Ignition and Combustion Characteristics of Aluminum Hydride-Based Kerosene Propellant
Abstract
1. Introduction
2. Experimental Methods
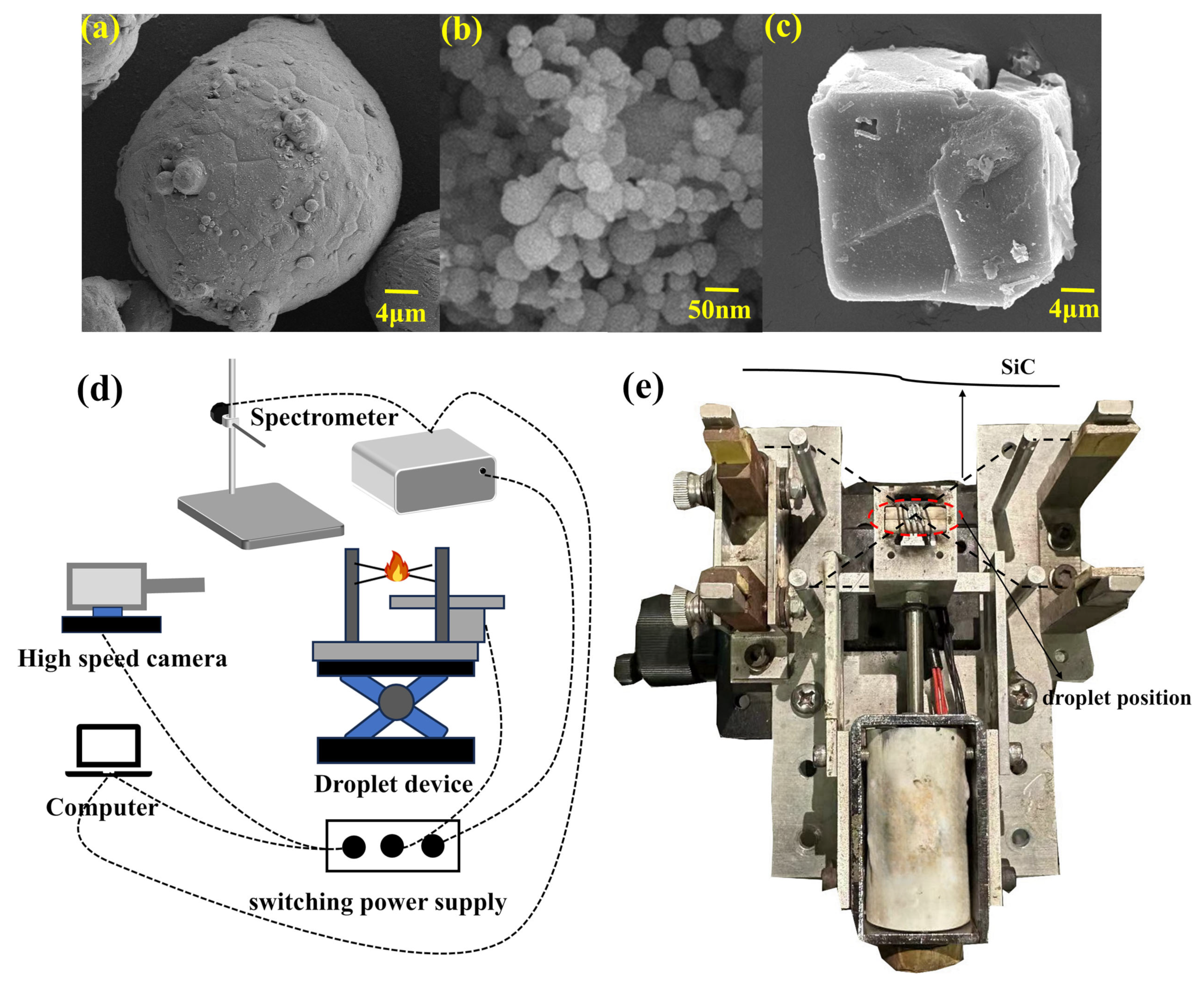
2.1. Materials and Preparation
2.2. Experimental Setup and Procedure
3. Results and Discussion
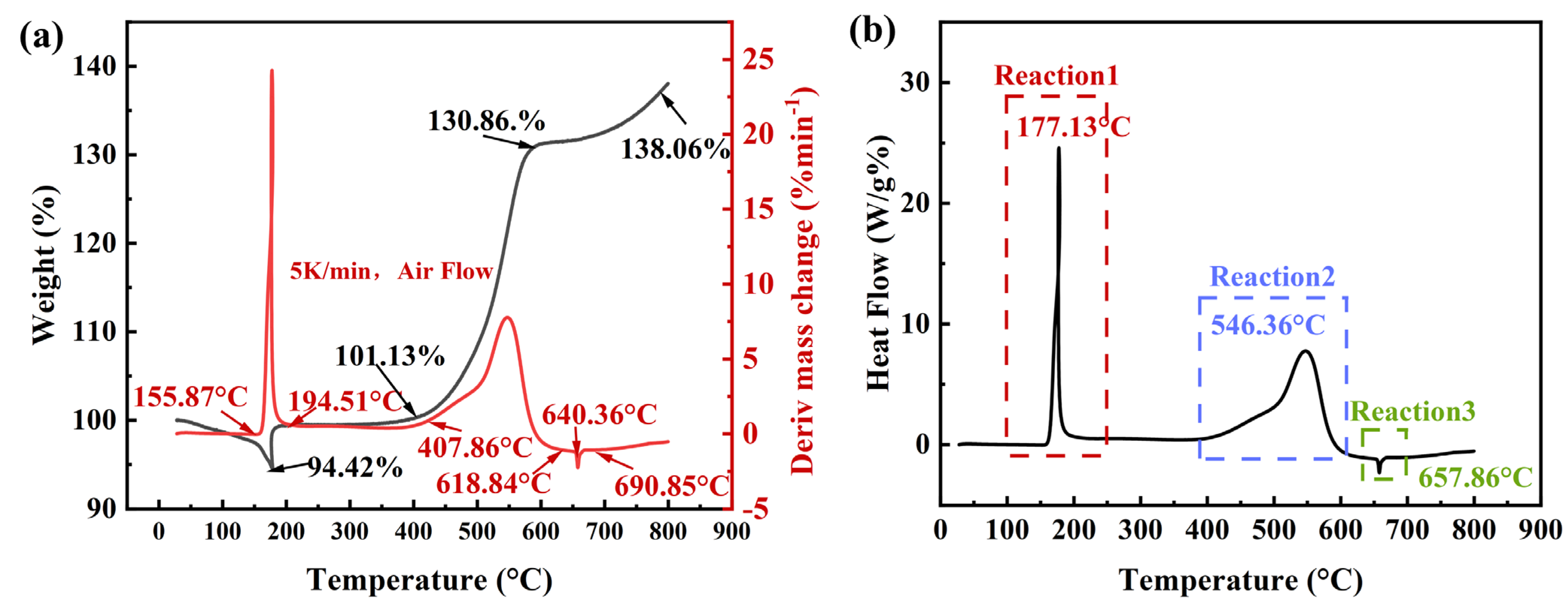
3.1. Thermal Oxidation Characteristic
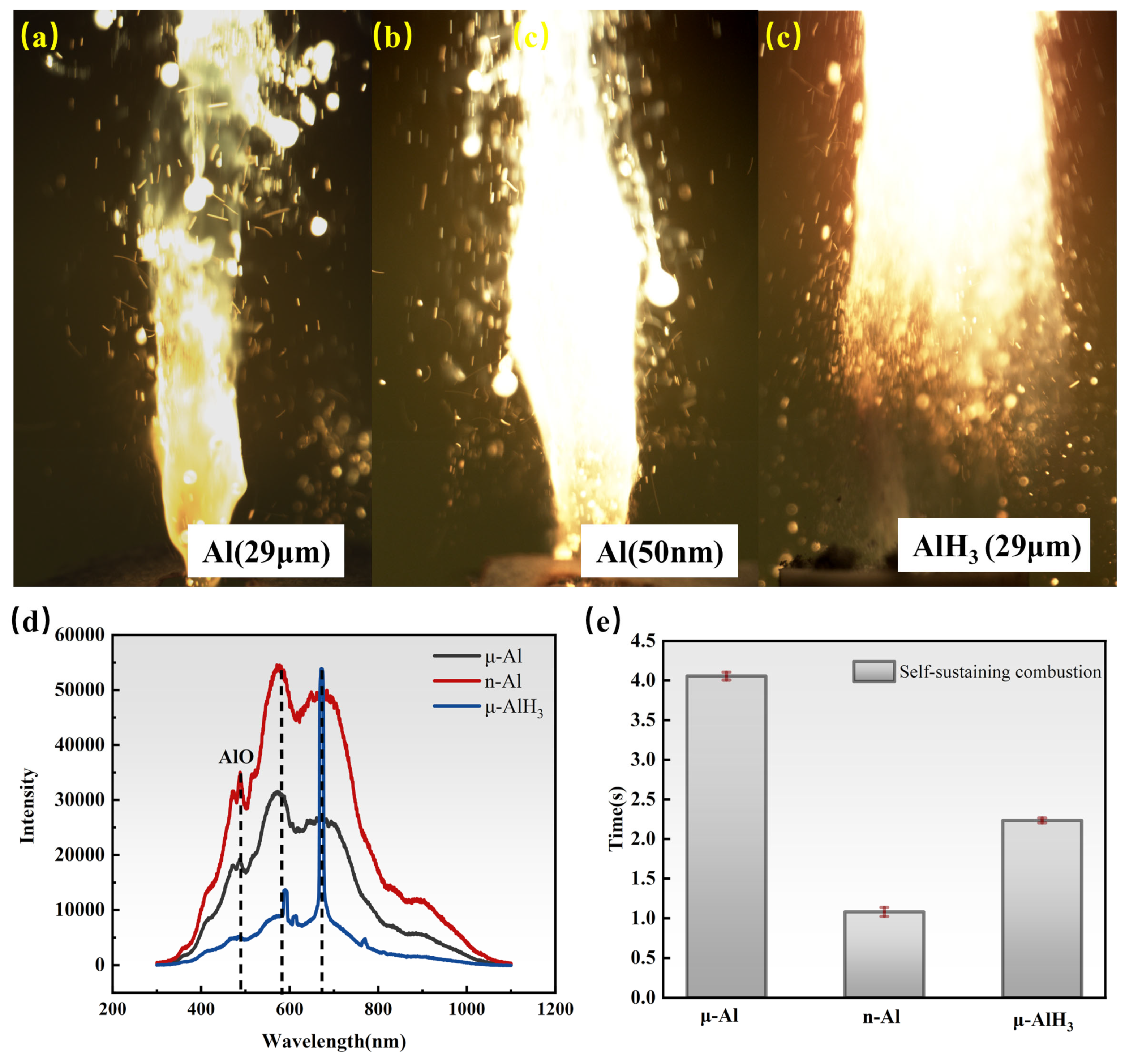
3.2. Laser Ignition Experiment
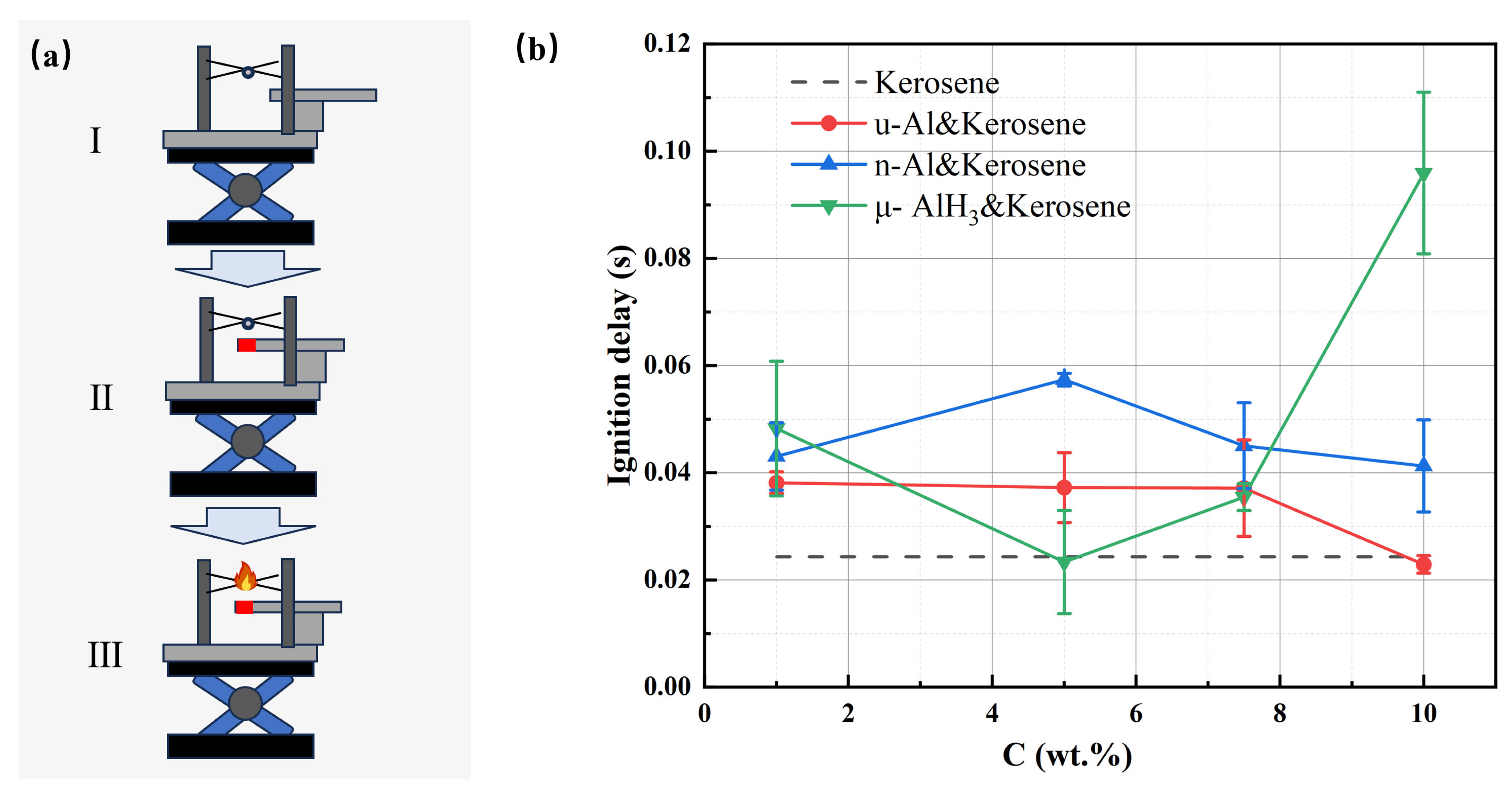
3.3. Droplet Ignition
3.4. Combustion Characteristics of Fuel Droplet
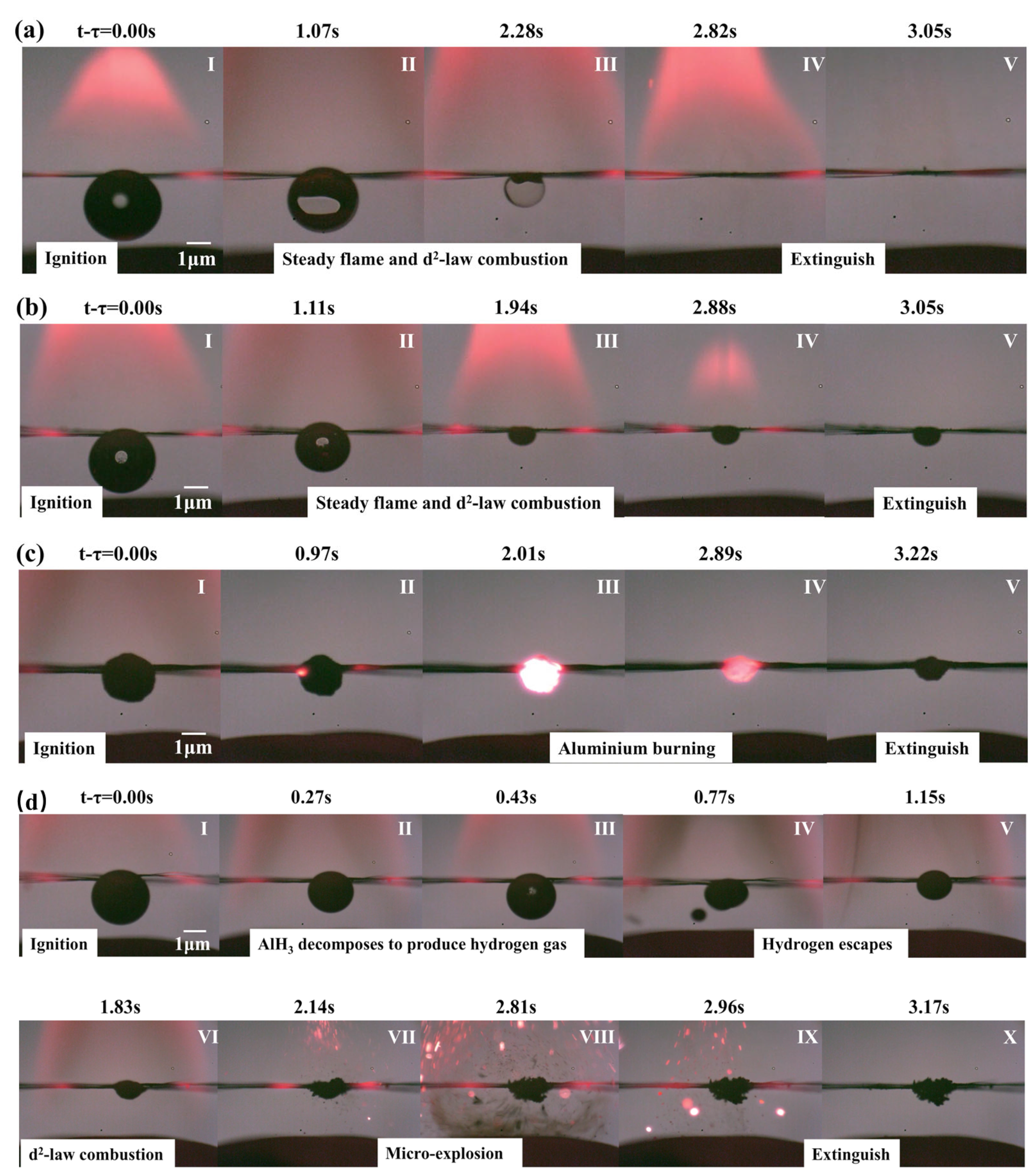
3.4.1. Kerosene Combustion
3.4.2. Combustion of μ-Al&Kerosene and n-Al&Kerosene
3.4.3. Combustion of μ-AlH3&Kerosene
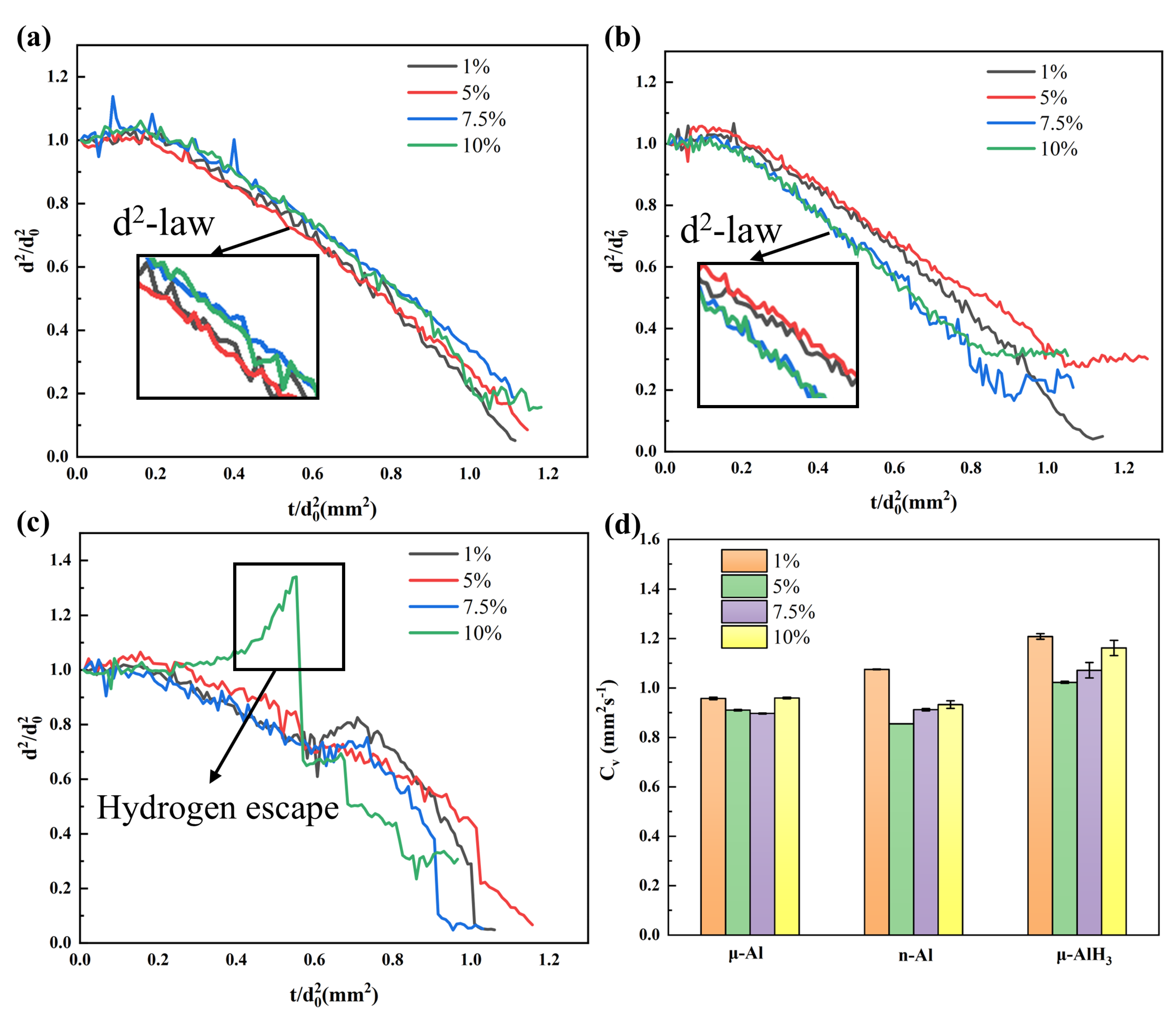
3.4.4. Combustion Rate Properties
3.5. Combustion Residue Morphology
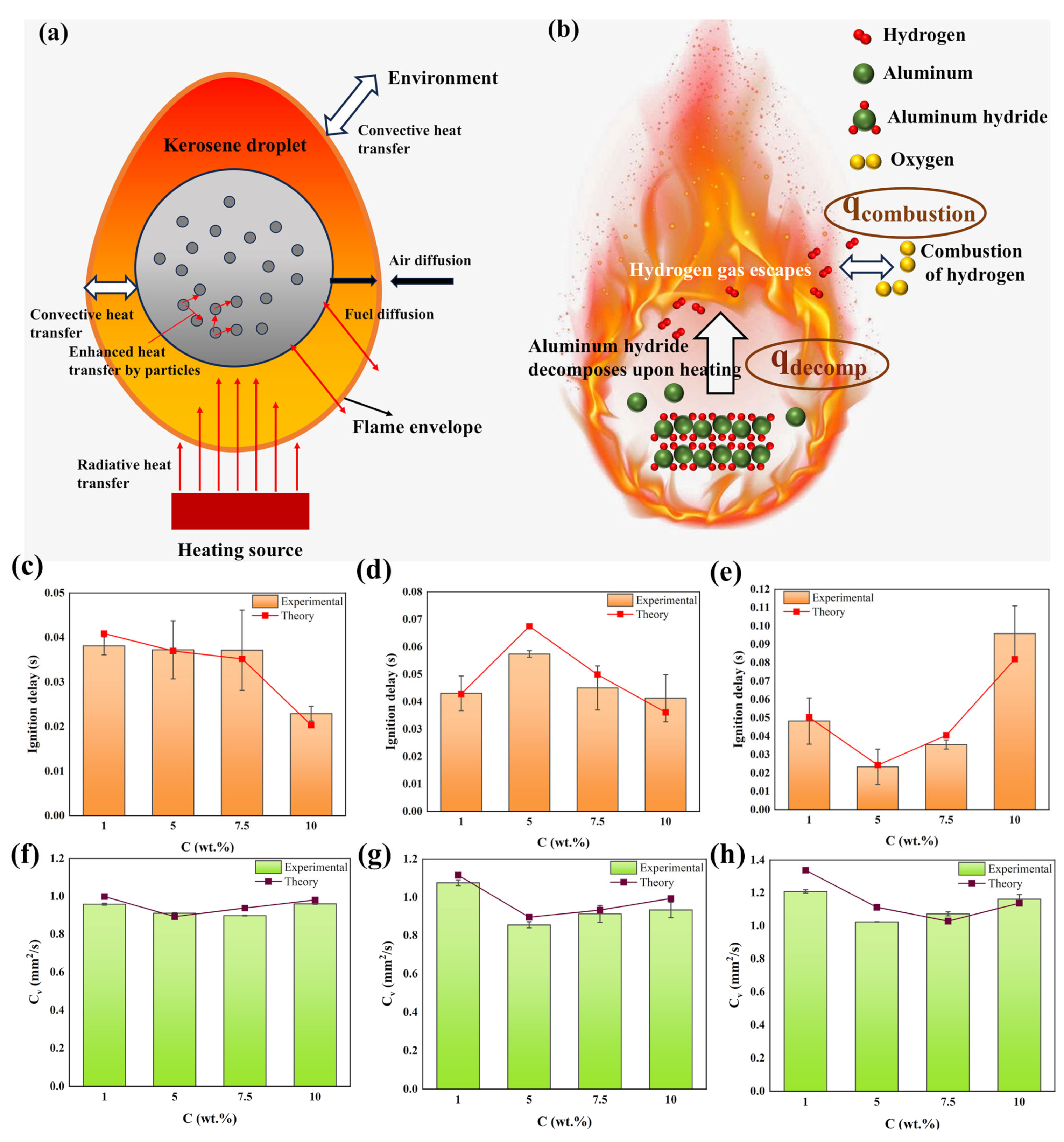
3.6. Ignition and Combustion Model of Kerosene Droplets Containing Aluminum Hydride
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Pan, L.; Wang, L.; Zou, J.-J. Review on synthesis and properties of high-energy-density liquid fuels: Hydrocarbons, nanofluids and energetic ionic liquids. Chem. Eng. Sci. 2018, 180, 95–125. [Google Scholar]
- Hui, X.; Kumar, K.; Sung, C.-J.; Edwards, T.; Gardner, D. Experimental studies on the combustion characteristics of alternative jet fuels. Fuel 2012, 98, 176–182. [Google Scholar]
- Wilson, G.R., III; Edwards, T.; Corporan, E.; Freerks, R.L. Certification of Alternative Aviation Fuels and Blend Components. Energy Fuels 2013, 27, 962–966. [Google Scholar]
- Chung, H.S.; Chen, C.S.H.; Kremer, R.A.; Boulton, J.R.; Burdette, G.W. Recent Developments in High-Energy Density Liquid Hydrocarbon Fuels. Energy Fuels 1999, 13, 641–649. [Google Scholar]
- Wei, S.; Sun, L.; Wu, L.; Yu, Z.; Zhang, Z. Study of combustion characteristics of diesel, kerosene (RP-3) and kerosene-ethanol blends in a compression ignition engine. Fuel 2022, 317, 123468. [Google Scholar]
- Billingsley, M.; Edwards, T.; Shafer, L.; Bruno, T. Extent and Impacts of Hydrocarbon Fuel Compositional Variability for Aerospace Propulsion Systems. In Proceedings of the 46th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Nashville, TN, USA, 25–28 July 2010. [Google Scholar]
- Pizzarelli, M.; Battista, F. Oxygen–methane rocket thrust chambers: Review of heat transfer experimental studies. Acta Astronaut. 2023, 209, 48–66. [Google Scholar]
- Jung, H.; Kittelson, D.B.; Zachariah, M.R. The influence of a cerium additive on ultrafine diesel particle emissions and kinetics of oxidation. Combust. Flame 2005, 142, 276–288. [Google Scholar]
- Jodłowski, P.J.; Jędrzejczyk, R.J.; Chlebda, D.K.; Dziedzicka, A.; Kuterasiński, Ł.; Gancarczyk, A.; Sitarz, M. Non-Noble Metal Oxide Catalysts for Methane Catalytic Combustion: Sonochemical Synthesis and Characterisation. Nanomaterials 2017, 7, 174. [Google Scholar] [CrossRef]
- Mandilas, C.; Karagiannakis, G.; Konstandopoulos, A.G.; Beatrice, C.; Lazzaro, M.; Di Blasio, G.; Molina, S.; Pastor, J.V.; Gil, A. Study of Basic Oxidation and Combustion Characteristics of Aluminum Nanoparticles under Enginelike Conditions. Energy Fuels 2014, 28, 3430–3441. [Google Scholar]
- Gan, Y.; Qiao, L. Combustion characteristics of fuel droplets with addition of nano and micron-sized aluminum particles. Combust. Flame 2011, 158, 354–368. [Google Scholar]
- Wang, H.; Jian, G.; Egan, G.C.; Zachariah, M.R. Assembly and reactive properties of Al/CuO based nanothermite microparticles. Combust. Flame 2014, 161, 2203–2208. [Google Scholar]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar]
- Granier, J.J.; Pantoya, M.L. Laser ignition of nanocomposite thermites. Combust. Flame 2004, 138, 373–383. [Google Scholar]
- Starik, A.M.; Savel’ev, A.M.; Titova, N.S. Specific features of ignition and combustion of composite fuels containing aluminum nanoparticles (Review). Combust. Explos. Shock. Waves 2015, 51, 197–222. [Google Scholar]
- Wang, X.; Zhang, J.; Ma, Y.; Wang, G.; Han, J.; Dai, M.; Sun, Z.Y. A comprehensive review on the properties of nanofluid fuel and its additive effects to compression ignition engines. Appl. Surf. Sci. 2020, 504, 144581. [Google Scholar]
- Mehta, R.N.; Chakraborty, M.; Parikh, P.A. Nanofuels: Combustion, engine performance and emissions. Fuel 2014, 120, 91–97. [Google Scholar]
- Guerieri, P.M.; Jacob, R.J.; DeLisio, J.B.; Rehwoldt, M.C.; Zachariah, M.R. Stabilized microparticle aggregates of oxygen-containing nanoparticles in kerosene for enhanced droplet combustion. Combust. Flame 2018, 187, 77–86. [Google Scholar]
- Xiu-Tian-Feng, E.; Pan, L.; Wang, F.; Wang, L.; Zhang, X.; Zou, J.J. Al-Nanoparticle-Containing Nanofluid Fuel: Synthesis, Stability, Properties, and Propulsion Performance. Ind. Eng. Chem. Res. 2016, 55, 2738–2745. [Google Scholar]
- Sundararaj, A.J.; Pillai, B.C.; Guna, K.R. Experimental investigation of effect of temperature on ignition behaviour of seeded refined kerosene. Thermochim. Acta 2020, 683, 178469. [Google Scholar]
- Allen, C.; Mittal, G.; Sung, C.-J.; Toulson, E.; Lee, T. An aerosol rapid compression machine for studying energetic-nanoparticle-enhanced combustion of liquid fuels. Proc. Combust. Inst. 2011, 33, 3367–3374. [Google Scholar]
- Javed, I.; Baek, S.W.; Waheed, K. Evaporation characteristics of heptane droplets with the addition of aluminum nanoparticles at elevated temperatures. Combust. Flame 2013, 160, 170–183. [Google Scholar]
- Liang, D.; Ren, K.; Wu, Z.; Jiang, Y.; Shen, D.; Li, H.; Liu, J. Combustion characteristics of oxygenated slurry droplets of nano-Al/EtOH and nano-Al/TPGME blends. Energy 2021, 220, 119693. [Google Scholar]
- Han, W.; Dai, B.; Liu, J.; Sun, Y.; Zhu, B.; Liu, X. Ignition and Combustion Characteristics of Heptane-Based Nanofluid Fuel Droplets. Energy Fuels 2019, 33, 10282–10289. [Google Scholar]
- Javed, I.; Baek, S.W.; Waheed, K. Autoignition and combustion characteristics of kerosene droplets with dilute concentrations of aluminum nanoparticles at elevated temperatures. Combust. Flame 2015, 162, 774–787. [Google Scholar]
- Sekoai, P.T.; Ouma CN, M.; Du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar]
- Ojha, P.K.; Karmakar, S. Boron for liquid fuel Engines-A review on synthesis, dispersion stability in liquid fuel, and combustion aspects. Prog. Aerosp. Sci. 2018, 100, 18–45. [Google Scholar]
- DeLuca, L.T.; Galfetti, L.; Severini, F.; Rossettini, L.; Meda, L.; Marra, G.; D’Andrea, B.; Weiser, V.; Calabro, M.; Vorozhtsov, A.B.; et al. Physical and ballistic characterization of AlH3-based space propellants. Aerosp. Sci. Technol. 2007, 11, 18–25. [Google Scholar]
- Meng, X.; Tian, H.; Zhu, H.; Wang, Z.; Yu, R.; Guo, Z.; Cai, G. Effects of aluminum and aluminum hydride additives on the performance of hybrid rocket motors based on 95% hydrogen peroxide. Aerosp. Sci. Technol. 2022, 130, 107914. [Google Scholar]
- Sun, M.; Wang, F.; Chen, Y.; Liao, X.; Liu, J. Thermal oxidation and combustion characteristics of single particle AlH3. Combust. Flame 2025, 274, 114038. [Google Scholar]
- Qin, W.; Guo, X.; Xiao, J.; Guan, Z.; Qiu, X.; Bai, F.-Q. Recent progress in model and dynamics research and its promotion to material development of energetic AlH3. Int. J. Hydrogen Energy 2024, 110, 430–444. [Google Scholar]
- Li, G.; Gao, W.; Jiang, H.; Liu, J. Enhancing combustion performance, hydrogen evolution stability and sintering resistance of AlH3-nanoparticles via Ni coating. Renew. Energy 2025, 248, 123086. [Google Scholar]
- Maehlen, J.P.; Yartys, V.A.; Denys, R.V.; Fichtner, M.; Frommen, C.; Bulychev, B.M.; Pattison, P.; Emerich, H.; Filinchuk, Y.E.; Chernyshov, D. Thermal decomposition of AlH3 studied by in situ synchrotron X-ray diffraction and thermal desorption spectroscopy. J. Alloys Compd. 2007, 446, 280–289. [Google Scholar]
- Xu, B.; Liu, J.; Wang, X. Preparation and thermal properties of aluminum hydride polymorphs. Vacuum 2014, 99, 127–134. [Google Scholar]
- Bazyn, T.; Eyer, R.; Krier, H.; Glumac, N. Combustion Characteristics of Aluminum Hydride at Elevated Pressure and Temperature. J. Propuls. Power 2004, 20, 427–431. [Google Scholar]
- Il’in, A.P.; Bychin, N.V.; Gromov, A.A. Products of combustion of aluminum hydride in air. Combust. Explos. Shock Waves 2001, 37, 490–491. [Google Scholar]
- Tian, H.; Wang, Z.; Guo, Z.; Yu, R.; Cai, G.; Zhang, Y. Effect of metal and metalloid solid-fuel additives on performance and nozzle ablation in a hydroxy-terminated polybutadiene based hybrid rocket motor. Aerosp. Sci. Technol. 2022, 123, 107493. [Google Scholar]
- Yuanlei, S.; Hammoodi, K.A.; Sajadi, S.M.; Rashid, F.L.; Li, Z.; Jasim, D.J.; Salahshour, S.; Esmaeili, S.; Sabetvand, R. The effect of initial conditions (temperature and pressure) on combustion of Fe-coated-aluminum hydride nanoparticles using the molecular dynamics approach. Case Stud. Therm. Eng. 2024, 53, 103901. [Google Scholar]
- Bazyn, T.; Krier, H.; Glumac, N. Combustion of nanoaluminum at elevated pressure and temperature behind reflected shock waves. Combust. Flame 2006, 145, 703–713. [Google Scholar]
- Young, G.; Piekiel, N.; Chowdhury, S.; Zachariah, M.R. Ignition Behavior of α-AlH3. Combust. Sci. Technol. 2010, 182, 1341–1359. [Google Scholar]
- Ao, W.; Liu, P.; Liu, H.; Wu, S.; Tao, B.; Huang, X.; Li, L.K.B. Tuning the agglomeration and combustion characteristics of aluminized propellants via a new functionalized fluoropolymer. Chem. Eng. J. 2020, 382, 122987. [Google Scholar]
- Ao, W.; Gao, Y.; Zhou, S.; Li, L.K.B.; He, W.; Liu, P.; Yan, Q.-L. Enhancing the stability and combustion of a nanofluid fuel with polydopamine-coated aluminum nanoparticles. Chem. Eng. J. 2021, 418, 129527. [Google Scholar]
- He, W.; Tao, B.; Yang, Z.; Yang, G.; Guo, X.; Liu, P.-J.; Yan, Q.-L. Mussel-inspired polydopamine-directed crystal growth of core-shell n-Al@PDA@CuO metastable intermixed composites. Chem. Eng. J. 2019, 369, 1093–1101. [Google Scholar]
- He, W.; Liu, P.J.; Gong, F.; Tao, B.; Gu, J.; Yang, Z.; Yan, Q.L. Tuning the Reactivity of Metastable Intermixed Composite n-Al/PTFE by Polydopamine Interfacial Control. ACS Appl. Mater. Interfaces 2018, 10, 32849–32858. [Google Scholar]
- Gao, Y.; Ao, W.; Li, L.K.B.; Zhou, S.; He, W.; Liu, P.; Yan, Q.-L. Catalyzed combustion of a nanofluid fuel droplet containing polydopamine-coated metastable intermixed composite n-Al/CuO. Aerosp. Sci. Technol. 2021, 118, 107005. [Google Scholar]
- Zhang, G.; Ao, W.; Fan, Z.; Zhang, Y.; Xu, Y.; Wang, F.; Liu, P.; Li, L.K.B. Acoustic excitation of an n-heptane droplet: Evaporation, ignition and combustion characteristics. Aerosp. Sci. Technol. 2023, 133, 108128. [Google Scholar]
- Zhao, Z.; Jiang, Y.; Li, S.; Liu, P.; Ren, P.; Ao, W. A study on the ignition and combustion properties of kerosene-based nanofluid fuels containing n-Al/graphene. Fuel 2025, 385, 134133. [Google Scholar]
- Lytvynov, L. 13–Aluminum Oxide. In Single Crystals of Electronic Materials; Fornari, R., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 447–485. [Google Scholar]
- Gervais, F. Aluminum Oxide (Al2O3). In Handbook of Optical Constants of Solids; Palik, E.D., Ed.; Academic Press: Boston, MA, USA, 1998; pp. 761–775. [Google Scholar]
- Glushkov, D.O.; Kuznetsov, G.V.; Nigay, A.G.; Yanovsky, V.A. Influence of gellant and drag-reducing agent on the ignition characteristics of typical liquid hydrocarbon fuels. Acta Astronaut. 2020, 177, 66–79. [Google Scholar]
- Li, H.-M.; Li, G.-X.; Li, L.; Yao, Z.-P. Experimental study on thermal ignition and combustion of droplet of ammonium dinitramide based liquid propellant in different oxidizing gas atmospheres. Acta Astronaut. 2020, 169, 40–49. [Google Scholar]
- Whang, J.-J.; Yukao, C.-Y.; Ho, J.-T.; Wong, S.-C. Experimental study of the ignition of single droplets under forced convection. Combust. Flame 1997, 110, 366–376. [Google Scholar]
- Galfetti, L.; DeLuca, L.T.; Severini, F.; Colombo, G.; Meda, L.; Marra, G. Pre and post-burning analysis of nano-aluminized solid rocket propellants. Aerosp. Sci. Technol. 2007, 11, 26–32. [Google Scholar]
- Faeth, G.M. Current Status of Droplet and Liquid Combustion. In Energy and Combustion Science; Chigier, N.A., Ed.; Pergamon: Oxford, UK, 1979; pp. 149–182. [Google Scholar]
- Li, C.; Wu, G.; Li, M.; Hu, C.; Wei, J. A heat transfer model for aluminum droplet/wall impact. Aerosp. Sci. Technol. 2020, 97, 105639. [Google Scholar]
- Zou, X.; Wang, N.; Wang, J.; Feng, Y.; Shi, B. A numerical investigation on heterogeneous combustion of aluminum nanoparticle clouds. Aerosp. Sci. Technol. 2021, 112, 106604. [Google Scholar]
- Janot, R.; Tang, W.S.; Clémençon, D.; Chotard, J.N. Catalyzed KSiH3 as a reversible hydrogen storage material. J. Mater. Chem. A 2016, 4, 19045–19052. [Google Scholar]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Rönnebro, E. Hydrogen Storage Properties of Nanosized MgH2–0.1TiH2 Prepared by Ultrahigh-Energy–High-Pressure Milling. J. Am. Chem. Soc. 2009, 131, 15843–15852. [Google Scholar]
- Wang, S.-P.; Zhang, A.M.; Liu, Y.-L.; Zhang, S.; Cui, P. Bubble dynamics and its applications. J. Hydrodyn. 2018, 30, 975–991. [Google Scholar]
- Amano, R.; Alkhalidi, A.; Miletta, B.; Li, J. Study of Air Bubble Creation for Aerospace Applications. In Proceedings of the 9th Annual International Energy Conversion Engineering Conference, American Institute of Aeronautics and Astronautics, San Diego, CA, USA, 31 July–3 August 2011. [Google Scholar]
- Flynn, H.G. Cavitation dynamics: II. Free pulsations and models for cavitation bubbles. J. Acoust. Soc. Am. 1975, 58, 1160–1170. [Google Scholar]

| Number | Droplet | Metal Particle | Metal Content |
|---|---|---|---|
| G-0 | kerosene | ||
| G-1 | kerosene | μ-Al | 1% |
| G-2 | kerosene | μ-Al | 5% |
| G-3 | kerosene | μ-Al | 7.5% |
| G-4 | kerosene | μ-Al | 10% |
| G-5 | kerosene | n-Al | 1% |
| G-6 | kerosene | n-Al | 5% |
| G-7 | kerosene | n-Al | 7.5% |
| G-8 | kerosene | n-Al | 10% |
| G-9 | kerosene | μ-AlH3 | 1% |
| G-10 | kerosene | μ-AlH3 | 5% |
| G-11 | kerosene | μ-AlH3 | 7.5% |
| G-12 | kerosene | μ-AlH3 | 10% |
| Samples | TG | DTG | |||
|---|---|---|---|---|---|
| Ti | Te | MC | Tp | Lmax | |
| AlH3 | 25.2 | 178.0 | −5.6 | 185.3 | 24.1 |
| 399.2 | 590.4 | 30.6 | 553.4 | 7.8 | |
| 590.5 | 810.1 | 7.2 | 654.3 | −2.8 | |
| Samples | Exotherm Peak | Endotherm Peak | ||||||
|---|---|---|---|---|---|---|---|---|
| Tp | Ti | Te | Q | Tp | Ti | Te | Q | |
| AlH3 | 177.1 | 158.0 | 192.2 | 24.5 | 657.9 | 645.8 | 667.8 | −2.3 |
| 546.4 | 400.1 | 605.5 | 7.7 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Hao, C.; Liu, Y.; Fu, Y.; Ao, W. Ignition and Combustion Characteristics of Aluminum Hydride-Based Kerosene Propellant. Aerospace 2025, 12, 891. https://doi.org/10.3390/aerospace12100891
Zhao J, Hao C, Liu Y, Fu Y, Ao W. Ignition and Combustion Characteristics of Aluminum Hydride-Based Kerosene Propellant. Aerospace. 2025; 12(10):891. https://doi.org/10.3390/aerospace12100891
Chicago/Turabian StyleZhao, Jiangong, Chenzhuo Hao, Yilun Liu, Yihao Fu, and Wen Ao. 2025. "Ignition and Combustion Characteristics of Aluminum Hydride-Based Kerosene Propellant" Aerospace 12, no. 10: 891. https://doi.org/10.3390/aerospace12100891
APA StyleZhao, J., Hao, C., Liu, Y., Fu, Y., & Ao, W. (2025). Ignition and Combustion Characteristics of Aluminum Hydride-Based Kerosene Propellant. Aerospace, 12(10), 891. https://doi.org/10.3390/aerospace12100891







