The Association of Serotonin Toxicity with Combination Linezolid–Serotonergic Agent Therapy: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process and Data Collection Process
2.5. Data Items
2.6. Study Risk of Bias Assessment
2.7. Effect Measures
2.8. Synthesis Methods
2.9. Reporting Bias Assessment
2.10. Certainty Assessment
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Title |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | p. 3 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | p. 3 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | p. 4 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | p. 4 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | p. 4 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | p. 5 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | p. 5 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | p. 4 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | p. 5 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | p. 5 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | p. 5 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | p. 5 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | p. 5 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Table 1 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | p. 5 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | p. 5 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | p. 5 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | p. 5 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | p. 5 |
| RESULTS | |||
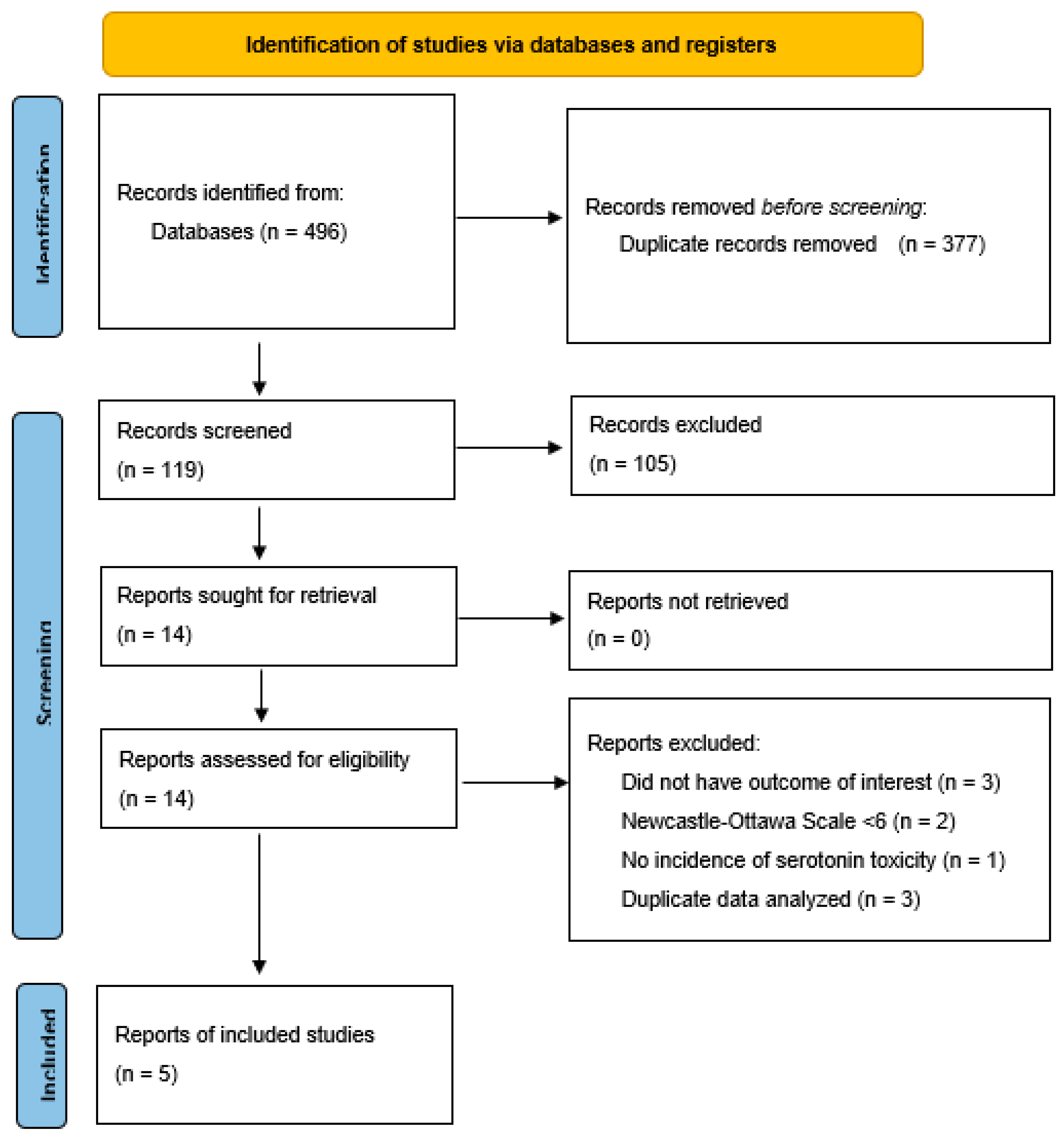
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | p. 6 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | p. 6, Table 1 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Appendix A Table A2 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Table 1 |
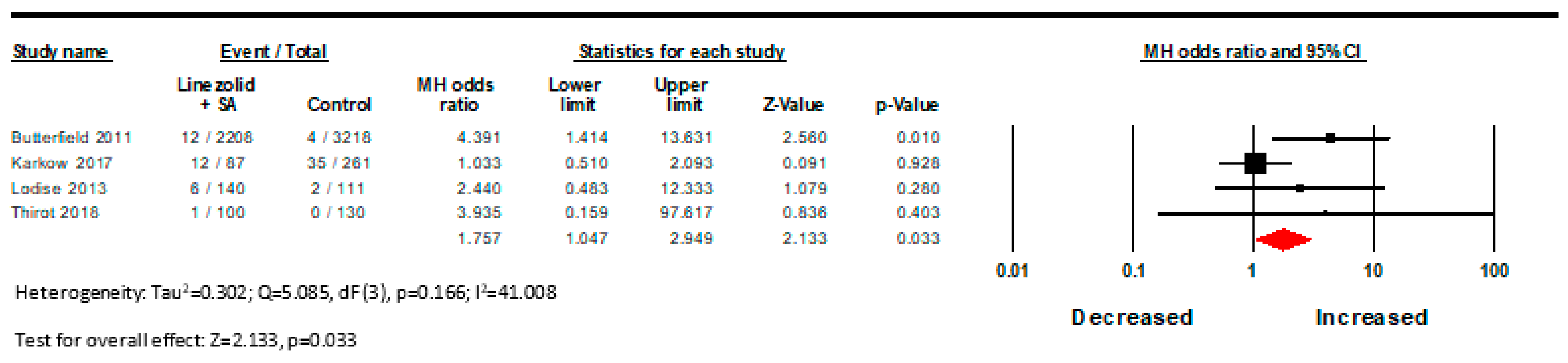
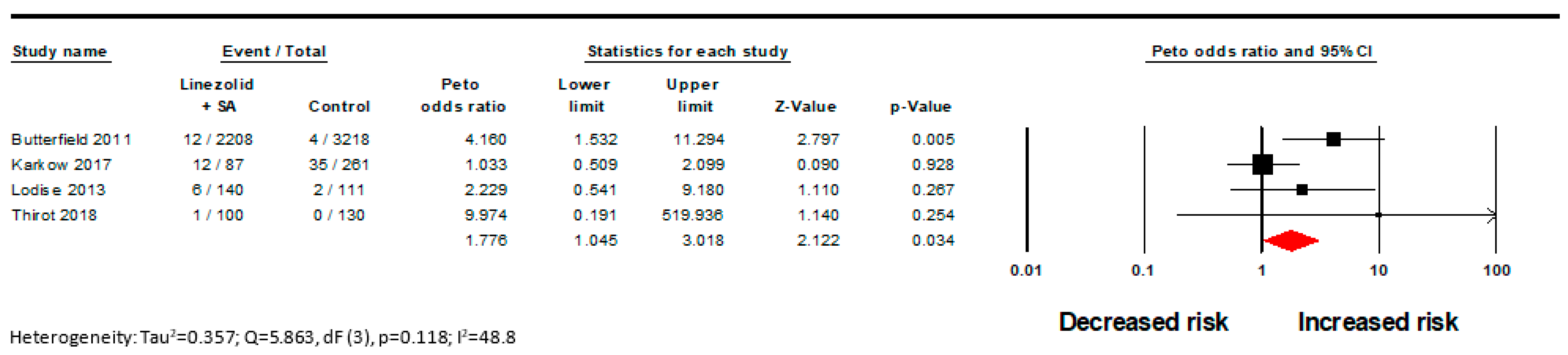
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | p. 6, Table 1 and Table 2, Figure 2 and Figure 3 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | p. 6, Figure 2 and Figure 3 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | p. 6, Figure 2 and Figure 3 | |
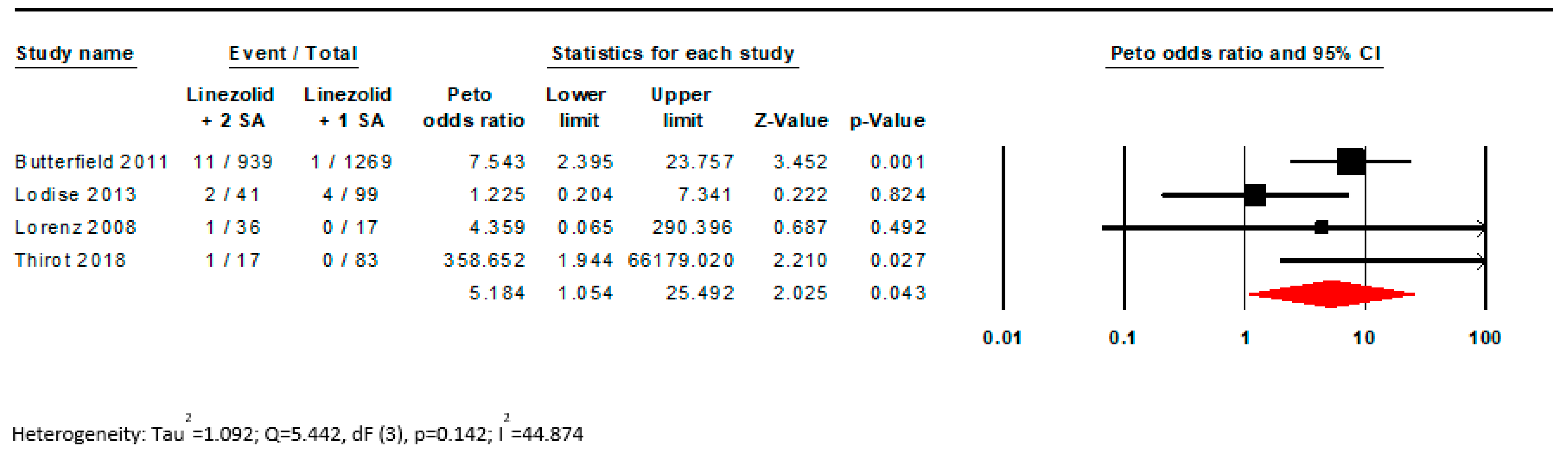
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | p. 6, Appendix A Figure A1 and Figure A2 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | n/a |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | p. 6, p. 7 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | p. 7, p. 8 |
| 23b | Discuss any limitations of the evidence included in the review. | p. 7, p. 8 | |
| 23c | Discuss any limitations of the review processes used. | p. 7, p. 8 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | p. 7, p. 8 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | p. 4 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | p. 4 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | n/a | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | p. 5 |
| Competing interests | 26 | Declare any competing interests of review authors. | p. 9 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | n/a |
| Domain | Butterfield 2011 [7] | Gatti 2020 [5] | Karkow 2017 [10] | Lodise 2013 [11] | Lorenz 2008 [12] | Quinn 2009 [6] | Thirot 2018 [13] | |
|---|---|---|---|---|---|---|---|---|
| Selection | Representativeness/case definition | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Selection of nonexposed/cases | 1 | 0 | 1 | 1 | 1 | 0 | 1 | |
| Ascertainment of exposure/selection of controls | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Baseline assessment/definition of controls | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Comparability | Confounders identified | 1 | 0 | 1 | 1 | 1 | 0 | 1 |
| Statistical adjustment | 0 | 0 | 0 | 1 | 0 | 1 | 1 | |
| Outcome/exposure | Outcome/exposure assessment | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Follow-up/method for ascertainment of exposure | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Adequacy of follow-up/nonresponse rate | 1 | 0 | 1 | 1 | 1 | 0 | 1 | |
| Total Score | 8 | 5 | 8 | 9 | 7 | 5 | 7 |
References
- Stalker, D.J.; Jungbluth, G.L. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin. Pharmacokinet. 2003, 42, 1129–1140. [Google Scholar] [CrossRef]
- Pea, F. Intracellular pharmacokinetics of antibacterials and their clinical implications. Clin. Pharmacokinet. 2018, 57, 177–189. [Google Scholar] [CrossRef]
- Pfizer. Linezolid [Package Insert]. Revised 9/2013. Available online: https://www.fda.gov/ (accessed on 1 January 2020).
- Food and Drug Administration. FDA Drug Safety Communication: Updated Information about the Drug Interaction between Linezolid (Zyvox) and Serotonergic Psychiatric Medications. Available online: www.fda.gov/Drugs/DrugSafety/ucm276251.htm (accessed on 1 March 2021).
- Gatti, M.; Raschi, E.; De Ponti, F. Serotonin syndrome by drug interactions with linezolid: Clues from pharmacovigilance-pharmacokinetic/pharmacodynamic analysis. Eur. J. Clin. Pharmacol. 2020, 77, 233–239. [Google Scholar] [CrossRef]
- Quinn, D.K.; Stern, T.A. Linezolid and serotonin syndrome. Prim. Care Companion J. Clin. Psychiatry 2009, 11, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.M.; Lawrence, K.R.; Reisman, A.; Huang, D.B.; Thompson, C.A.; Lodise, T.P. Comparison of serotonin toxicity with concomitant use of either linezolid or comparators and serotonergic agents: An analysis of Phase III and IV randomized clinical trial data. J. Antimicrob. Chemother. 2012, 67, 494–502. [Google Scholar] [CrossRef]
- Clarke, C.; Finnegan, M.; O’dwyer, A.M.; Mc Donald, C.; O’connell, B.; Cooney, J. Co-prescribing of linezolid and serotonergic agents in a general hospital setting. Ir. J. Psychol. Med. 2014, 31, 191–193. [Google Scholar] [CrossRef]
- Go, A.C.; Golightly, L.K.; Barber, G.R.; Barron, M.A. Linezolid interaction with serotonin reuptake inhibitors: Report of two cases and incidence assessment. Drug Metab. Drug Interact. 2010, 25, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Karkow, D.C.; Kauer, J.F.; Ernst, E.J. Incidence of serotonin syndrome with combined use of linezolid and serotonin reuptake inhibitors compared with linezolid monotherapy. J. Clin. Psychopharmacol. 2017, 37, 518–523. [Google Scholar] [CrossRef]
- Lodise, T.P.; Patel, N.; Rivera, A.; Tristani, L.; Lazariu, V.; Vandewall, H.; McNutt, L.A. Comparative evaluation of serotonin toxicity among veterans affairs patients receiving linezolid and vancomycin. Antimicrob. Agents Chemother. 2013, 57, 5901–5911. [Google Scholar] [CrossRef]
- Lorenz, R.A.; Vandenberg, A.M.; Canepa, E.A. Serotonergic antidepressants and linezolid: A retrospective chart review and presentation of cases. Int. J. Psychiatry Med. 2008, 38, 81–90. [Google Scholar] [CrossRef]
- Thirot, H.; Holemans, X.; Jacobs, F.; Briquet, C.; Frippiat, F.; Spinewine, A.; Van Bambeke, F.; Tulkens, P. Is the risk of linezolid to cause serotonin syndrome real in routine clinical practice? In Proceedings of the International Society of Pharmacovigilance 18th Annual Meeting, Geneva, Switzerland, 11–14 November 2018.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 July 2023).
- Balduzzi, S.; Rucker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid.-Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 19 October 2009).
- Melsen, W.G.; Bootsma, M.C.J.; Rovers, M.M.; Bonten, M.J.M. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 2014, 20, 123–129. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2009, 1, 97–111. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Rind, D.; Devereaux, P.J.; Montori, V.M.; Freyschuss, B.; Vist, G.; et al. GRADE guidelines 6. Rating the quality of evidence—Imprecision. J. Clin. Epidemiol. 2011, 64, 1283–1293. [Google Scholar] [CrossRef]
- Efthimiou, O. Practical guide to the meta-analysis of rare events. Evid.-Based Ment. Health 2018, 21, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Serghiou, S.; Goodman, S.N. Random-effects meta-analysis: Summarizing evidence with caveats. JAMA 2019, 321, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Updated February 2021; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 1 July 2023).
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; updated February 2022; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 1 July 2023).
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October 2013; The GRADE Working Group. 2013. Available online: www.guidelinedevelopment.org/handbook (accessed on 1 August 2023).
- McMaster University and Evidence Prime. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. 2022. Available online: www.gradepro.org (accessed on 1 August 2023).
- Huang, V.; Gortney, J.S. Risk of serotonin syndrome with concomitant administration of linezolid and serotonin agonists. Pharmacotherapy 2006, 26, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.R.; Adra, M.; Gillman, P.K. Serotonin toxicity associated with the use of linezolid: A review of postmarketing data. Clin. Infect. Dis. 2006, 42, 1578–1583. [Google Scholar] [CrossRef]
- Ramsey, T.D.; Lau, T.T.; Ensom, M.H. Serotonergic and adrenergic drug interactions associated with linezolid: A critical review and practical management approach. Ann. Pharmacother. 2013, 47, 543–560. [Google Scholar] [CrossRef]
- Gun, Z.U.; Bahcecioglu, O.; Gok, S. Linezolid drug interactions: A retrospective study. Med. Sci. 2020, 320, 9190. [Google Scholar] [CrossRef]
- Taylor, J.J.; Wilson, J.W.; Estes, L.L. Linezolid and serotonergic drug interactions: A retrospective survey. Clin. Infect. Dis. 2006, 43, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, E.; Isbister, G.; Sibbritt, D.; Dawson, A.; Whyte, I. The hunter serotonin toxicity criteria: Simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003, 96, 635–642. [Google Scholar] [CrossRef]
- Martin, J.P.; Herberg, J.T.; Slatter, J.G.; Dupuis, M.J. Although a novel microtiter-plate assay demonstrates that linezolid (PBU-100766) is a weak, competitive (reversible) inhibitor of human monoamine oxidase (MAO A), no clinical evidence of MAO A inhibition in clinical trials has been observed. In Proceedings of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 24–27 September 1998; p. 27. [Google Scholar]
- Humphrey, S.J.; Curry, J.T.; Turman, C.N.; Stryd, R.P. Cardiovascular sympathomimetic amine interactions in rats treated with monoamine oxidase inhibitors and the novel oxazolidinone antibiotic linezolid. J. Cardiovasc. Pharmacol. 2001, 37, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, P.E.; Antal, E.J.; Welshman, I.R.; Batts, D.H.; Hopkins, N.K. Linezolid: Pharmacokinetic and pharmacodynamic evaluation of coadministration with pseudoephedrine HCl phenylpropanolamine HCl, and dextromethorphan HBr. J. Clin. Pharmacol. 2001, 41, 563–572. [Google Scholar] [CrossRef]
- MacGowan, A.P. Pharmacokinetic and pharmacodynamics profile of linezolid in healthy volunteers and patients with Gram-positive infections. J. Antimicrob. Chemother. 2003, 51, ii17–ii25. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Country | Setting | Design | Definition of Exposure | Definition of Outcome | ST Rate, Cases per 1000 Patients (n/N) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| LZD + SA | LZD + no SA | LZD + 1 SA | LZD + >1 SA | |||||||
| Butterfield et al. [7] | 2011 | United States | Phase III/IV RCTs | Retrospective cohort | Received any SA | Satisfy HSTC * or Sternbach criteria | 5.4 (12/2208) | 1.2 (4/3218) | 0.8 (1/1269) | 11.7 11/939 |
| Karkow et al. [10] | 2017 | United States | Inpatients, University of Iowa Hospitals and Clinics | Retrospective matched cohort | Received LZD with or within 14 days of SA | Satisfy HSTC or Sternbach criteria | 138 (12/87) | 134 (35/261) | - | - |
| Lodise et al. [11] | 2013 | United States | Inpatients, VISN-2 † | Retrospective matched cohort | At least 1 LZD dose + SA from −35 to + 7 days post treatment | Satisfy HSTC or Sternbach criteria | 42.8 (6/140) | 18 (2/111) | 40 (4/99) | 49 (2/41) |
| Lorenz et al. [12] | 2008 | United States | Inpatients, MUSC ‡ | Retrospective cohort | SA concurrent or within 14 days of LZD | HSTC or surrogate signs/symptoms | 18.9 (1/53) | - | 0 (0/17) | 27.8 (1/36) |
| Thirot et al. [13] | 2018 | Belgium | Inpatients,4 hospital centers | Retrospective cohort | LZD + SA | Not reported | 10 (1/100) | 0 (0/130) | 0 (0/83) | 58.8 (1/17) |
| Pooled incidence | 12.3 (32/2588) | 11 (41/3720) | 3.4 (5/1468) | 14.5 (15/1033) | ||||||
| Certainty Assessment | # of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | [Intervention] | [Comparison] | Relative (95% CI) | Absolute (95% CI) | |
| Serotonin Toxicity in LZD + SA versus LZD alone | |||||||||||
| 4 | Observational studies | Not serious | Not serious | Not serious | Serious a | All plausible residual confounding would suggest spurious effect, while no effect was observed | 31/2535 (1.2%) | 41/3720 (1.1%) | OR 1.750 (1.047 to 2.949) | 8 more per 1000 (from 1 more to 21 more) | ⨁⨁◯◯ Low |
| Serotonin Toxicity with LZD + 1 SA versus LZD + multiple SA | |||||||||||
| 4 | Observational studies | Not serious | Not serious | Not serious | Serious a | Strong association all plausible residual confounding would suggest spurious effect, while no effect was observed | 15/1015 (1.5%) | 5/1486 (0.3%) | OR 6.770 (2.240 to 20.447) | 19 more per 1000 (from 4 more to 61 more) | ⨁⨁⨁◯ Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
SanFilippo, S.; Turgeon, J.; Michaud, V.; Nahass, R.G.; Brunetti, L. The Association of Serotonin Toxicity with Combination Linezolid–Serotonergic Agent Therapy: A Systematic Review and Meta-Analysis. Pharmacy 2023, 11, 182. https://doi.org/10.3390/pharmacy11060182
SanFilippo S, Turgeon J, Michaud V, Nahass RG, Brunetti L. The Association of Serotonin Toxicity with Combination Linezolid–Serotonergic Agent Therapy: A Systematic Review and Meta-Analysis. Pharmacy. 2023; 11(6):182. https://doi.org/10.3390/pharmacy11060182
Chicago/Turabian StyleSanFilippo, Savanna, Jacques Turgeon, Veronique Michaud, Ronald G. Nahass, and Luigi Brunetti. 2023. "The Association of Serotonin Toxicity with Combination Linezolid–Serotonergic Agent Therapy: A Systematic Review and Meta-Analysis" Pharmacy 11, no. 6: 182. https://doi.org/10.3390/pharmacy11060182
APA StyleSanFilippo, S., Turgeon, J., Michaud, V., Nahass, R. G., & Brunetti, L. (2023). The Association of Serotonin Toxicity with Combination Linezolid–Serotonergic Agent Therapy: A Systematic Review and Meta-Analysis. Pharmacy, 11(6), 182. https://doi.org/10.3390/pharmacy11060182









