the United Kingdom and Saudi Arabia. Eur. J. Clin. Pharmacol. 2012, 68, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; Martus, P.; Odin, P.; Schaefer, M. Drug-related problems in Parkinson's disease: The role of community pharmacists in primary care. Int. J. Clin. Pharm. 2011, 33, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Smythe, M.A.; Shah, P.P.; Spiteri, T.L.; Lucarotti, R.L.; Begle, R.L. Pharmaceutical care in medical progressive care patients. Ann. Pharmacother. 1998, 32, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Somers, A.; Robays, H.; De Paepe, P.; Van Maele, G.; Perehudoff, K.; Petrovic, M. Evaluation of clinical pharmacist recommendations in the geriatric ward of a Belgian university hospital. Clin. Interv. Aging 2013, 8, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Spinewine, A.; Dhillon, S.; Mallet, L.; Tulkens, P.M.; Wilmotte, L.; Swine, C. Implementation of ward-based clinical pharmacy services in Belgium-description of the impact on a geriatric unit. Ann. Pharmacother. 2006, 40, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Stafford, A.C.; Tenni, P.C.; Peterson, G.M.; Jackson, S.L.; Hejlesen, A.; Villesen, C.; Rasmussen, M. Drug-related problems identified in medication reviews by Australian pharmacists. Pharm. World Sci. 2009, 31, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.; Stafford, A.; Hughes, J.; Angley, M.; Bereznicki, L.; Peterson, G. Drug-related problems identified in post-discharge medication reviews for patients taking warfarin. Int. J. Clin. Pharm. 2011, 33, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Stemer, G.; Laml-Wallner, G.; Kuegler, I.; Poelzleitner, P.; Messner, S.; Steininger, S.; Dolinar, E.; Zehetmayer, S. Comprehensive evaluation of clinical pharmacists' interventions in a large Austrian tertiary care hospital. Eur. J. Hosp. Pharm. 2012, 1–6. [Google Scholar] [CrossRef]
- Pichala, P.T.; Kumar, B.M.; Zachariah, S.; Thomas, D.; Saunchez, L.; Gerardo, A.U. An interventional study on intensive care unit drug therapy assessment in a rural district hospital in India. J. Basic Clin. Pharm. 2013, 4, 64–67. [Google Scholar] [PubMed]
- Williams, M.; Peterson, G.M.; Tenni, P.C.; Bindoff, I.K.; Stafford, A.C. DOCUMENT: A system for classifying drug-related problems in community pharmacy. Int. J. Clin. Pharm. 2012, 34, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hepler, C.D.; Strand, L.M. Opportunities and responsibilities in pharmaceutical care. Am. J. Hosp. Pharm. 1990, 47, 533–543. [Google Scholar] [PubMed]
- Strand, L.M.; Morley, P.C.; Cipolle, R.J.; Ramsey, R.; Lamsam, G.D. Drug-related problems: Their structure and function. Dalian Inst. Chem. Phys. 1990, 24, 1093–1097. [Google Scholar] [CrossRef]
- Cipolle, R.J.; Strand, L.M.; Morley, P.C. Pharmaceutical Care Practice; McGraw-Hill: New York, NY, USA, 1998; pp. 78–79. [Google Scholar]
- Pharmaceutical Care Network Europe. DRP-Classification V6.02. Available online: http://www.pcne.org/working-groups/2/drug-related-problems (accessed on 24 April 2017).
- Schaefer, M. Discussing basic principles for a coding system of drug-related problems: The case of PI-Doc. Pharm. World Sci. 2002, 24, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, J.T.; Schmader, K.E.; Samsa, G.P.; Weinberger, M.; Uttech, K.M.; Lewis, I.K.; Cohen, H.J.; Feussner, J.R. A method for assessing drug therapy appropriateness. J. Clin. Epidemiol. 1992, 45, 1045–1051. [Google Scholar] [CrossRef]
- Allenet, B.; Bedouch, P.; Rose, F.X.; Escofier, L.; Roubille, R.; Charpiat, B.; Juste, M.; Conort, O. Validation of an instrument for the documentation of clinical pharmacists’ interventions. Pharm. World Sci. 2006, 28, 181–188. [Google Scholar] [CrossRef] [PubMed]
- ASHP guidelines on a standardized method for pharmaceutical care. In Best Practices for Health-System Pharmacy; Deffenbaugh, J. (Ed.) American Society of Health-System Pharmacists: Bethesda, MD, USA, 1996; pp. 109–111. [Google Scholar]
- Eadon, H. Assessing the quality of ward pharmacists’ interventions. Int. J. Pharm. Pract. 1992, 145–147. [Google Scholar] [CrossRef]
- Kjeldsen, L.J.; Birkholm, T.; Fischer, H.; Graabæk, T.; Hansen, M.K.; Kibsdal, K.P.; Ravn-Nielsen, L.V.; Truelshøj, T.H. A national drug related problems database: Evaluation of use in practice, reliability and reproducibility. Int. J. Clin. Pharm. 2014, 36, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.R.; Andersen, S.E.; Rasmussen, M.; Honore, P.H. Clinical pharmacist service in the acute ward. Int. J. Clin. Pharm. 2013, 35, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.; Eickhoff, C.; Klotz, J.M.; Schulz, M.; Radziwill, R. Development of a classification system for drug-related problems in the hospital setting (APS-Doc) and assessment of the interrater reliability. J. Clin. Pharm. Ther. 2012, 37, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Somers, A.; Mallet, L.; van der Cammen, T.; Robays, H.; Petrovic, M. Applicability of an adapted medication appropriateness index for detection of drug-related problems in geriatric inpatients. Am. J. Geriatr. Pharmacother. 2012, 10, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Hoth, A.B.; Carter, B.L.; Ness, J.; Bhattacharyya, A.; Shorr, R.I.; Rosenthal, G.E.; Kaboli, P.J. Development and reliability testing of the clinical pharmacist recommendation taxonomy. Pharmacotherapy 2007, 27, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Bedouch, P.; Allenet, B.; Grass, A.; Labarère, J.; Brudieu, E.; Bosson, J.L.; Calop, J. Drug-related problems in medical wards with a computerized physician order entry system. J. Clin. Pharm. Ther. 2009, 34, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.H.; Charpiat, B.; Catoire, C.; Juste, M.; Roubille, R.; Rose, F.X.; Chanoine, S.; Bosson, J.L.; Conort, O.; Allenet, B.; et al. Tools for Assessing Potential Significance of Pharmacist Interventions: A Systematic Review. Drug Saf. 2016, 39, 131–146. [Google Scholar] [CrossRef] [PubMed]
| Reference (Author and Year) | Country/Setting | Included Patients (pts) | Mean Age (Years) | Gender (Male) | Type of Intervention | Number of DRPs or Suggested Interventions 1 | Acceptance Rate of DRPs 2 | Implemen-tation Rate 2 | Link between Clinical Significance and Type of DRPs |
|---|---|---|---|---|---|---|---|---|---|
| Alagiriswami (2009) [40] | India One hospital Medicine wards | 189 pts | 49.8 | 57.8% | Medication review | 261 DRPs | 87% n = 261 | 81% n = 261 | No |
| Alderman (1997) [41] | Australia One hospital Acute-care, psychiatric inpatients | 69 pts with DRPs | 66.8 | 75% | Medication review | 187 DRPs | 92% n = 204 | Missing | No |
| Blix (2006) [42] | Norway Five hospitals Six internal medicine and two rheumatology departments | 672 pts with DRPs | Missing | Missing | Pharmacist contribution in therapeutic hospital team. | 2128 DPRs | 92% n = 1583 | 67% n = 1583 | No |
| Bondesson (2013) [43] | Sweden One hospital One internal medicine ward | 141 pts (70 IG, 71 CG) | 81.6 years (IG: 81.9, CG: 81.3) | 36% (IG: 33%, CG: 39%) | Integrated medication management | 690 DRPs | 93% n = 450 | Missing | Yes |
| Bondesson (2012) [44] | Sweden One hospital Two internal medicine wards | 132 pts | 81 | 48% | Medication review and medication reconciliation | 197 suggested interventions 127 DRPs assessed for clinical significance | 90% n = 197 | 90% n = 197 | Yes |
| Castelino (2011) [45] | India One hospital Renal unit | 308 pts reviewed | Age groups | 67.8% | Medication review | 327 DRPs | 97% n = 259 | 81% n = 259 | No |
| Celin (2012) [46] | India One hospital Medicine and neurology wards | 108 pts | Age groups | 68.5% | Pharmaceutical care | 80 DRPs | 97% n = 80 | 88% n = 80 | No |
| Chua (2012) [47] | Malaysia 44 primary care clinics | 477 pts | 47.9 | 60.2% | Pharmaceutical care | 706 DRPs | Missing | 87% n = 388 | No |
| Elliott (2011) [48] | Australia One hospital, Aged care assessment clinic and memory disorder clinic | 46 pts | 82 | 26% | Medication history and medication review | 113 DRPs | Missing | Missing | Yes |
| Granaas (1999) [49] | UK One general practice surgery | 285 pts | 65 (median) | 38% | Pharmaceutical review of repeat prescriptions | IG: 90 CG: 86 | IG: 86% (n = 90) CG: 13% (n = 86) | Missing | Yes |
| Granas (2010) [50] | Norway 23 community pharmacies | 73 pts (43 pts with DRPs) | Missing | Missing | Medication review | 88 DRPs | Missing | Missing | No |
| Kassam (2007) [51] | Canada One hospital Outpatient diabetes clinic | 105 pts with DRPs | Missing | Missing | Pharmacist contribution to multidicsiplinary diabetes team | 276 DRPs | Missing | Missing | No |
| Kumar (2013) [52] | India One hospital General medicine wards | 240 pts (49 pts with DRPs) | Age groups | 61.3% | Routine monitoring of patients’ medication | 71 DRPs | 90% n = 71 | 71% n = 71 | No |
| Kumar (2012) [53] | India One hospital Medicine wards | 189 pts with DRPs | 49.8 | 57.8% | Pharmaceutical care | 261 DRPs | 87% n = 227 | Missing | No |
| Kwint (2012) [54] | The Netherlands 10 community pharmacies | 155 pts | 76 (median) | 46% | Home medicines review | 1565 DRPs | Missing | Missing | No |
| Mekonnen (2013) [55] | Ethiopia One hospital Internal medicine ward | 48 pts with DRPs | 38 | 33.3% | Pharmaceutical care services including involvement in ward rounds, medication review and discharge counselling | 94 DRPs | 68% n = 149 | Missing | No |
| Rashed (2012) [56] | UK and Saudi Arabia Two hospitals Medical ward, paediatric intensive care unit (PICU) and neonatal intensive care unit (NICU) | 737 pts (333 pts with DRPs) | 2.3 (median) | 58.1% | Medication reivew | 478 DRPs | Missing | Missing | No |
| Schröder (2011) [57] | Germany Community pharmacies Patients with idiopathic Parkinson’s disease | 113 pts | 71.50 | 52.2% | ‘‘Drug service’’ or ‘‘pharmaceutical management’’ including medication history and medication review | 331 DRPs | Missing | Missing | No |
| Simioni (1996) [58] | Australia One hospital Medical ward | 157 pts (CG: 80, IG: 77) | Missing (CG: 68.5, IG: 69.0) | Missing (CG: 62.5%, IG: 46.8%) | Pharmaceutical care plans | IG: 154 DRPs CG: 99 DRPs | IG: 86% (n = 131) CG: 82% (n = 89) | Missing | No |
| Smythe (1998) [59] | USA One hospital Medical progressive care patients | 287 pts (IG: 152 pts included, 131 evaluated, CG: 135) | Missing | Missing | Pharmaceutical care | 818 DRPs | 86% n = 235 | Missing | No |
| Somers (2013) [60] | Belgium One hospital Geriatric ward | 100 pts | 81.4 | 52% | Medication review | 304 DRPs | 60% n = 304 | Missing | No |
| Spinewine (2006) [61] | Belgium One hospital Acute geriatric unit | 101 pts | 82.2 | 27% | Pharmaceutical care from admission to discharge including participation at ward rounds | 1066 DRPs | 88% (+7.2% partially accepted) n = 1066 | Missing | Yes |
| Stafford (2009) [62] | Australia Home-dwelling (HD) and residential care-facility (RC) patients | Missing | 78.1 (HD: 73.9, RC: 83.9) | 31.6 (HD: 44.2%, RC: 13.5%) | Medication reviews | 1038 DRPs | Missing | Missing | Yes |
| Stafford (2011) [63] | Australi Community pharmacy practice | 129 pts | Missing | Missing | Home medicines reviews | 157 warfarin-associated DRPs | Missing | Missing | No |
| Stemer (2012) [64] | Austria One hospital Six different wards (1 psyciatric, 1 surgery and 4 medicine) | Missing | Missing | Missing | Clinical pharmacy service at ward rounds | 478 DRPs | 55% n = 478 | Missing | No |
| Tejashwani Pichala (2013) [65] | India One hospital Intensive care unit | 72 pts | Age groups | 59.7% | Clinical pharmacy service at ward rounds | 243 DRPs | 47% n = 243 | Missing | One example |
| Williams (2012) [66] | Australia 185 community pharmacies | Missing | Missing | Missing | Medication reviews | 5948 DRPs | Missing | Missing | Some data |
| Categorisation System Used | Untreated Indication | Improper Drug Selection | Subtherapeutic Dose | Failure to Receive Drug | Overdosage/over Dose | Adverse Drug Reaction | Drug Interaction | Drug Use without Indication | Sub-Optimal Compliance | Non-optimal Dosing | Monitoring | Medication Error | Patient Education Required | Specific Information/Therapy Discussion | Literature Search | Drug duplication/Class Duplication | Medication Management Problem | Improper Duration | Drug Use Problem/Improper Drug Use | Most Cost-Effective Agent Available | Contraindication | No specific Problem | Other | In Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alagiriswami (2009) N = 189 | a | X | X | X | X | X | X | X | X | X | 9 | ||||||||||||||
| Castelino (2011) N = 308 | a | X | X | X | X | X | X | X | X | X | XX | X | X | 13 | |||||||||||
| Kumar (2012) N = 189 | a | X | X | X | X | X | X | X | X | X | 9 | ||||||||||||||
| Spinewine (2006) N = 101 | a, h, i | X | (X) 1 | X | XX | X | (X) 1 | X | X | XX | X | X | XX | X | X | X | 17 | ||||||||
| Alderman (1997) N = 69 | b | X | X | X | X | X | X | X | X | 8 | |||||||||||||||
| Blix (2006) N = 672 | b | X | X | X | X | XX | X | X | X | X | X | X | X | X | 14 | ||||||||||
| Elliott (2011) N = 46 | b | X | X | X | X | X | X | X | X | X | 9 | ||||||||||||||
| Simioni (1996) N = 157 | b | X | X | X | X | X | X | XXX | X | X | 11 | ||||||||||||||
| Bondesson (2013) N = 141 | c | X | X | X | X | X | X | X | 7 | ||||||||||||||||
| Bondesson (2012) N = 132 | c | X | X | X | X | X | X | X | 7 * | ||||||||||||||||
| Celin (2012) N = 108 | c | X | X | X | X | X | X | X | X | X | 9 | ||||||||||||||
| Mekonnen (2013) N = 48 | c | X | X | X | X | X | X | X | 7 | ||||||||||||||||
| Chua (2012) N = 477 | d | X | X | X | X | X | X | 6 | |||||||||||||||||
| Granas (2010) N = 73 | d | X | X | X | X | X | X | 6 | |||||||||||||||||
| Rashed (2012) N = 737 | d | X | X | X | X | X | X | 6 | |||||||||||||||||
| Kwint (2012) N = 155 | f | (X) 1 | X | (X) 1 | X | X | X | X | X | 7 | |||||||||||||||
| Stafford (2009) N = missing | f | X | X | X | X | X | X | X | X 2 | 8 | |||||||||||||||
| Stafford (2011) N = 129 | f | X | X | X | X | X | X | X | X 2 | 8 | |||||||||||||||
| Williams (2012) N = missing | f | X | X | X | X | X | X | X | X 2 | 8 | |||||||||||||||
| Schröder (2011) N = 113 | g | X | X | X | X | X | X | 6 | |||||||||||||||||
| Stemer (2012) N = missing | j | X | X | X | X | X | XXXX | X | X | X | X | X | X | X | X | 17 | |||||||||
| Tejashwani Pichala (2013) N = 72 | k | XX | X | X | XX | X | X | 8 | |||||||||||||||||
| Granaas (1999) N = 285 | e | X | X | X | X | XX | (X) 3 | (X) 3 | X | X | X | X | X | X | 13 | ||||||||||
| Kassam (2007) N = 105 | e | X | X | X | X | X | X | X | X | 8 | |||||||||||||||
| Kumar (2013) N = 240 | e | (X) 1 | X | (X) 1 | X | X | X | X | X | X | 8 | ||||||||||||||
| Smythe (1998) N = 287 | e | X | X | X | X | X | XX | X | X | X | 10 | ||||||||||||||
| Somers (2013) N = 100 | e | X | X | X | X | X | XX | XX | X | 10 |
| Categories Used | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ref of categorization system | Assessment methods for clinical significance in current study * | No. DRP (x) or recommendations (y) where clinical significance was assessed | Extremelyimportant/Significant/life threatening/Possibly life-saving/extreme +deleterious/Type A (%) | Major/Severe/High/Very significant/possibly very important relevance/Type B (%) | Moderate/Significant/Definitely clinically significant/Medium/possibly important relevance/Type C (%) | Minor/Mild/Somewhat significant/Minimal clinical significance/Type D (%) | Low/Probably clinically insignificant/possibly low relevance (%) | Nill/No significance/Not relevant (%) | Adverse significance (%) | |
| Alagiriswami (2009) N = 189 | Missing | 4 | 261 | . | 11 | 60 | 29 | . | . | . |
| Alderman (1997) N = 69 | Missing | 5 | 187 | . | 20 | 59 | 21 | . | . | . |
| Castelino (2011) N = 308 | Alderman | 5 | 327 | . | 10 | 16 | 74 | . | . | . |
| Kassam (2007) N = 105 | Alderman | 3 | 276 | . | 31 | 69 | 0 | . | . | . |
| Kumar (2013) N = 240 | Alagiriswami | 5 | 71 | . | 13 | 48 | 39 | . | . | . |
| Kumar (2012) N = 189 | Missing | 1 | 261 | . | 11 | 60 | 29 | . | . | . |
| Celin (2012) N = 108 | Missing | 5 | 12 ### | . | 0 | 17 | 83 | . | . | . |
| Rashed (2012) N = 737 | Dean and Barber | 3 | 474 | . | 0 | 27 | 72 | . | . | . |
| Blix (2006) N = 672 | Missing | 2 | 373 | 6 | 44 | 40 | 10 | . | . | . |
| Mekonnen (2013) N = 48 | Missing | 2 | 94 | 5 | 49 | 27 | 19 | . | . | . |
| Schröder (2011) N = 113 | van Mil | 2 | 331 | 5 | 27 | 29 | 39 | . | . | . |
| Bondesson (2013) N = 141 | Hatoum | 2 | 733 # | 0.3 | 12 | 32 | 29 | . | 27 | . |
| Bondesson (2012) N = 132 | Hatoum | 2 | 127y ## | 0 | 7 | 51 | 20 | . | 18 | 3 |
| Simioni (1996) N = 157 | Hatoum | 4 | 253+ | 0.4 | 14 | 52 | 16 | . | 18 | 0 |
| Stemer (2012) ++ N = missing | Hatoum | 4 | 478 | 0 | 5 | 38 | 32 | . | 25 | <1 |
| Chua (2012) N = 477 | Stubbs | 3 | 706 | 0.2 | . | 9 | 39 | 52 | . | . |
| Elliott (2011) N = 46 | Standards Australia | 2 | 113 | 2 | 33 | 57 | . | 9 | . | . |
| Granaas (1999) N = 285 | Eadon | 2 | 75 | *** | . | . | . | . | . | . |
| Granas (2010) N = 73 | Missing | 3 | 80 | *** | . | . | . | . | . | . |
| Kwint (2012) N = 155 | Missing | 4 | 1.565 | . | 42 | ? | . | ? | . | . |
| Smythe (1998) N = 287 | Missing | 3 | 818 | . | 4 | 43 | 29 | . | 20 | 4 |
| Somers (2013) ++ N = 100 | Overhage | 3 | 302 | . | 4 | 53 | . | 38 | 4 | 0.3 |
| Spinewine (2006) N = 101 | van Mill/Hatoum | 2 | 334 +++ | 0.4 + 0.1 = 0.5 | 29 | 68 | 3 | . | . | . |
| Stafford (2009) N = missing | Peterson | 1 | 1.038 | . | 30 | ? | ? | ? | . | |
| Stafford (2011) N = 129 | Peterson | 4 | 157 | . | ? | 79 | 19 | ? | ? | . |
| Williams (2012) N = missing | Peterson | 1 | 2535 | . | 43 | ? | ? | ? | . | |
| Tejashwani Pichala (2013) N = 72 | Missing | 5 | 192 | 0 | 17 | 61 | 22 | . | . | . |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kjeldsen, L.J.; Nielsen, T.R.H.; Olesen, C. Investigating the Relative Significance of Drug-Related Problem Categories. Pharmacy 2017, 5, 31. https://doi.org/10.3390/pharmacy5020031
Kjeldsen LJ, Nielsen TRH, Olesen C. Investigating the Relative Significance of Drug-Related Problem Categories. Pharmacy. 2017; 5(2):31. https://doi.org/10.3390/pharmacy5020031
Chicago/Turabian StyleKjeldsen, Lene Juel, Trine Rune Høgh Nielsen, and Charlotte Olesen. 2017. "Investigating the Relative Significance of Drug-Related Problem Categories" Pharmacy 5, no. 2: 31. https://doi.org/10.3390/pharmacy5020031
APA StyleKjeldsen, L. J., Nielsen, T. R. H., & Olesen, C. (2017). Investigating the Relative Significance of Drug-Related Problem Categories. Pharmacy, 5(2), 31. https://doi.org/10.3390/pharmacy5020031





