Abstract
There are three known clinical syndromes of leishmaniasis: cutaneous (CL), mucocutaneous (MCL), and visceral disease (VL). In MCL and VL, treatment must be systemic (either oral or intravenous), while CL treatment options vary and include observation-only localized/topical treatment, oral medications, or parenteral drugs. Leishmaniasis treatment is difficult, with several factors to be considered. First, the efficacy of treatments varies among different species of parasites prevalent in different areas on the globe, with each species having a unique clinical presentation and resistance profile. Furthermore, leishmaniasis is a neglected tropical disease (NTD), resulting in a lack of evidence-based knowledge regarding treatment. Therefore, physicians often rely on case reports or case series studies, in the absence of randomized controlled trials (RCT), to assess treatment efficacy. Second, defining cure, especially in CL and MCL, may be difficult, as death of the parasite can be achieved in most cases, while the aesthetic result (e.g., scars) is hard to predict. This is a result of the biological nature of the disease, often diagnosed late in the course of disease (with possible keloid formation, etc.). Third, physicians must consider treatment ease of use and the safety profile of possible treatments. Thus, topical or oral treatments (for CL) are desirable and promote adherence. Fourth, the cost of the treatment is an important consideration. In this review, we aim to describe the diverse treatment options for different clinical manifestations of leishmaniasis. For each currently available treatment, we will discuss the various considerations mentioned above (efficacy, ease of use, safety, and cost).
1. Introduction
Leishmaniasis is a neglected tropical disease (NTD), with a spectrum of clinical syndromes. Visceral leishmaniasis (VL), also called Kala-Azar, expresses the dissemination of the Leishmania parasite throughout the reticuloendothelial system [1]. Mucocutaneous leishmaniasis (MCL) is defined as the involvement of naso-oropharyngeal or laryngeal mucosa and creation of ulcers or lesions [2]. Cutaneous leishmaniasis (CL), the most common clinical syndrome in many affected regions, is characterized by painless and chronic ulcers at infected sites of sand fly bites. Cutaneous leishmaniasis has a variable clinical manifestation, linked to several factors, including the individual host immune response [3].
The approach to leishmaniasis diagnosis has undergone profound transformation with the integration of molecular diagnostic tools, such as PCR [1]. In contrast to the traditional diagnostic approach, relying on the microscopic pathological examination of parasites in tissue biopsies from infected lesions, molecular testing provides a conclusive identification of the specific species. This holds considerable significance, as distinct species are associated with the varying clinical manifestations mentioned [4]. The ability to pinpoint the involved species enables healthcare professionals to make targeted treatment decisions [1].
Once the specific species causing the disease has been identified, the physician must address different considerations when choosing the most appropriate treatment option. The first consideration and most eminent of all is the treatment efficacy against the species recognized. It is important to recall that the same parasite species might have a unique clinical presentation and resistance profile in different areas across the globe [5]. Due to the lack of evidence-based knowledge regarding treatment, physicians often rely on case reports or case series studies in the absence of randomized controlled trials (RCTs) to assess treatment efficacy [6,7]. Furthermore, the evaluation of drug efficacy is even more challenging because of the biological nature of the disease. Even though death of the parasite can be achieved in most cases, the aesthetic result (e.g., scars) can vary [8,9]. The pathophysiology of CL and MCL involves complicated mechanisms of host–parasite interactions [8,10,11]. The immune response plays a crucial role in disease progression. In CL, an exaggerated Th1 response is associated with healing, whereas a Th2-dominated response may lead to chronicity [9,10]. Similarly, MCL is characterized by an inadequate immune response, allowing for parasite dissemination to mucosal tissues. Moreover, the delayed diagnosis, often associated with leishmaniasis, allows for the formation of complications, such as keloids and disfiguring scars, particularly in CL cases.
The treatment of leishmaniasis is not without potential side effects. Common side effects of anti-leishmanial drugs include systemic effects (e.g., gastrointestinal disturbances such as nausea, vomiting, and abdominal pain, hepatotoxicity, renal toxicity), as well as skin reactions. Furthermore, given the prolonged treatment courses often required, patients may experience cumulative toxicity. It is crucial for healthcare providers to monitor patients closely during treatment and to balance the benefits of therapy with potential adverse effects.
The ease of use of leishmaniasis treatment is a critical consideration, with topical or oral administration often favoured to enhance patient adherence and facilitate effective management of the disease. While VL and MCL must include systemic treatment by oral or parenteral administration, CL can be treated by other options, such as cream or spray.
Finally, the cost of treatment constitutes a significant factor for consideration. As previously highlighted, the prevalence of the disease is widespread in third-world countries. While the treatment is efficacious, its affordability poses a challenge for many patients. Global health organizations are actively working to tackle this issue. For instance, the Pan American Health Organization (PAHO) has recently issued new guidelines advocating for a briefer and safer treatment regimen for VL in the Americas, thereby enhancing accessibility for individuals in need [12].
In this comprehensive narrative review, our objective is to delineate the array of treatment modalities available for addressing the distinct clinical manifestations of VL, MCL and CL. For each existing therapeutic option, we will thoroughly explore four key considerations, including drug efficacy, potential adverse side effects, ease of administration, and cost of treatment. This review takes a practical approach in detailing the considerations for choosing specific treatment, which hopefully will allow physicians in different settings worldwide a judicious management of simple and complex leishmaniasis cases.
2. Visceral Leishmaniasis
Visceral leishmaniasis (VL) is a potentially fatal disease, ranking second and seventh in terms of mortality and loss of disability-adjusted life years among tropical diseases, respectively [13,14]. According to the World Health Organization (WHO), almost 13,000 cases of VL were documented in 2020, with more than 90% of them in only seven endemic countries: Brazil, Ethiopia, India, Kenya, Somalia, South Sudan, and Sudan [15,16]. Visceral leishmaniasis is mainly caused by Leishmania donovani and Leishmania infantum, the latter also called Leishmania chagasi in South America. It is critical to recall that all species mentioned can also cause non-visceral manifestations, CL and MCL included [17,18,19,20]. This fact emphasizes the importance of diagnosis of the specific species involved, allowing the physician to identify cases when visceral disease is possible, and to treat accordingly to achieve prevention. Similarly, VL may rarely be caused by leishmania species that are usually causing CL, like Leishmania tropica [21,22,23]. As mentioned above, due to the possible grave outcome of VL, a systemic option is the treatment of choice [24,25]. Correspondingly, this increases physicians’ willingness to cope with relatively severe adverse effects of effective treatment.
2.1. Oral Medications
Miltefosine is an oral treatment for VL, with cure rates of 98% [26]. In a phase III trial, the drug demonstrated an efficacy ranging from 94% to 97% for children after 6 months of follow-up, as determined by the density of parasites in bone marrow and/or splenic aspirates. The effectiveness of miltefosine is comparable to that of other agents, given intravenously (IV), such as amphotericin B and liposomal amphotericin B [27,28]. These remarkable cure rates are unparalleled by other oral drugs, and therefore, make miltefosine an attractive option for VL treatment (Table 1).

Table 1.
Systemic treatment (IV and oral) of leishmaniasis.
Toxic effects associated with miltefosine have been tolerable and reversible, although the therapeutic window appears to be narrow [29]. In a phase III trial, gastrointestinal symptoms like vomiting and diarrhoea were observed. Additionally, some patients experienced reversible hepatotoxicity and nephrotoxicity, as indicated by elevated levels of liver transaminases, urea, and creatinine, which typically normalized by the end of the second week of therapy [27,30]. Therefore, the drug is not approved for the treatment of children younger than 12 years old [1]. Nevertheless, although highly effective, relatively safe, and comfortable for patient use, access to miltefosine remains far from secure for some of those needing it the most, with a cost of hundreds of US dollars per treatment in India, for example [31].
Due to the high cost of miltefosine and prior to its development, other oral medications have been suggested as possible treatment for VL. These included anti-fungal “azoles” agents (e.g., itraconazole), antibiotics (e.g., azithromycin), and other drugs (e.g., allopurinol). Despite the benign side effects profile and relatively cheap cost of the drugs mentioned, these treatments were not proven to have reasonable efficacy in RCTs [32,33,34], thus limiting their widespread use.
2.2. IV Treatment
Liposomal amphotericin B has emerged as a promising intravenous treatment for visceral leishmaniasis. This was driven by the fact that liposomal amphotericin B was found to be as effective as amphotericin B deoxycholate, but much safer. Indeed, efficacy studies have shown notable success, with high cure rates, of liposomal amphotericin B for VL treatment [35]. The side effect profile of the drug is marked by tolerable and reversible toxic effects, including fever, chills, vomiting, and reversible nephrotoxicity [36]. While liposomal amphotericin B may be associated with a higher cost compared to some other treatment options (i.e., amphotericin B deoxycholate), its efficacy and relatively manageable side effects contribute to its favourable consideration. It is important to recall that as administration is parenteral, necessitating hospitalization to administer the drug, it might affect patient adherence.
Although the mechanism is unclear, pentavalent antimonial compounds have been the mainstay of the treatment of VL, MCL, and CL for approximately half a century [37]. Research from 1998 in Sudan revealed that almost all (98%) of 1593 VL patients treated with sodium stibogluconate (antimony–carbohydrate complex) between 1989 and 1995 responded well to treatment. The side effect profile includes pain and swelling upon injection site (the drug can be given by IV or intramuscular [IM] route). Other adverse events noted are pancreatitis, leukopenia, headache, lethargy, myalgia, arthralgia, diarrhoea, nausea, vomiting, nephrotoxicity, and elevation of liver enzymes. The cost of treatment is relatively cheap, compared with miltefosine and Liposomal amphotericin B [31,36,37].
2.3. Clinical Correlation and Integration
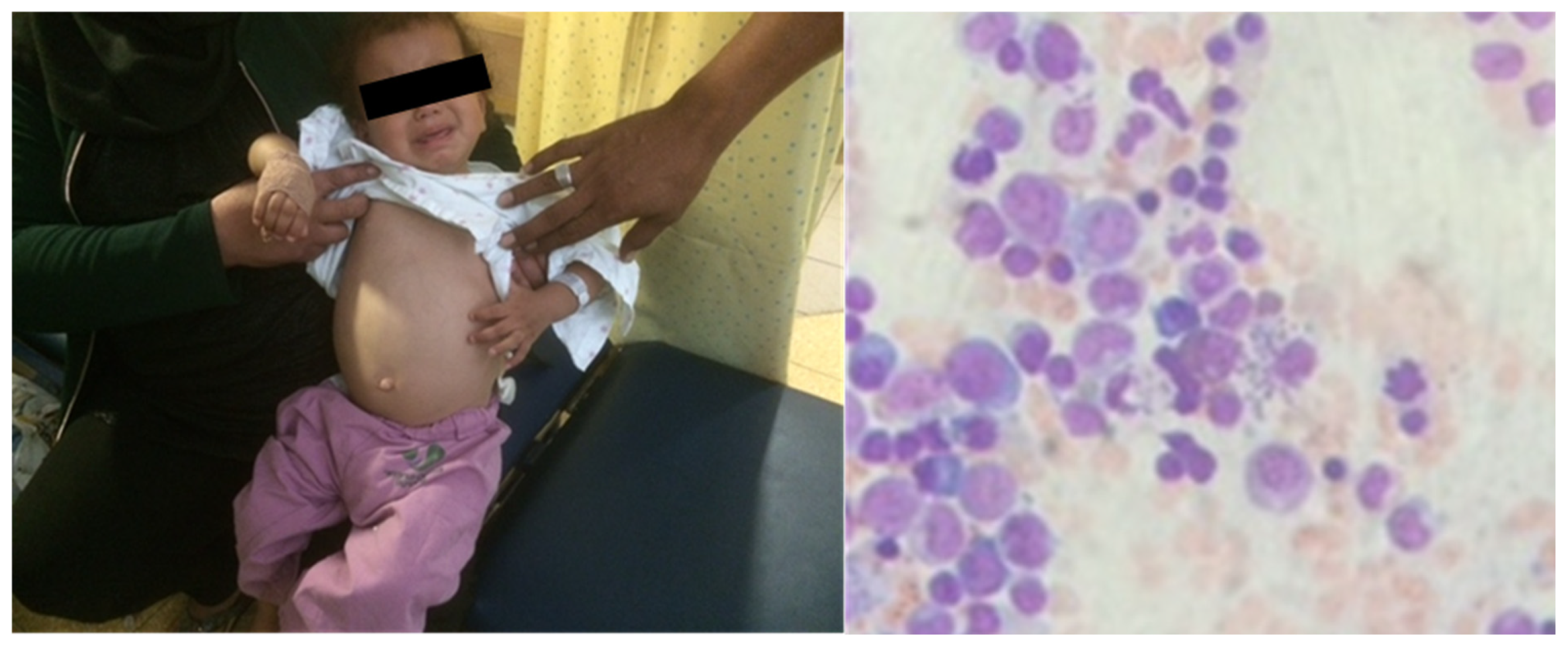
A 4-year-old patient arrived at our clinic, in a leishmaniasis endemic region, with complaints of prolonged fever (>1 month), abdominal pain and a “swollen belly”. The child was febrile and looked fatigued. A physical examination revealed hepatosplenomegaly. On bone marrow biopsy, characteristic leishmaniasis amastigotes were observed on microscope examination (Figure 1). The PCR result was positive for Leishmania infantum. The patient was diagnosed with VL due to an L. infantum infection.
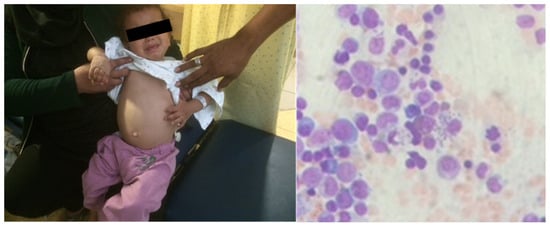
Figure 1.
Visceral leishmaniasis in a 4-year-old patient. Hepatosplenomegaly (left) and amastigotes in bone marrow biopsy (right) are notable.
The child was treated with IV Liposomal amphotericin B. This therapeutic option is highly effective against this pathogen, as proven in RCTs; in contrast to the favourite oral drug of choice, miltefosine, it is used in young children <12 years of age. With efficacy considerations dominating the decision-making process, we believe that IV Liposomal amphotericin B is the only acceptable option in the case of VL in young children, despite safety concerns (mandating strict monitoring) and the need for hospitalization.
3. Mucocutaneous Leishmaniasis
Mucocutaneous leishmaniasis is a chronic inflammatory process involving the nasal, pharyngeal, and laryngeal mucosa, which can lead to extensive tissue destruction [38,39]. Patients should be monitored for associated complications, particularly if the disease progresses. MCL is potentially life-threatening and therefore requires systemic treatment. The disease can develop post-infection from Leishmania species of the Viannia subgenus, typically found in the Americas, including L. braziliensis, L. amazonensis, L. panamensis, and L. guyanensis [40,41]. The clinical progression to mucosal disease depends on a combination of host cell-mediated immunity and parasite virulence [42]. Among a population of infected individuals with cutaneous manifestation, infection progresses to the mucosa in 1–10% of patients [43]. The specific host factors that determine which patients will develop MCL are still unclear. Adequate systemic treatment of cutaneous leishmaniasis caused by these species may reduce the risk for mucosal disease, but some risk may remain. The common presenting symptoms include persistent nasal congestion, erythema, and erosions [38,39]. Ulcers can be seen around the nares and lips as the disease progresses. These findings are sometimes mistakenly interpreted for impetigo contagiosum, forcing the physician to be aware of local epidemiology and leishmania species [44].
3.1. Oral Treatment
As presented for VL, miltefosine given orally is the treatment of choice. Other treatments elaborated for VL are possible for MCL disease as well (itraconazole, azithromycin, and allopurinol) [1,12,45,46,47].
New effective and safe oral drugs are necessary to address the needs of VL patients in developing countries [48].
3.2. IV Treatment
Based on a WHO recommendation, pentavalent antimonial compounds are the most commonly used parenteral medication followed by liposomal amphotericin B [1,12,33,49]. There are no sufficient data about MCL cure rates. Treatment failure and relapse are common [50,51].
Intravenous liposomal amphotericin B cure rates suggest efficacy of 80 to 90% for MCL, similar to rates reported for other antileishmanial drugs (i.e., pentavalent antimonial compounds) [52].
3.3. Clinical Correlation and Integration
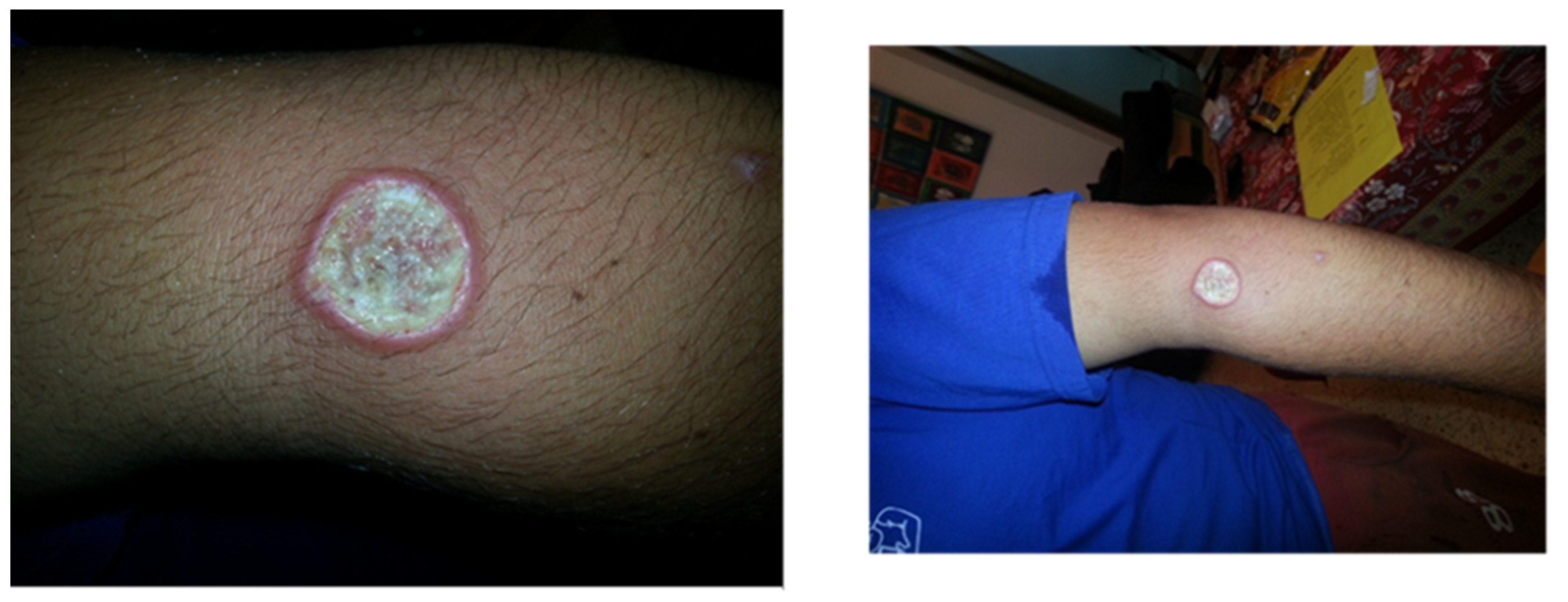
A 24-year-old student arrived at our clinic with a recent appearance of an ulcer on his left arm. He just returned from a trip to South and Central America during a summer vacation, including visits to Brazil, Panama, and Guatemala. Physical examination revealed an ulcer, as presented in Figure 2. On biopsy from the lesion, a molecular test was performed, and the PCR result was positive for Leishmania braziliensis. The patient was concerned about systemic treatment adverse events, and asked whether topical treatment is sufficient.
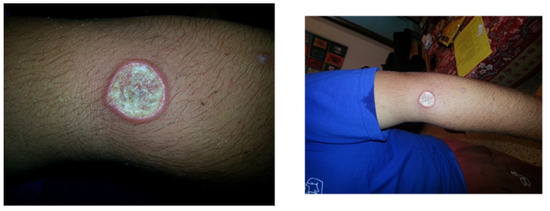
Figure 2.
Cutaneous leishmaniasis in a 24-year-old student diagnosed with L. braziliensis without adequate systemic treatment; patient is at risk for developing MCL.
Mucosal leishmaniasis usually becomes clinically evident within several years of the original cutaneous lesions if not treated or treated not optimally [45,53]. Detecting a species, which can potentially cause MCL, should lead the treating physician to try and reduce future complications. Adequate systemic treatment of cutaneous leishmaniasis caused by these species may reduce the risk for the development of mucosal disease [1]. Miltefosine or other systemic drugs found to be effective as discussed above are the treatment of choice.
4. Cutaneous Leishmaniasis
Cutaneous leishmaniasis is the most common presentation of leishmaniasis, with an estimated 600,000 to 1 million new patients annually worldwide, with eight countries contributing 90% of CL cases: Afghanistan, Algeria, Brazil, Iran, Pakistan, Peru, Saudi Arabia, and Syria. [54]. Notably, the clinical findings of CL depend, among other factors, on the species involved [15]. Lesions caused by L. tropica and L. major usually self-heal within a year but tend to leave permanent scars. In contrast, lesions by L. aethiopica take years to heal and can develop into severe oral–nasal MCL and diffuse forms of CL [15]. While not posing an immediate threat to life, the recognition and treatment of CL hold significance due to its potential association with the formation of permanent scars. Such scarring can result in aesthetic impairment, diminished quality of life, and enduring psychological consequences, thereby highlighting the importance of addressing this condition [43]. Consequently, the willingness of physicians to administer systemic drugs with relatively severe side effects rely on a combination of often contradicting factors, balancing disease severity and perception by the patient with potential adverse events [15].
The complexity of CL underscores the challenges faced by physicians in their attempt to achieve a definitive cure [55]. The mere eradication of the parasite does not necessarily translate into the desired aesthetic result, an aspect subjectively evaluated by the patient. The scarring process itself relies on tangled relationships between diverse factors, such as the time to diagnosis, the unique host immune response, and the parasite-specific virulence [42]. Certain species, such as L. major, exclusively induce cutaneous manifestations. Conversely, other species can manifest as CL during the initial stages of MCL [38]. L. tropica infection, prevalent in numerous regions, predominantly results in CL, yet it may rarely progress to VL [56]. Consequently, local treatment is usually adequate for CL, but vigilant monitoring is imperative to avert potential complications.
Various treatment modalities exist for CL. In instances where patient concerns about aesthetic impairment are minimal and there is a low risk of disease progression (e.g., lesions in concealed areas caused by L. major), choosing a watchful waiting approach is a valid option.
Topical treatments are the most common therapeutic options used, but they often bear the possibility of limited efficacy and local adverse events. In selected cases, especially where additional symptoms arise or if the patient has risk factors for disease progression, the physician may opt for systemic treatment [1,6]. Factors associated with choosing systemic treatment may be related to the nature of the skin condition (e.g., multiple lesions, facial lesions, or lesions in hard-to-reach areas, like the eyelid), as well as to patient characteristics (e.g., age, immunosuppression, or prior treatment failure). Finally, physicians may consider systemic treatment when the molecular diagnosis is positive for species associated with VL or MCL [57,58].
The complementary treatment of CL, addressing secondary bacterial infections and residual scarring, is crucial for optimization of the overall therapeutic approach. Antibiotics, both administered locally and systemically, play a significant role in managing bacterial complications that may arise concurrently with leishmaniasis [59]. Given the susceptibility of open lesions to bacterial colonization, especially skin pathogens such as Staphylococcus aureus, timely intervention with antibiotics can prevent the exacerbation of the infection, enhance wound healing, and contribute to the overall recovery of the affected individuals [60]. Furthermore, the incorporation of anti-keloid treatment represents another dimension in the complementary strategy for leishmaniasis management [61]. Keloid formation, characterized by the overgrowth of scar tissue beyond the boundaries of the original wound, is a potential consequence of CL. Anti-keloid treatments aim to mitigate excessive scarring, thereby improving the aesthetic outcome for patients.
4.1. Local/Topical Treatment
4.1.1. Non-Selective Treatments
Thermotherapy can be a treatment of localized CL. Research conducted in Brazil evaluated its safety and efficacy and found that although most lesions achieved full healing, patients suffered from side effects including itching, burning sensation, pain, and blisters [62] (Table 2).

Table 2.
Local treatment of CL.
Cryotherapy, often using liquid nitrogen, is another possible cost-effective and accessible treatment for CL, with variable efficacy. A meta-analysis (2016) comparing clinical trials reveals that the respective per lesion efficacies of 67.3% and 67.7% were reported for cryotherapy and pentavalent antimonial, respectively [63,64]. The most common reported side effects for cryotherapy were hypopigmentation or reversible hyperpigmentation, erythema, oedema, and pain.
Notably, both thermotherapy and cryotherapy are non-selective treatments. This means that besides killing the parasite, the surrounding tissue cells are also affected and destroyed, causing oedema, erythema and pain, highlighting the need for more directed therapy [63].
Ultraviolet (UV) radiation, a potent suppressor of cell-mediated immune responses, is considered to have some relevance to the treatment of CL. However, the literature on this treatment option is currently anecdotal [65].
4.1.2. Selective Treatments
The WHO recommends using either intra-lesion or systemic pentavalent antimonials (e.g., pentostam) for CL, depending on the specific species and clinical features [12]. In the case of intra-lesional administration, the suggested approach involves injecting 1–3 mL of the medication under and around the lesion until the surface becomes pale, with repeat injections every 5–7 days, totalling 2–5 times. A review from 1999 found that intra-lesional antimonials were successful in partially or completely curing 72–97% of lesions caused by L. major [66]. Frequent pain during injections was reported among children [66]. Other reported adverse events include bacterial super-infections, signs of stibio-intolerance in cephalic locations, and progression to more advanced lesions [67]. When choosing treatment by intra-lesional antimonials, the most eminent factor to consider is the procedure’s ease of use. A repeated regimen of injections can be challenging in children, and anaesthesia may be needed for the feasibility of the process, forcing strict monitoring and hospitalization.
Synthesized nitric oxide-releasing chitosan nanoparticles (NONPs) showed potential in vitro activity against L. amazonensis and in infected murine models [68]. Thus, NONPs may be suitable for topical use, and although this path looks promising, further research is needed to achieve precise compounds and administration protocols or devices for clinical use.
Topical paromomycin-containing cream offers a non-systemic treatment for CL. El-On and colleagues developed a formulation with 15% paromomycin in white soft paraffin, also incorporating 12% methyl benzethonium chloride [69]. This formulation proved more effective than no treatment for L. major infections in Israel and superior to a vehicle-control treatment for L. mexicana and L. braziliensis infections in Guatemala [70]. However, due to the high cost and considerable irritancy and intolerance associated with methyl benzethonium chloride (observed in up to 75% of patients), the formulation is infrequently used. A RCT conducted in Tunisia established the efficacy of paromomycin–gentamicin and paromomycin alone for treating ulcerative L. major disease [71].
Few reports discuss the use of topical liposomal amphotericin B (LAmB) in treating CL, in contrast to the proven success of intravenous LAmB for VL [72,73,74]. Horev et al. explored this alternative treatment, emphasizing its potential effectiveness, ease of use, and safety. This RCT involved the evaluation of 13 patients with a total of 39 lesions caused by Leishmania major, demonstrating LAmB efficacy with only mild and localized side effects [75].
4.2. Oral Treatment
Systemic treatment for CL is mainly reserved, as discussed above, for complicated cases with previous treatment failure or with specific host and parasite risk factors for treatment failure [1,6]. Briefly, these factors include CL with multiple lesions, facial lesions, lesions in hard-to-reach areas, patients’ young age, immunosuppression and disease caused by leishmania species capable of causing MCL or VL [55,56].
Similar to VL, miltefosine given orally is the treatment of choice [12].
4.3. IV Treatment
Liposomal amphotericin B has emerged as a promising intravenous treatment for CL when a physician decides that systemic treatment is needed [32,35].
4.4. Clinical Correlation and Integration
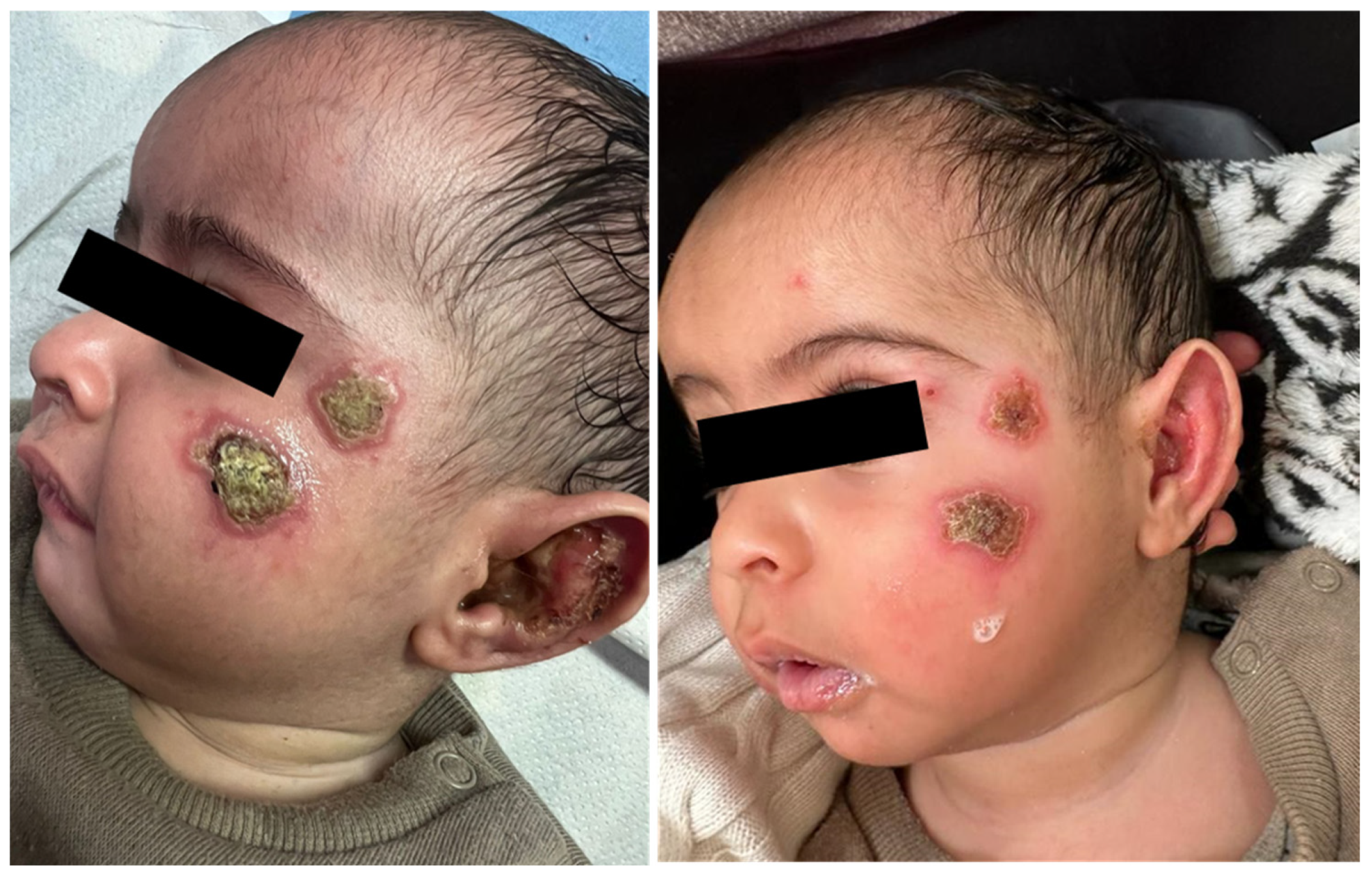
A 6-month-old infant arrived at our clinic with a recent appearance of multiple facial lesions, as seen in Figure 3 (left). In a biopsy from the lesion, the PCR test was positive for Leishmania major.
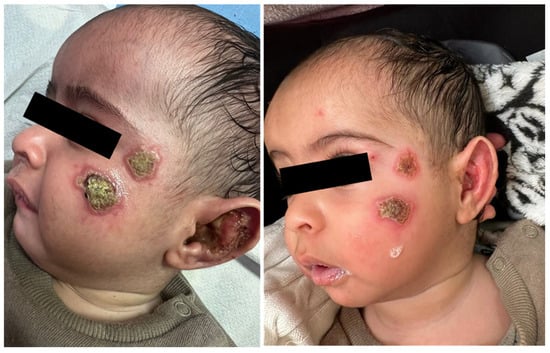
Figure 3.
Cutaneous leishmaniasis in a 6-month-old infant, diagnosed with L. major infection (left). After one week of systemic treatment with IV Liposomal amphotericin B, the patient is showing clinical improvement (right).
This patient presented several factors favouring systemic treatment, including a very young age, multiple facial lesions, and lesions in a hard-to-reach area (the ear auricle). The child was treated with IV Liposomal amphotericin B. This therapeutic option is highly effective against this pathogen, and in contrast to the oral drug of choice, miltefosine, it may be used in children <12 years of age. Figure 3 (right) presents evidence of clinical improvement after one week of treatment.
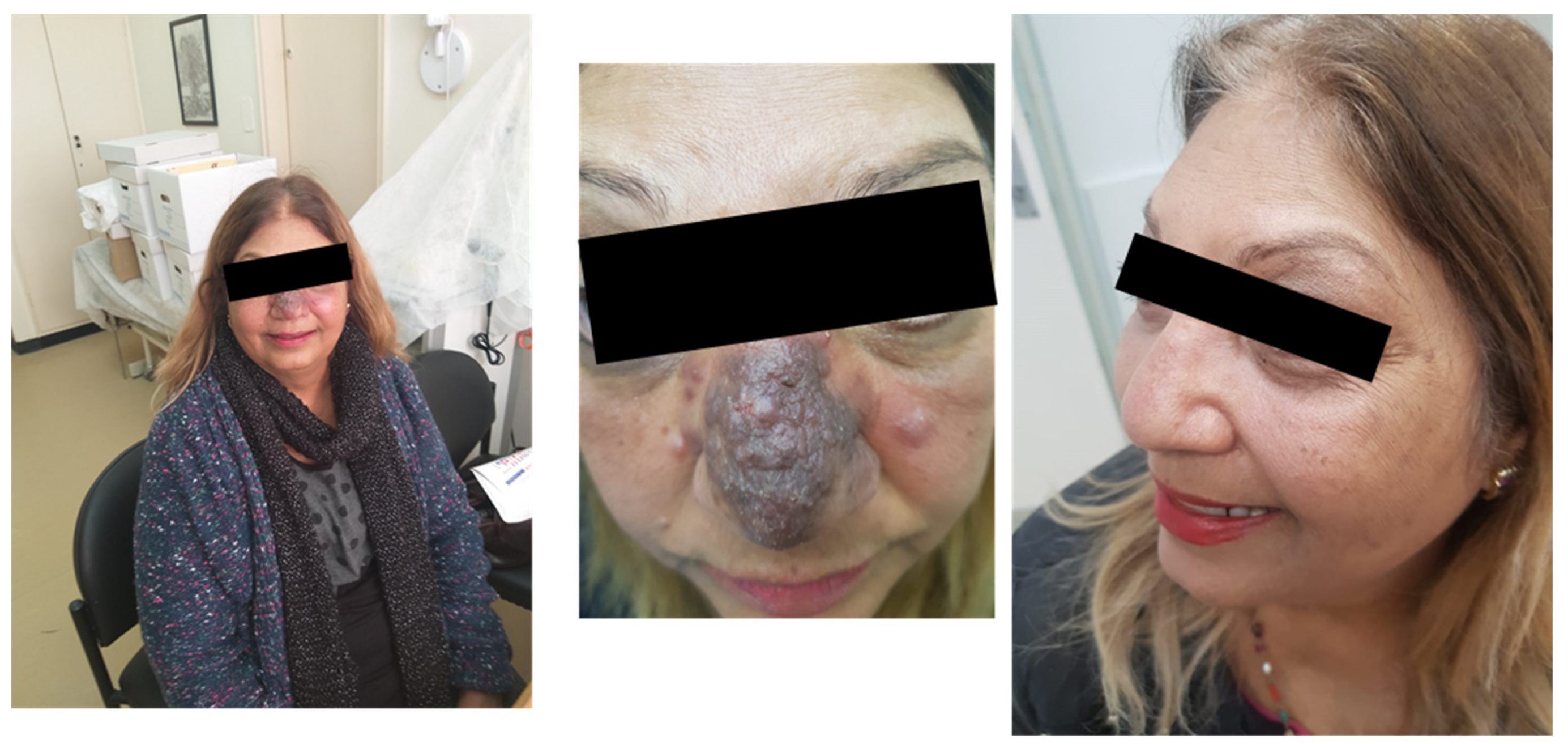
A 54-year-old patient, diagnosed with L. major infection, came to our clinic with a facial lesion on her nose, shown in Figure 4 (left).
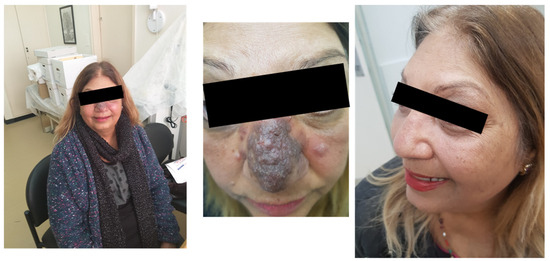
Figure 4.
Cutaneous leishmaniasis in a 54-year-old patient (left, middle). Following miltefosine treatment, the patient showed improvement (right).
Due to the aesthetic nature of the lesion, we decided to use IV Liposomal amphotericin B for treatment. This option is effective, relatively convenient, and generally safe. However, the patient experienced mild side effects, and there was no improvement several weeks following treatment. After consulting the patient, we decided to treat her with miltefosine, an expensive oral medication. Following 28 days of miltefosine treatment, a remarkable improvement was observed, as shown in Figure 4 (right). Notably, changing the treatment might have caused the improvement, but it is also possible that the first drug had some positive (late) effects as well.
5. Summary
In this narrative review article on leishmaniasis treatment, the multifaceted landscape of available options is presented. Despite a plethora of choices, a definitive ideal drug for leishmaniasis, and especially for CL, remains elusive. Managing leishmaniasis necessitates a nuanced decision-making process, taking into account various host-dependent and pathogen-dependent factors. Looking forward, there is a significant need for the development of a locally applicable, highly efficacious drug with minimal adverse events. Finally, there is a critical need for more affordable and readily accessible drugs for CL, MCL and VL.
Author Contributions
M.S. and S.B.-S. have equally contributed to conceptualisation, methodology, data curation, writing, reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
The patients or the legal guardians of minors have given informed consent to the publication of the figures.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.M.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin. Infect. Dis. 2016, 63, e202–e264. [Google Scholar] [CrossRef]
- Abadias-Granado, I.; Diago, A.; Cerro, P.A.; Palma-Ruiz, A.M.; Gilaberte, Y. Cutaneous and Mucocutaneous Leishmaniasis. Actas Dermo-Sifiliográficas (Engl. Ed.) 2021, 112, 601–618. [Google Scholar] [CrossRef]
- Mortazavi, H.; Sadeghipour, P.; Taslimi, Y.; Habibzadeh, S.; Zali, F.; Zahedifard, F.; Rahmati, J.; Kamyab, K.; Ghandi, N.; Zamanian, A.; et al. Comparing acute and chronic human cutaneous leishmaniasis caused by Leishmania major and Leishmania tropica focusing on arginase activity. J. Eur. Acad Dermatol. Venereol. 2016, 30, 2118–2121. [Google Scholar] [CrossRef]
- Galluzzi, L.; Ceccarelli, M.; Diotallevi, A.; Menotta, M.; Magnani, M. Real-time PCR applications for diagnosis of leishmaniasis. Parasites Vectors 2018, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Azam, M.; Ramesh, V.; Singh, R. Unusual Observations in Leishmaniasis-An Overview. Pathogens 2023, 12, 297. [Google Scholar] [CrossRef]
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. QJM 2014, 107, 7–14. [Google Scholar] [CrossRef]
- Pace, D. Leishmaniasis. J. Infect. 2014, 69 (Suppl. S1), S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.P.; Fong, D. Cell Biology of Host-Parasite Membrane Interactions in Leishmaniasis. In Ciba Foundation Symposium 99-Cytopathology of Parasitic Disease; John Wiley & Sons, Ltd.: Chichester, UK, 1983; Volume 99, pp. 113–137. [Google Scholar] [CrossRef]
- Lamotte, S.; Spath, G.F.; Rachidi, N.; Prina, E. The enemy within: Targeting host-parasite interaction for antileishmanial drug discovery. PLoS Neglected Trop. Dis. 2017, 11, e0005480. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Bravo, C.; Koh, E.Y.; Tan, K.S.W. The roles of parasite-derived extracellular vesicles in disease and host-parasite communication. Parasitol. Int. 2021, 83, 102373. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, K. Exploring the paradox of defense between host and Leishmania parasite. Int. Immunopharmacol. 2022, 102, 108400. [Google Scholar] [CrossRef]
- Pan American Health Organization. Guideline for the Treatment of Leishmaniasis in the Americas, 2nd ed.; Pan American Health Organization: Washington, DC, USA, 2022. [Google Scholar]
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, N.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Postigo, J.A.; Jain, S.; Maia-Elkhoury, A.M.A.N.; Valadas, S.; Warusavithana, S.; Osman, M.; Lin, Z.; Beshah, A.; Yajima, A.; Gasimov, E. Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap/Surveillance mondiale de la leishmaniose: 2019–2020, une periode de reference pour la feuille de route a l’horizon 2030. Wkly. Epidemiol. Rec. 2021, 96, 401–420. [Google Scholar]
- Ben-Shimol, S.; Sagi, O.; Horev, A.; Avni, Y.S.; Ziv, M.; Riesenberg, K. Cutaneous leishmaniasis caused by Leishmania infantum in Southern Israel. Acta Parasitol. 2016, 61, 855–858. [Google Scholar] [CrossRef]
- Kumar, N.P.; Srinivasan, R.; Anish, T.S.; Nandakumar, G.; Jambulingam, P. Cutaneous leishmaniasis caused by Leishmania donovani in the tribal population of the Agasthyamala Biosphere Reserve forest, Western Ghats, Kerala, India. J. Med. Microbiol. 2015, 64, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Astman, N.; Warshavsky, K.; Barzilai, A.; Meningher, T.; Avni, D.; Schwartz, E. Cutaneous Leishmaniasis Caused by Leishmania infantum, Israel, 2018–2021. Emerg. Infect. Dis. 2023, 29, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Thakur, L.; Singh, K.K.; Kushwaha, H.R.; Sharma, S.K.; Shankar, V.; Negi, A.; Verma, G.; Kumari, S.; Jain, A.; Jain, M. Leishmania donovani Infection with Atypical Cutaneous Manifestations, Himachal Pradesh, India, 2014–2018. Emerg. Infect. Dis. 2020, 26, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Teke, T.A.; Metin Timur, O.; Gayretli Aydin, Z.G.; Oz, N.; Bayhan, G.I.; Yilmaz, N.; Mungan, M.; Tanir, G. Three Pediatric Cases of Leishmaniasis with Different Clinical Forms and Treatment Regimens. Turkiye Parazitol. Derg. 2015, 39, 147–150. [Google Scholar] [CrossRef]
- Magill, A.J.; Grogl, M.; Gasser, R.A., Jr.; Sun, W.; Oster, C.N. Visceral infection caused by Leishmania tropica in veterans of Operation Desert Storm. N. Engl. J. Med. 1993, 328, 1383–1387. [Google Scholar] [CrossRef]
- Ozbilgin, A.; Tunali, V.; Cavus, I.; Tetik, A.V.; Dinc, M.; Yalcin, T.; Gunduz, C.; Beyaz, M.; Kose, S. Visceral Leishmaniasis Caused by Leishmania Tropica. Acta Parasitol. 2023, 68, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.W. Treatment of visceral leishmaniasis (kala-azar): A decade of progress and future approaches. Int. J. Infect. Dis. 2000, 4, 158–177. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.L.; Guerin, P.J.; Gerstl, S.; Haaskjold, A.A.; Rottingen, J.A.; Sundar, S. Treatment options for visceral leishmaniasis: A systematic review of clinical studies done in India, 1980–2004. Lancet Infect. Dis. 2005, 5, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.K.; Singh, Z. Miltefosine: First Oral Drug for Treatment of Visceral Leishmaniasis. Med. J. Armed Forces India 2006, 62, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, E. Oral miltefosine treatment in children with visceral leishmaniasis: A brief review. Braz. J. Infect. Dis. 2008, 12, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Kumar, R.; Jaiswal, B.P.; Singh, U.K. Miltefosine: An oral drug for visceral leishmaniasis. Indian J. Pediatr. 2004, 71, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef]
- More, B.; Bhatt, H.; Kukreja, V.; Ainapure, S.S. Miltefosine: Great expectations against visceral leishmaniasis. J. Postgrad. Med. 2003, 49, 101–103. [Google Scholar] [CrossRef]
- Sunyoto, T.; Potet, J.; Boelaert, M. Why miltefosine-a life-saving drug for leishmaniasis-is unavailable to people who need it the most. BMJ Glob. Health 2018, 3, e000709. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Krome, A.K.; Al-Obaidi, I.; Batchelor, H.; Vaillant, M.; Hoerauf, A.; Opoku, N.O.; Kuesel, A.C. The pipeline for drugs for control and elimination of neglected tropical diseases: 2. Oral anti-infective drugs and drug combinations for off-label use. Parasites Vectors 2023, 16, 394. [Google Scholar] [CrossRef]
- Taheri, A.R.; Rad, S.S.; Molkara, S. Systemic Treatments of Leishmaniasis: A Narrative Review. Rev. Clin. Med. 2019, 6, 91. [Google Scholar]
- Teixeira, A.C.; Paes, M.G.; Guerra Jde, O.; Prata, A.; Silva-Vergara, M.L. Low efficacy of azithromycin to treat cutaneous leishmaniasis in Manaus, AM, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2007, 49, 235–238. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Liposomal amphotericin B and leishmaniasis: Dose and response. J. Glob. Infect. Dis. 2010, 2, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.R.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome((R))): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Scholar, E. Sodium Stibogluconate. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Machado-Coelho, G.L.; Caiaffa, W.T.; Genaro, O.; Magalhaes, P.A.; Mayrink, W. Risk factors for mucosal manifestation of American cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Weigle, K.; Saravia, N.G. Natural history, clinical evolution, and the host-parasite interaction in New World cutaneous Leishmaniasis. Clin. Dermatol. 1996, 14, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, S.; Lawn, S.D.; Kanagalingam, J.; Grant, H.; Lockwood, D.N. Mucocutaneous leishmaniasis: An imported infection among travellers to central and South America. BMJ 2004, 329, 842–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Konecny, P.; Stark, D.J. An Australian case of New World cutaneous leishmaniasis. Med. J. Aust. 2007, 186, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Volpedo, G.; Pacheco-Fernandez, T.; Holcomb, E.A.; Cipriano, N.; Cox, B.; Satoskar, A.R. Mechanisms of Immunopathogenesis in Cutaneous Leishmaniasis And Post Kala-azar Dermal Leishmaniasis (PKDL). Front. Cell. Infect. Microbiol. 2021, 11, 685296. [Google Scholar] [CrossRef] [PubMed]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef]
- Amato, V.S.; Tuon, F.F.; Imamura, R.; Abegao de Camargo, R.; Duarte, M.I.; Neto, V.A. Mucosal leishmaniasis: Description of case management approaches and analysis of risk factors for treatment failure in a cohort of 140 patients in Brazil. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Saenz, R.E.; de Rodriguez, C.G.; Johnson, C.M.; Berman, J.D. Efficacy and toxicity of pentostam against Panamanian mucosal leishmaniasis. Am. J. Trop. Med. Hyg. 1991, 44, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Amato, V.S.; Graf, M.E.; Siqueira, A.M.; Nicodemo, A.C.; Amato Neto, V. Treatment of New World cutaneous leishmaniasis—A systematic review with a meta-analysis. Int. J. Dermatol. 2008, 47, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.; Biggart, A.; Be, C.; Srinivas, H.; Hein, A.; Caridha, D.; Sciotti, R.J.; Pybus, B.; Kreishman-Deitrick, M.; Bursulaya, B.; et al. Discovery and Characterization of Clinical Candidate LXE408 as a Kinetoplastid-Selective Proteasome Inhibitor for the Treatment of Leishmaniases. J. Med. Chem. 2020, 63, 10773–10781. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, E. Treatment strategies for mucocutaneous leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Gomes-Silva, A.; Da-Cruz, A.M.; Duarte, M.I.; Neto, V.A.; Amato, V.S. Local immunological factors associated with recurrence of mucosal leishmaniasis. Clin. Immunol. 2008, 128, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Zajtchuk, J.T.; Casler, J.D.; Netto, E.M.; Grogl, M.; Neafie, R.C.; Hessel, C.R.; de Magalhaes, A.V.; Marsden, P.D. Mucosal leishmaniasis in Brazil. Laryngoscope 1989, 99, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Chivinski, J.; Nathan, K.; Naeem, F.; Ekmekjian, T.; Libman, M.D.; Barkati, S. Intravenous Liposomal Amphotericin B Efficacy and Safety for Cutaneous and Mucosal Leishmaniasis: A Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2023, 10, ofad348. [Google Scholar] [CrossRef]
- Parasites-Leishmaniasis. CDC Centers for Disease Control and Prevention. Resources for Health Professionals. 2023. Available online: https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html (accessed on 2 January 2024).
- de Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef]
- Gurel, M.S.; Tekin, B.; Uzun, S. Cutaneous leishmaniasis: A great imitator. Clin. Dermatol. 2020, 38, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, F.; Koltas, I.S.; Alabaz, D.; Uzun, S.; Karakas, M. Clinical manifestations and genetic variation of Leishmania infantum and Leishmania tropica in Southern Turkey. Exp. Parasitol. 2015, 154, 67–74. [Google Scholar] [CrossRef]
- de Vries, H.J.; Reedijk, S.H.; Schallig, H.D. Cutaneous leishmaniasis: Recent developments in diagnosis and management. Am. J. Clin. Dermatol. 2015, 16, 99–109. [Google Scholar] [CrossRef]
- Machado, G.U.; Prates, F.V.; Machado, P.R.L. Disseminated leishmaniasis: Clinical, pathogenic, and therapeutic aspects. An. Bras. Dermatol. 2019, 94, 9–16. [Google Scholar] [CrossRef]
- Ziaie, H.; Sadeghian, G. Isolation of bacteria causing secondary bacterial infection in the lesions of Cutaneous Leishmaniasis. Indian J. Dermatol. 2008, 53, 129–131. [Google Scholar] [CrossRef]
- Layegh, P.; Ghazvini, K.; Moghiman, T.; Hadian, F.; Zabolinejad, N.; Pezeshkpour, F. Bacterial contamination in cutaneous leishmaniasis: Its effect on the lesions’ healing course. Indian J. Dermatol. 2015, 60, 211. [Google Scholar] [CrossRef] [PubMed]
- Jebran, A.F.; Schleicher, U.; Steiner, R.; Wentker, P.; Mahfuz, F.; Stahl, H.C.; Amin, F.M.; Bogdan, C.; Stahl, K.W. Rapid healing of cutaneous leishmaniasis by high-frequency electrocauterization and hydrogel wound care with or without DAC N-055: A randomized controlled phase IIa trial in Kabul. PLoS Neglected Trop. Dis. 2014, 8, e2694. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, S.; Costa, C.H.N. Treatment of cutaneous leishmaniasis with thermotherapy in Brazil: An efficacy and safety study. An. Bras. Dermatol. 2018, 93, 347–355. [Google Scholar] [CrossRef]
- Linquest, L.A.; Hickham, L.C.; Richardson, B.J.; Hickham, P.R. Successful Treatment of Cutaneous Leishmaniasis With Cryotherapy. Cureus 2023, 15, e41871. [Google Scholar] [CrossRef]
- Lopez-Carvajal, L.; Cardona-Arias, J.A.; Zapata-Cardona, M.I.; Sanchez-Giraldo, V.; Velez, I.D. Efficacy of cryotherapy for the treatment of cutaneous leishmaniasis: Meta-analyses of clinical trials. BMC Infect. Dis. 2016, 16, 360. [Google Scholar] [CrossRef]
- Giannini, S.H. Effects of ultraviolet B irradiation on cutaneous leishmaniasis. Parasitol. Today 1992, 8, 44–48. [Google Scholar] [CrossRef]
- Moskowitz, P.F.; Kurban, A.K. Treatment of cutaneous leishmaniasis: Retrospectives and advances for the 21st century. Clin. Dermatol. 1999, 17, 305–315. [Google Scholar] [CrossRef]
- Kharfi, M.; Benmously, R.; El Fekih, N.; Daoud, M.; Fitouri, Z.; Mokhtar, I.; Ben Becher, S.; Kamoun, M.R. Childhood leishmaniasis: Report of 106 cases. Dermatol. Online J. 2004, 10, 6. [Google Scholar] [CrossRef]
- Cabral, F.V.; Pelegrino, M.T.; Seabra, A.B.; Ribeiro, M.S. Nitric-oxide releasing chitosan nanoparticles towards effective treatment of cutaneous leishmaniasis. Nitric Oxide 2021, 113–114, 31–38. [Google Scholar] [CrossRef]
- El-On, J.; Jacobs, G.P.; Witztum, E.; Greenblatt, C.L. Development of topical treatment for cutaneous leishmaniasis caused by Leishmania major in experimental animals. Antimicrob. Agents Chemother. 1984, 26, 745–751. [Google Scholar] [CrossRef] [PubMed]
- El-On, J.; Livshin, R.; Even-Paz, Z.; Hamburger, D.; Weinrauch, L. Topical treatment of cutaneous leishmaniasis. J. Invest. Dermatol. 1986, 87, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Ben Salah, A.; Ben Messaoud, N.; Guedri, E.; Zaatour, A.; Ben Alaya, N.; Bettaieb, J.; Gharbi, A.; Belhadj Hamida, N.; Boukthir, A.; Chlif, S.; et al. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N. Engl. J. Med. 2013, 368, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Noursadeghi, M.; Boyle, J.; Davidson, R.N. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br. J. Dermatol. 2005, 153, 203–205. [Google Scholar] [CrossRef] [PubMed]
- del Rosal, T.; Artigao, F.B.; Miguel, M.J.; de Lucas, R.; del Castillo, F. Successful treatment of childhood cutaneous leishmaniasis with liposomal amphotericin B: Report of two cases. J. Trop. Pediatr. 2010, 56, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Pavlotsky, F.; Leshem, E.; Ephros, M.; Trau, H.; Schwartz, E. Liposomal amphotericin B treatment of cutaneous leishmaniasis due to Leishmania tropica. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 973–977. [Google Scholar] [CrossRef]
- Horev, A.; Sagi, O.; Zur, E.; Ben-Shimol, S. Topical liposomal amphotericin B gel treatment for cutaneous leishmaniasis caused by Leishmania major: A double-blind, randomized, placebo-controlled, pilot study. Int. J. Dermatol. 2023, 62, 40–47. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).