Improving Asthma Management: Patient–Pharmacist Partnership Program in Enhancing Therapy Adherence
Abstract
1. Introduction
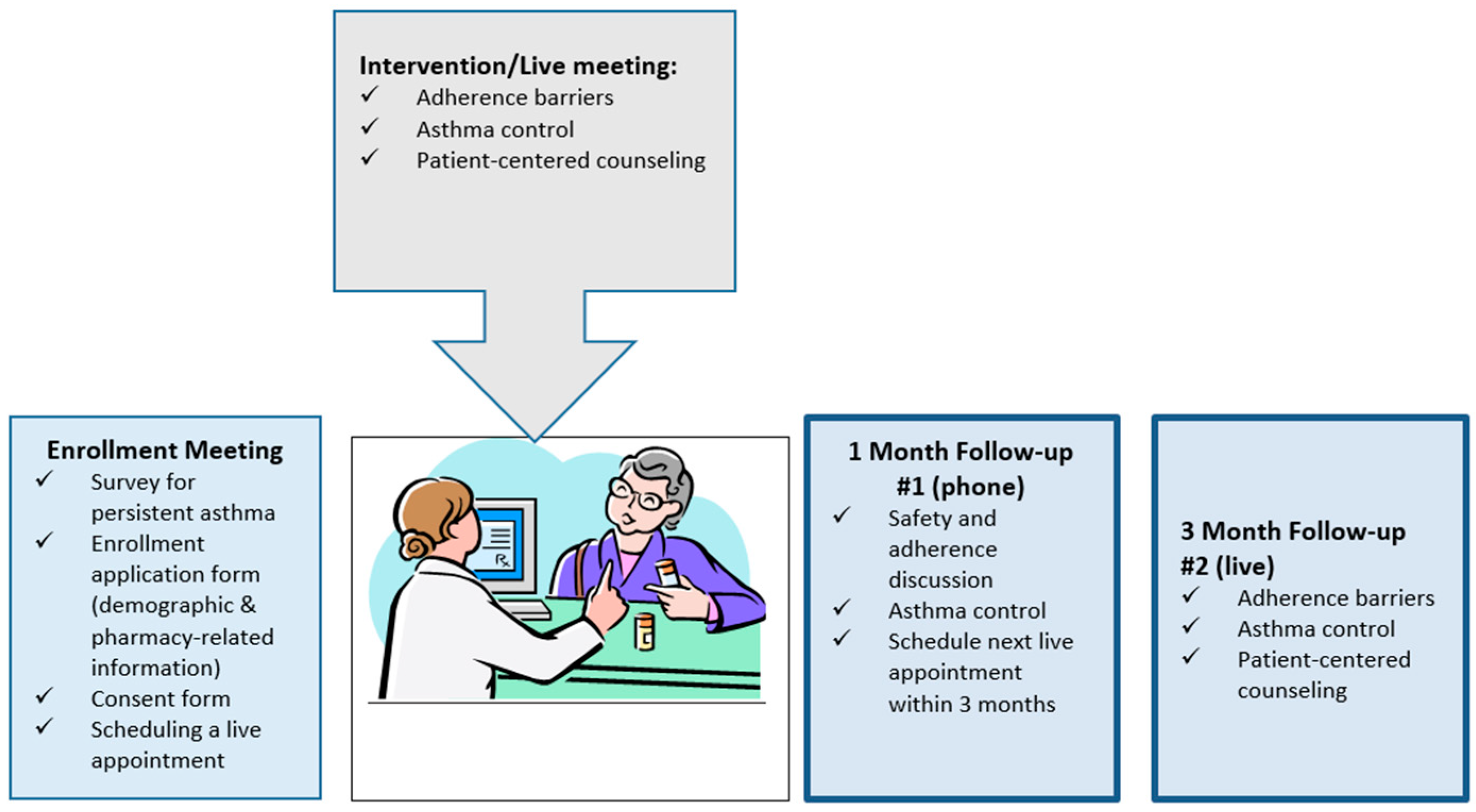
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Survey #1: Your Asthma Experiences
| Note: Inhaler below Refers to CONTROLLER Inhaler | Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree |
| I just forget to use my inhaler some of the time | ☐ | ☐ | ☐ | ☐ | ☐ |
| I run out of my inhaler because I don’t get refills on time | ☐ | ☐ | ☐ | ☐ | ☐ |
| Using my inhaler more than once a day is inconvenient | ☐ | ☐ | ☐ | ☐ | ☐ |
| I feel confident that my inhaler will help me | ☐ | ☐ | ☐ | ☐ | ☐ |
| I know how to use my inhaler correctly | ☐ | ☐ | ☐ | ☐ | ☐ |
| I have an Asthma Action Plan and know if I am reaching my goals | ☐ | ☐ | ☐ | ☐ | ☐ |
| I have someone I can call with questions about my inhaler | ☐ | ☐ | ☐ | ☐ | ☐ |
| My doctor/nurse and I work together to make decisions | ☐ | ☐ | ☐ | ☐ | ☐ |
| I only use my inhaler when I am having symptoms such as shortness of breath, coughing, wheezing, or chest tightness | ☐ | ☐ | ☐ | ☐ | ☐ |
| Have you… | In the last week | In the last month | In the last 3 months | >3 months ago | Never |
| Used your inhaler more or less often than prescribed? | ☐ | ☐ | ☐ | ☐ | ☐ |
| Skipped or stopped using your inhaler because you didn’t think it was working? | ☐ | ☐ | ☐ | ☐ | ☐ |
| Skipped or stopped using your inhaler because it made you feel bad? | ☐ | ☐ | ☐ | ☐ | ☐ |
| Skipped, stopped, not refilled, or used less of inhaler because of the cost? | ☐ | ☐ | ☐ | ☐ | ☐ |
| Not had your inhaler with you when it was time to use it? | ☐ | ☐ | ☐ | ☐ | ☐ |
| Every day | Once a week | Once a month | When asthma worsens | I don’t have one | |
| How often do you use your peak flow meter? | ☐ | ☐ | ☐ | ☐ | ☐ |
| ☐ Hypertension | ☐ Dyslipidemia | ☐ Diabetes | ☐ Depression |
| ☐ Chronic Pain | ☐ Gastrointestinal disorder | ☐ Thyroid disorder | ☐ Heart disease |
| ☐ Arthritis | ☐ Obesity | ☐ Other, Please specify _______________________ | |
References
- Tan, H.; Sarawate, C.; Singer, J.; Elward, K.; Cohen, R.I.; Smart, B.A.; Busk, M.F.; Lustig, J.; O’Brien, J.D.; Shatz, M. Impact of asthma controller medications on clinical, economic, and patient-reported outcomes. Mayo Clin. Proc. 2009, 84, 675–684. [Google Scholar] [CrossRef]
- Van Dijk, B.C.P.; Svedsater, H.; Heddini, A.; Nelsen, L.; Balradj, J.S.; Alleman, C. Relationship between the Asthma Control Test (ACT) and other outcomes: A targeted literature review. BMC Pulm. Med. 2020, 79. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Health Outcomes. Available online: https://www.cdc.gov/asthma/data-visualizations/health-outcomes.htm (accessed on 20 January 2022).
- Barnes, C.B.; Ulrik, C.S. Asthma and Adherence to Inhaled Corticosteroids: Current Status and Future Perspectives. Respir. Care 2015, 60, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.; Stevenson, M.; McClean, E.; Heaney, L.G. The prevalence of nonadherence in difficult asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Oppenheimer, J.J. Medication adherence in the asthmatic child and adolescent. Curr. Allergy Asthma Rep. 2011, 11, 454–464. [Google Scholar] [CrossRef]
- Jentzsch, N.S.; Camargos, P.A.; Colosimo, E.A.; Bousquet, J. Monitoring adherence to beclomethasone in asthmatic children and adolescents through four different methods. Allergy 2009, 64, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.G.; Bender, S.E. Patient-identified barriers to asthma treatment adherence: Responses to interviews, focus groups, and questionnaires. Immunol. Allergy Clin. North. Am. 2005, 25, 107–130. [Google Scholar] [CrossRef]
- Jácome, C.; Almeida, R.; Pereira, A.M.; Amaral, R.; Vieira-Marques, P.; Mendes, S.; Alves-Correia, M.; Ferreira, J.A.; Lopes, I.; Gomes, J.; et al. Monitoring adherence to asthma inhalers using the InspirerMundi app: Analysis of real-world, medium-term feasibility studies. Front Med. Technol. 2021, 3. [Google Scholar] [CrossRef]
- Bozek, A.; Jarzab, J. Adherence to asthma therapy in elderly patients. J. Asthma. 2010, 47, 162–165. [Google Scholar] [CrossRef]
- Howell, G. Nonadherence to medical therapy in asthma: Risk factors, barriers, and strategies for improving. J. Asthma. 2008, 45, 723–729. [Google Scholar] [CrossRef]
- Ponieman, D.; Wisnivesky, J.P.; Leventhal, H.; Musumeci-Szabó, T.J.; Halm, E.A. Impact of positive and negative beliefs about inhaled corticosteroids on adherence in inner-city asthmatic patients. Ann. Allergy Asthma Immunol. 2009, 103, 38–42. [Google Scholar] [CrossRef]
- Gatwood, J.; Bailey, J.E. Improving medication adherence in hypercholesterolemia: Challenges and solutions. Vasc. Health Risk Manag. 2014, 10, 615–625. [Google Scholar] [CrossRef] [PubMed]
- García-Cárdenas, V.; Sabater-Hernández, D.; Kenny, P.; Martínez-Martínez, F.; Faus, M.J.; Benrimoj, S.I. Effect of a pharmacist intervention on asthma control. A cluster randomised trial. Respir. Med. 2013, 107, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Armour, C.; Bosnic-Antisevich, S.; Brillant, M.; Burton, D.; Emmerton, L.; Krass, I.; Saini, B.; Smith, L.; Stewart, K. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax. 2007, 62, 496–502. [Google Scholar] [CrossRef]
- Goeman, D.; Jenkins, C.; Crane, M.; Paul, E.; Douglas, J. Educational intervention for older people with asthma: A randomised controlled trial. Patient Educ. Couns. 2013, 93, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Serhal, S.; Saini, B.; Bosnic-Anticevich, S.; Krass, I.; Emmerton, L.; Bereznicki, B.; Bereznicki, L.; Weier, N.; Mitchell, B.; Wilson, F.; et al. A Novel Multi-Mode Education Program to Enhance Asthma Care by Pharmacists. Am. J. Pharm. Educ. 2021, 16. [Google Scholar] [CrossRef] [PubMed]
- Witry, M.J.; Doucette, W.R.; Zhang, Y.; Farris, K.B. Multiple Adherence Tool Evaluation Study (MATES). J. Manag. Care Pharm. 2014, 20, 734–740. [Google Scholar] [CrossRef]
- Giraud, V.; Allaert, F.A.; Roche, N. Inhaler technique and asthma: Feasability and acceptability of training by pharmacists. Respir. Med. 2011, 105, 1815–1822. [Google Scholar] [CrossRef]
- Saini, B.; LeMay, K.; Emmerton, L.; Krass, I.; Smith, L.; Bosnic-Anticevich, S.; Stewart, K.; Burton, D.; Armour, C. Asthma disease management-Australian pharmacists’ interventions improve patients’ asthma knowledge and this is sustained. Patient Educ. Couns. 2011, 83, 295–302. [Google Scholar] [CrossRef]
- Ulrik, C.S.; Backer, V.; Søes-Petersen, U.; Lange, P.; Harving, H.; Plaschke, P.P. The patient’s perspective: Adherence or non-adherence to asthma controller therapy? J. Asthma. 2006, 43, 701–704. [Google Scholar] [CrossRef]
- Raynor, D.K.; Savage, I.; Knapp, P.; Henley, J. We are the experts: People with asthma talk about their medicine information needs. Patient Educ. Couns. 2004, 53, 167–174. [Google Scholar] [CrossRef]
- Schatz, M.; Kosinski, M.; Yarlas, A.S.; Hanlon, J.; Watson, M.E.; Jhingran, P. The minimally important difference of the Asthma Control Test. J. Allergy Clin. Immunol. 2009, 124, 719–723. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Sorkness, C.A.; Li, J.T.; Marcus, P.; Murray, J.J.; Nathan, R.A.; Kosinski, M.; Pendergraft, T.B.; Jhingran, P. Asthma control test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J. Allergy Clin. Immunol. 2006, 117, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Matza, L.S.; Park, J.; Coyne, K.S.; Skinner, E.P.; Malley, K.G.; Wolever, R.Q. Derivation and validation of the ASK-12 adherence barrier survey. Ann. Pharmacother. 2009, 43, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Makhinova, T.; Barner, J.C.; Brown, C.M.; Richards, K.M.; Rascati, K.L.; Barnes, J.N.; Nag, A. Adherence enhancement for patients with asthma in community pharmacy practice: Tools development and pharmacists’ feedback. J. Pharm. Health Serv. Res. 2018, 9, 215–226. [Google Scholar] [CrossRef]
- Doucette, W.R.; Farris, K.B.; Youland, K.M.; Newland, B.A.; Egerton, S.J.; Barnes, J.M. Development of the Drug Adherence Work-up (DRAW) tool. J. Am. Pharm. Assoc. 2012, 52, e199–e204. [Google Scholar] [CrossRef]
- Makhinova, T.; Barner, J.C.; Brown, C.M.; Richards, K.M.; Rascati, K.L.; Rush, S.; Nag, A. Examination of barriers to medication adherence, asthma management, and control among community pharmacy patients with asthma. J. Pharm. Pract. 2020, 34, 515–522. [Google Scholar] [CrossRef]
- Gaude, G.S.; Hattiholi, J.; Chaudhury, A. Role of health education and self-action plan in improving the drug compliance in bronchial asthma. J. Family Med. Prim. Care 2014, 3, 33–38. [Google Scholar] [CrossRef]
- Yawn, B.P.; Rank, M.A.; Cabana, M.D.; Wollan, P.C.; Juhn, Y.J. Adherence to asthma guidelines in children, tweens, and adults in primary care settings: A practice-based network assessment. Mayo Clin. Proc. 2016, 91, 411–421. [Google Scholar] [CrossRef]
- Boulet, L.P.; Boulay, M.E.; Guylaine, G.; Battisti, L.; Chabot, V.; Beauchesne, M.; Villeneuve, D.; Côté, P. Benefits of an asthma education program provided at primary care sites on asthma outcomes. Respir. Med. 2015, 109, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Wiener-Ogilvie, S.; Pinnock, H.; Huby, G.; Sheikh, A.; Partridge, M.R.; Gillies, J. Do practices comply with key recommendations of the British Asthma Guideline? If not, why not? Prim. Care Respir. J. 2007, 16, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Coffman, J.M.; Sumino, K.; Cabana, M.D. Patient reminder systems and asthma medication adherence: A systematic review. J. Asthma. 2014, 51, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Halm, E.A.; Mora, P.; Leventhal, H. No symptoms, no asthma: The acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest 2006, 129, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Sofianou, A.; Martynenko, M.; Wolf, M.S.; Wisnivesky, J.P.; Krauskopf, K.; Wilson, E.A.H.; Goel, M.S.; Leventhal, H.; Halm, E.A.; Federman, A.D. Asthma beliefs are associated with medication adherence in older asthmatics. J. Gen. Intern. Med. 2013, 28, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Guenette, L.; Breton, M.C.; Grégoire, J.P.; Jobin, M.; Bolduc, Y.; Boulet, L.; Dorval, E.; Moisan, J. Effectiveness of an asthma integrated care program on asthma control and adherence to inhaled corticosteroids. J. Asthma. 2015, 52, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.D.; Tian, L. Promoting adherence: Effects of theory-based asthma education. Clin. Nurs. Res. 2004, 13, 69–89. [Google Scholar] [CrossRef]
- Price, D.; Bosnic-Anticevich, S.; Briggs, A.; Chrystyn, H.; Rand, C.; Scheuch, G.; Bousquet, J. Inhaler competence in asthma: Common errors, barriers to use and recommended solutions. Respir. Med. 2013, 107, 37–46. [Google Scholar] [CrossRef]
- Young, H.N.; Kanchanasuwan, S.; Cox, E.D.; Moreno, M.M.; Havican, N.S. Barriers to medication use in rural underserved patients with asthma. Res. Soc. Adm. Phar. 2015, 11, 909–914. [Google Scholar] [CrossRef][Green Version]
| Variable | Respondents (N = 17) | Non-Respondents (N = 19) | p-Value | ||
|---|---|---|---|---|---|
| Age (years), mean (SD) | 41.7 | 16.6 | 38.9 | 13.3 | 0.58 |
| N | % | N | % | ||
| Female | 10 | 58.8 | 14 | 73.7 | 0.34 |
| Race/ethnicity | N/A 1 | ||||
| Caucasian | 7 | 41.2 | 12 | 63.2 | |
| African American | 2 | 11.8 | 2 | 10.5 | |
| Hispanic/Latino | 3 | 17.6 | 5 | 26.3 | |
| Other | 5 | 29.4 | - | ||
| Level of education | N/A 1 | ||||
| Primary | - | 1 | 5.3 | ||
| Some high school | 3 | 17.6 | 1 | 5.3 | |
| High school | 3 | 17.6 | 2 | 10.5 | |
| Some college | 5 | 29.4 | 3 | 15.8 | |
| College | 4 | 23.5 | 8 | 42.1 | |
| Postgraduate | 2 | 11.8 | 4 | 21.0 | |
| Chronic condition(s) | N/A 1 | ||||
| None 2 | 6 | 35.3 | 7 | 36.8 | |
| Hypertension | 4 | 23.5 | 6 | 31.6 | |
| Diabetes | 1 | 5.9 | 2 | 10.5 | |
| Dyslipidemia | 1 | 5.9 | 4 | 21.0 | |
| Other 3 | 8 | 47.1 | 11 | 57.9 | |
| ACT, mean (SD) | 15.1 | 3.5 | 19.4 | 3.7 | 0.001 |
| Control level | 0.001 | ||||
| ≤19 (uncontrolled) | 16 | 94.1 | 8 | 42.1 | |
| >19 (controlled) | 1 | 5.9 | 11 | 57.9 | |
| Barrier to adherence score, mean (SD) 4 | 31.2 | 7.2 | 28.7 | 7.9 | 0.33 |
| Number of barriers, mean (SD) | 4.2 | 2.5 | 3.7 | 2.3 | 0.5 |
| Pre Period | Post Period | p-Value 3 | |||
|---|---|---|---|---|---|
| Individual Barriers | Median 1 (IQR) | Barrier Present 2 N (%) | Median 1 (IQR) | Barrier Present2 N (%) | |
| 2.0 (3.0) | 7 (41.2%) | 2.0 (2.0) | 6 (35.3%) | 0.8174 |
| 2.0 (1.0) | 4 (23.5%) | 2.0 (2.0) | 4 (23.5%) | 0.9863 |
| 2.0 (2.0) | 5 (29.4%) | 2.0 (2.0) | 5 (29.4%) | 0.9844 |
| 2.0 (1.0) | 1 (5.9%) | 1.0 (1.0) | 1 (5.9%) | 0.6250 |
| 1.0 (1.0) | 1 (5.9%) | 1.0 (1.0) | 1 (5.9%) | 1.0 |
| 4.0 (1.0) | 9 (52.9%) | 2.0 (1.0) | 3 (17.6%) | 0.0034 |
| 2.0 (1.0) | 3 (17.6%) | 2.0 (0) | 1 (5.9%) | 0.0972 |
| 3.0 (1.0) | 4 (23.5%) | 2.0 (1.0) | 0 (0%) | 0.0010 |
| 2.0 (2.0) | 6 (35.3%) | 2.0 (3.0) | 6 (35.3%) | 0.9922 |
| 3.0 (3.0) | 8 (47.1%) | 1.0 (3.0) | 7 (41.2%) | 0.7500 |
| 1.0 (0) | 1 (5.9%) | 1.0 (0) | 0 (0%) | 0.7500 |
| 1.0 (0) | 0 (0%) | 1.0 (0) | 0(0%) | 1.0 |
| 1.0 (0) | 1 (5.9%) | 1.0 (0) | 0 (0%) | 0.6250 |
| 2.0 (3.0) | 6 (35.3%) | 1.0 (3.0) | 6 (35.3%) | 0.8232 |
| Total Barrier Scale Score 4 | 30.0 (8.0) | 29.0 (10.0) | 0.0530 | ||
 Inconvenience/forgetfulness subscale.
Inconvenience/forgetfulness subscale.  Treatment beliefs subscale.
Treatment beliefs subscale.  Behavior subscale.
Behavior subscale.| Pre Median (IQR) | 3-Month Post Median (IQR) | p-Value 3 | |
|---|---|---|---|
| ACT | 16.0 (3.0) | 18.0 (8.0) | 0.060 |
| Barrier score | |||
| Overall 1 | 30.0 (8.0) | 29.0 (10.0) | 0.053 |
| Subscales 2 | |||
| Behavior | 1.8 (0.8) | 1.6 (1.2) | 0.370 |
| Forgetfulness | 2.3 (1.0) | 2.3 (1.0) | 0.772 |
| Beliefs | 2.3 (0.5) | 2.2 (0.8) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhinova, T.; Barner, J.C.; Brown, C.M.; Richards, K.M.; Rascati, K.L.; Nag, A. Improving Asthma Management: Patient–Pharmacist Partnership Program in Enhancing Therapy Adherence. Pharmacy 2022, 10, 34. https://doi.org/10.3390/pharmacy10010034
Makhinova T, Barner JC, Brown CM, Richards KM, Rascati KL, Nag A. Improving Asthma Management: Patient–Pharmacist Partnership Program in Enhancing Therapy Adherence. Pharmacy. 2022; 10(1):34. https://doi.org/10.3390/pharmacy10010034
Chicago/Turabian StyleMakhinova, Tatiana, Jamie C. Barner, Carolyn M. Brown, Kristin M. Richards, Karen L. Rascati, and Arpita Nag. 2022. "Improving Asthma Management: Patient–Pharmacist Partnership Program in Enhancing Therapy Adherence" Pharmacy 10, no. 1: 34. https://doi.org/10.3390/pharmacy10010034
APA StyleMakhinova, T., Barner, J. C., Brown, C. M., Richards, K. M., Rascati, K. L., & Nag, A. (2022). Improving Asthma Management: Patient–Pharmacist Partnership Program in Enhancing Therapy Adherence. Pharmacy, 10(1), 34. https://doi.org/10.3390/pharmacy10010034






