Germination Phenological Response Identifies Flora Risk to Climate Change
Abstract
:1. Introduction
2. Methods
2.1. Species and Study Area Selection
2.2. Germination Experiment
2.3. Statistical Analysis
2.4. Mechanistic Model
2.5. Climate Parameters and Scenarios
3. Results
3.1. Climatic Condition of the Sites
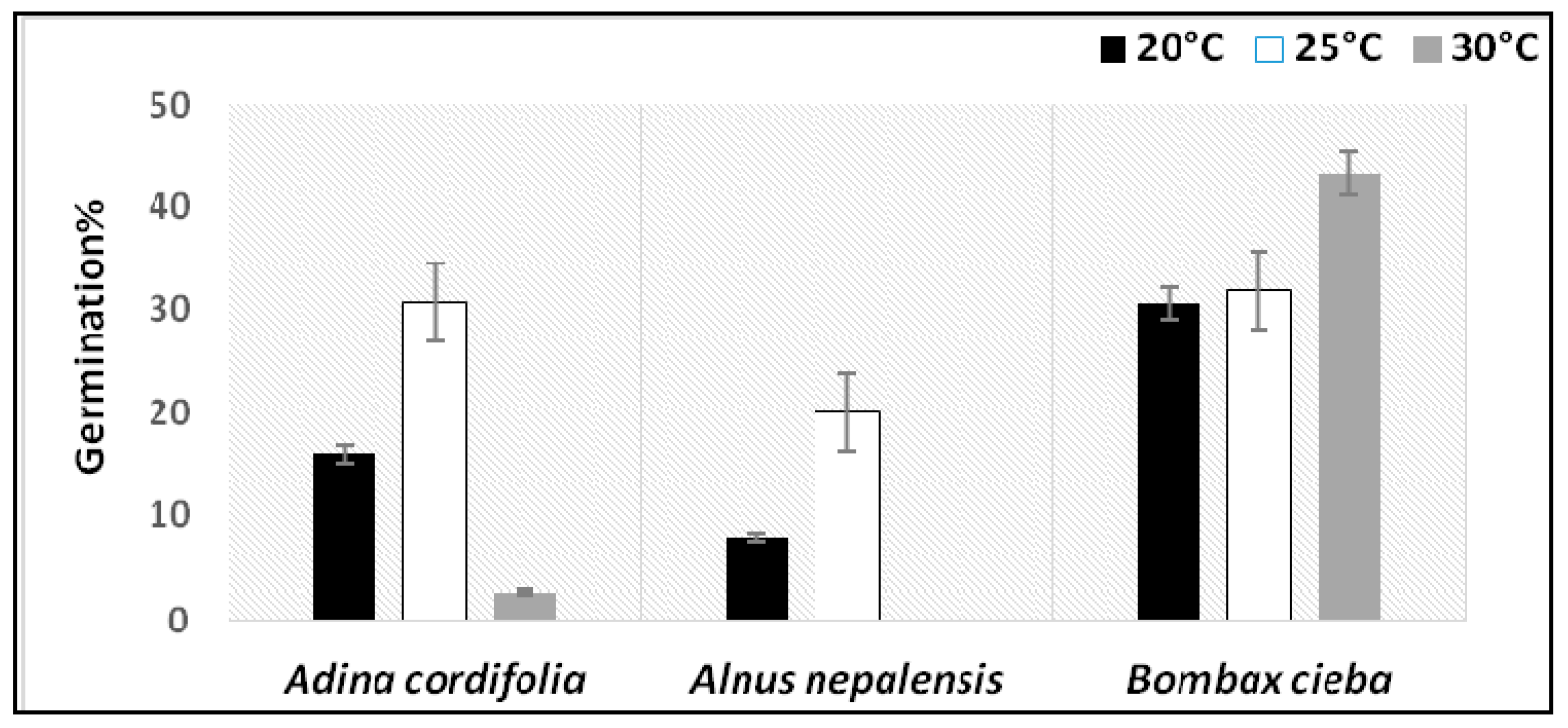
3.2. Experimental Germination Result
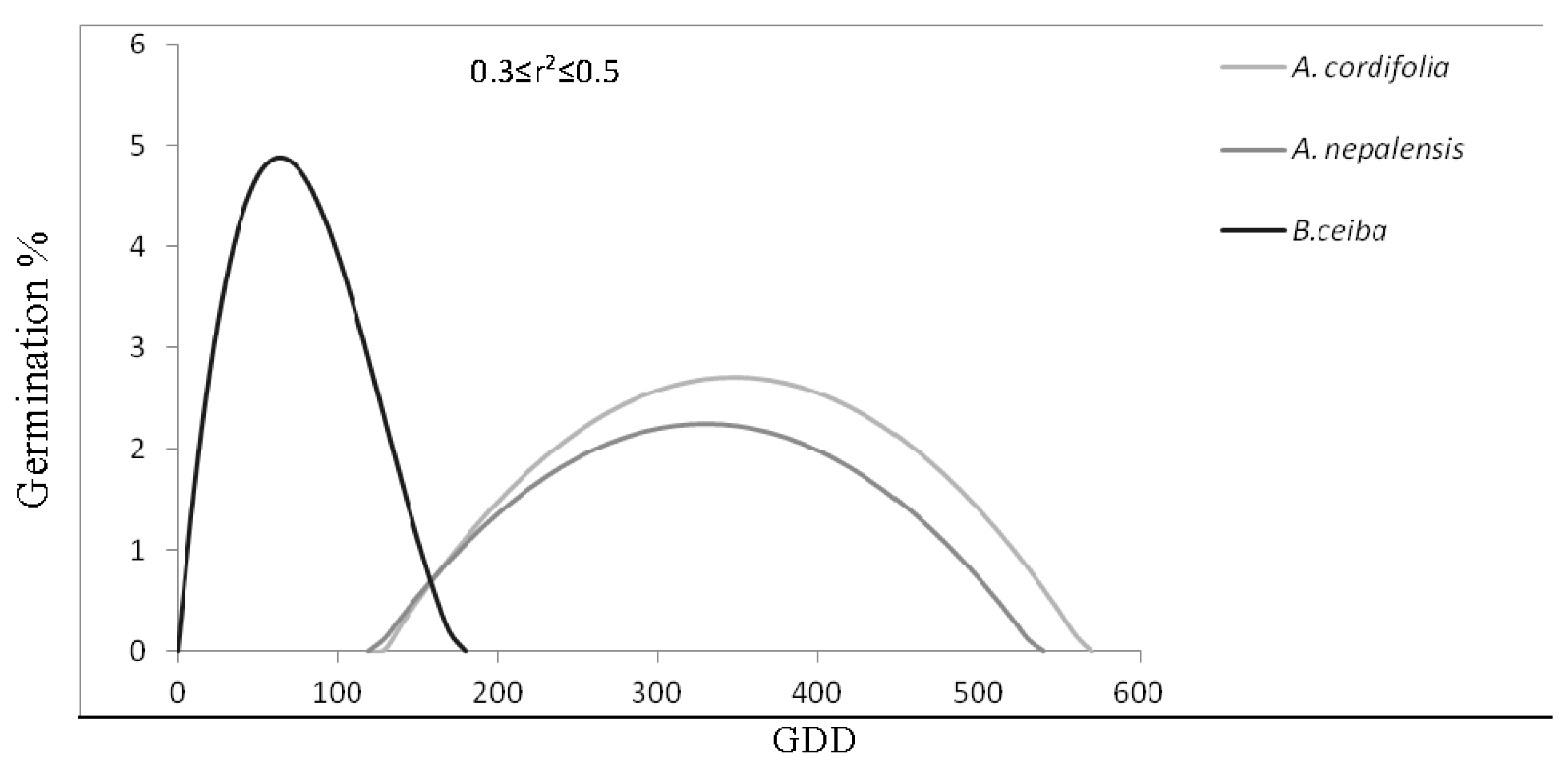
3.3. Species General Response:TACA-GEM
4. Discussion
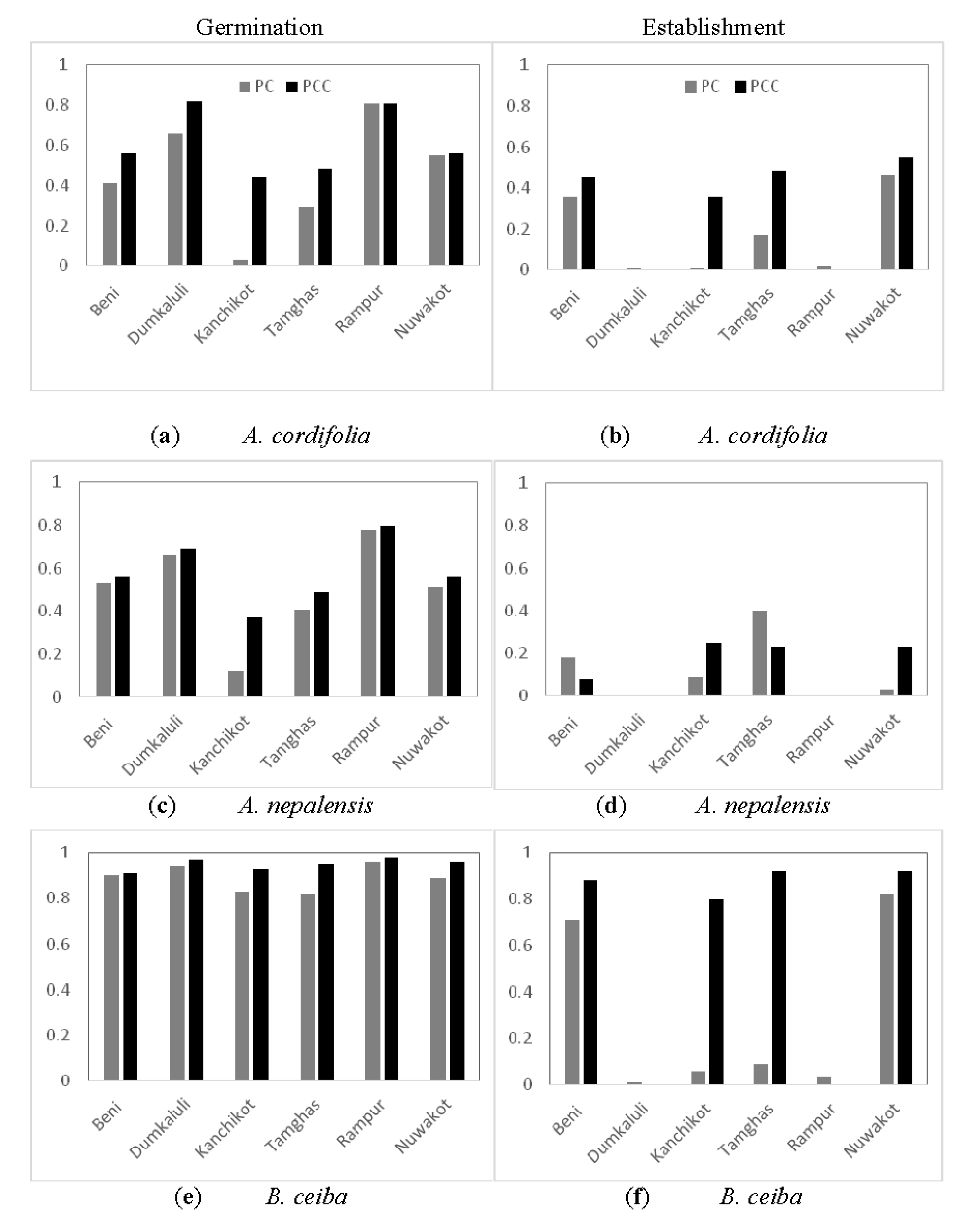
4.1. Spatial Response
4.2. Species-Specific Response
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Munang, R.; Thiaw, I.; Alverson, K.; Mumba, M.; Liu, J.; Rivington, M. Climate change and ecosystem based adaptation: A new pragmatic approach to buffering climate change impacts. Curr. Opin. Environ. Sustain. 2013, 5, 67–71. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.; Almond, R.E.; Baillie, J.E.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) IPCC (Intergovernmental Panel on Climate Change) Summary for Policymakers. In Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Shrestha, A.B.; Wake, C.P.; Mayewski, P.A.; Dibb, J.E. Maximum temperature trends in the Himalaya and its vicinity: An analysis based on temperature records from Nepal for the period 1971–1994. J. Clim. 1999, 12, 2775–2786. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B. Climatic warming in the Tibetan Plateau during recent decades Xiaodong. Int.J. Climatol. 2000, 20, 1729–1742. [Google Scholar] [CrossRef]
- Yan, L.; Liu, X. Has climatic warming over the Tibetan plateau paused or continued in recent years? J. Earth Ocean Atmos. Sci. 2014, 1, 13–28. [Google Scholar]
- Chaudhary, P.; Bawa, K.S. Local perceptions of climate change validated by scientific evidence in the Himalayas. Biol. Lett. 2011, 7, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.B.; Wake, C.P.; Dibb, J.E.; Mayewski, P.A. Precipitation fluctuation in the Nepal Himalaya and its vicinity and relationship with some large scale climatological parameters. Int. J Climatol. 2000, 20, 317–327. [Google Scholar] [CrossRef]
- HMG/MOPE (HMGN/Ministry of Population and Environment). Initial National Communication Report to the Conference of the parties of the United Nations Framework Convention of Climate Change (UNFCCC); HMGN/Ministry of Population and Environment: Singha Durbar, Nepal, 2004.
- Du, M.Y.; Kawashima, S.; Yonemura, S.; Zhang, X.Z.; Chen, S.B. Mutual influence between human activities and climate change in the Tibetan Plateau during recent years. Glob. Planet Chang. 2004, 41, 241–249. [Google Scholar] [CrossRef]
- Baidya, S.K.; Shrestha, M.L.; Sheikh, M.M. Trends in daily climatic extremes of temperature and precipitation in Nepal. J. Hydrol. Meteorol. 2008, 5, 38–51. [Google Scholar]
- Alamgir, M.; Pretzsch, J.; Turton, S.M. Climate change effects on community forests: Finding through user’s lens and local knowledge. Small-Scale For. 2014, 13, 445–460. [Google Scholar] [CrossRef]
- MOE (Ministry of Environment). National Adaptation Program of Action to Climate Change; Ministry of Environment: Kathmandu, Nepal, 2010.
- Secretariat, C.B.D. Secretariat of the Convention on Biological Diversity; Secretariat of the Convention on Biological Diversity: Montreal, MB, Canada, 2009. [Google Scholar]
- Meyer, S.E.; Monsen, S.B.; McArthur, E.D. Germination response of Artemisia tridentata (Asteraceae) to light and chill: Patterns of between-population variation. Bot. Gaz. 1990, 151, 176–183. [Google Scholar] [CrossRef]
- Adler, P.B.; Lambers, J.H.R. The influence of climate and species composition on the population dynamics of ten prairie forbs. Ecology. 2008, 89, 3049–3060. [Google Scholar] [CrossRef]
- Meyer, S.E.; Allen, P.S.; Beckstead, J. Seed germination regulation in Bromustectorum (Poaceae) and its ecological significance. Oikos 1997, 78, 475–485. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Germination ecophysiology of herbaceous plant species in a temperate region. Am. J. Bot. 1988, 75, 286–305. [Google Scholar] [CrossRef]
- Walk, J.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Benitze-Malvido, J. Impact of forest fragmentation on seedling abundance in a tropical rain forest. Conserv. Biol. 1998, 2, 380–389. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; White, P.S. The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: San Diego, CA, USA, 1985. [Google Scholar]
- Lawes, M.J.; Joubert, R.; Griffiths, M.E.; Boudreau, S.; Chapman, C.A. The effect of the spatial scale of recruitment on tree diversity in Afromontane forest fragments. Biol. Conserv. 2007, 139, 447–456. [Google Scholar] [CrossRef]
- Galán, C.; Alcázar, P.; Oteros, J.; García-Mozo, H.; Aira, M.J.; Belmonte, J.; De la Guardia, C.D.; Fernández-González, D.; Gutierrez-Bustillo, M.; Moreno-Grau, S.; et al. Airborne pollen trends in the Iberian Peninsula. Sci. Total Environ. 2016, 550, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Koller, D.; Mayer, A.M.; Poljakoff-Mayber, A.; Klein, S. Seed Germination. Ann. Rev. Plant Physiol. 1962, 13, 437–464. [Google Scholar] [CrossRef]
- Ross, M.A.; Harper, J.L. Occupation of biological space during seedling establishment. J. Ecol. 1972, 60, 77–88. [Google Scholar] [CrossRef]
- Boojh, R.; Ramakrishnan, P.S. Temperature responses to seed germination in two closely related tree species of Schima, Reinw. Curr. Sci. 1981, 50, 416–418. [Google Scholar]
- Kozlowski, T.T. Growth and Development of Trees; Academic Press: New York, NY, USA, 1971; Volume 1. [Google Scholar]
- Kozlowski, T.T. Tree Growth and Environmental Stresses; Washington University Press: Scattle, WA, USA, 1979. [Google Scholar]
- Chmielewski, F.M. Phenology in Agriculture and Horticulture. In Phenology: An Integrative Environmental Science, 2nd ed.; Schwartz, M.D., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 539–562. [Google Scholar]
- Nitschke, C.R.; Hickey, G.M. Assessing the Vulnerability of Victoria’s Central Highlands Forests to Climate Change; Technical Report; Department of Sustainability and Environment: Melbourne, Australia, 2007. [Google Scholar]
- Nitschke, C.R.; Innes, J.L. A tree and climate assessment tool for modelling ecosystem response to climate change. Ecol. Model. 2008, 210, 263–277. [Google Scholar] [CrossRef]
- Nitschke, C.R.; Amoroso, M.; Coates, K.D.; Astrup, R. The influence of climate change, site type and disturbance on stand dynamics in northwest British Columbia, Canada. Ecosphere 2012, 3, 1–21. [Google Scholar] [CrossRef]
- Mok, H.F.; Arndt, S.K.; Nitschke, C.R. Modelling the potential impact of climate variability and change on species regeneration potential in the temperate forests of south-eastern Australia. Glob. Chang Biol. 2012, 18, 1053–1072. [Google Scholar] [CrossRef]
- Rawal, D.S.; Kasel, S.; Keatley, M.R.; Nitschke, C.R. Environmental effects on germination phenology of co-occurring eucalypts: Implications for regeneration under climate change. Int. J. Biometeorol. 2015, 59, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Thapa, G.J.; Wikramanayake, E.; Forrest, J. Climate-Change Impacts on the Biodiversityof the Terai Arc Landscape and the Chitwan-Annapurna Landscape; WWF Nepal, Hariyoban Program: Kathmandu, Nepal, 2013. [Google Scholar]
- Chaudhary, R.P. Biodiversity in Nepal: Status and Conservation, India; Tecpress Books: Bangkok, Thailand, 1998. [Google Scholar]
- Tamrakar, P.R. State of Forest Genetic Resources Conservation and Management in Nepal; Forest Genetic Resources Working Papers; Working Paper FGR/69E Forest Resources Division FAO; FAO: Rome, Italy, 2003. [Google Scholar]
- Bell, D.T.; Rokich, D.P.; McChesney, C.J.; Plummer, J.A. Effects of temperature, light and gibberellic acid on the germination of seeds of 43 species native to Western Australia. J. Veg. Sci. 1995, 6, 797–806. [Google Scholar] [CrossRef]
- ISTA. International Seed Testing Association; ISTA Publications: Bassersdorf, Switzerland, 2011. [Google Scholar]
- Ecocrop, FAO. Available online: www.ecocrop.fao.org (accessed on 12 October 2016).
- Factnet Winrock International. Available online: www.factnet.winrock.org (accessed on 15 October 2016).
- World Agroforestry Center. Available online: http://worldagroforestry.org (accessed on 16 October 2016).
- Ranieri, B.D.; Pezzini, F.F.; Garcia, Q.S.; Chautems, A.; Franca, M.G.C. Testing the regeneration niche hypothesis with Gesneriaceae (Tribe Sinningiae) in Brazil: Implications for the conservation of rare species. Austral Ecol. 2012, 37, 125–133. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life tables. J. Roy. Stat. Soc. 1976, 34, 187–220. [Google Scholar]
- SPSS IBM Corp. IBM SPSS Statistics for Windows, Version 20.0; SPSS IBM Corp: Armonk, NY, USA, 2011. [Google Scholar]
- Shahba, M.A.; Qian, Y.L. Effect of seedling date, seeding rate and seeding treatment on saltgrass seed germination and establishment. Crop Sci. 2008, 48, 2453–2458. [Google Scholar] [CrossRef]
- Wielgolaski, F.E. Starting dates and basic temperatures in phenological observations of plants. Int. J. Biometeorol. 1999, 43, 1432–3471. [Google Scholar] [CrossRef]
- Green, D.S. Controls of growth phenology vary in seedlings of three, co-occurring ecologically distinct northern conifers. Tree Physiol. 2007, 27, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.H. Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MA, USA, 2009. [Google Scholar]
- Government of Nepal, Ministry of Population and Environment, Department of Hydrology and Meteorology, Hydrology Division, Flood Forecasting Section. Available online: http://hydrology.gov.np (accessed on 25 August 2017).
- Ministry of forest and soil conservation, Nepal. Available online: www.forestrynepal.org (accessed on 30 May 2016).
- Danida Forest Seed Center. Available online: www.dfsc.dk (accessed on 21 June 2016).
- Bargali, K.; Singh, S.P. Germination behaviour of some leguminous and actinorhizal plants of Himalaya: Effect of temperature and medium. Trop. Ecol. 2007, 48, 99–105. [Google Scholar]
- Joshi, L.R. An Introduction about Bombax ceibaLinn. Non Timber Forest Products; Institute of Forestry, Pokhara Campus: Pokhara, Nepal, 2010. [Google Scholar]
- Fennessey, N.M.; Kirshen, P.H. Evaporation and evapo-transpiration under climate change in New England. J. Water Res. Plan. Manag. 1994, 120, 48–69. [Google Scholar] [CrossRef]
- Falloon, P.; Betts, R. Climate impacts on European agriculture and water management in the context of adaptation and mitigation—The importance of an integrated approach. Sci. Total Environ. 2010, 408, 5667–5687. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.M.; Daws, I.; Hay, F.R. Seed-based approach for identifying flora at risk from climate warming. Austral Ecol. 2011, 36, 923–935. [Google Scholar] [CrossRef]
- Tao, F.; Yokozawa, M.; Liu, J.; Zhang, Z. Climate—Crop yield relationships at provincial scales in China and the impacts of recent climate trends. Clim. Res. 2008, 38, 83–94. [Google Scholar] [CrossRef]
- Venkateswarlu, B.; Shanker, A.K. Climate change and agriculture: Adaptation and mitigation strategies. Indian J. Agron. 2009, 54, 226–230. [Google Scholar]
- PIER. Pacific Islands Ecosystems at Risk. USA: Institute of Pacific Islands Forestry. Available online: http://www.hear.org/pier/index.html (accessed on 25 August 2017).
| Species | Climatic Zone | Optimal Temp. Range (°C) | Absolute Temp. Range (°C) | Optimal Rainfall Range (mm/yr-1) | Altitude Range (m) |
|---|---|---|---|---|---|
| Adina cordifolia (Karma) a,c | tropical wet/dry and wet | 25–35 | 5–47 | 800–2000 | 0–800 |
| Alnusnepalensis (Utis) a,b | tropical wet/dry, steppe or semiarid, subtropical humid, temperate, oceanic/continental, temperate with humid winters/dry winters | 13–26 | 4–36 | 1000–2000 | 500–3000 b |
| Bombaxceiba (Simal) a | tropical wet/dry, steppe or semiarid | 28–42 | 5–49 | 750–4000 | 0–1500 |
| Serial Number | District Name | Geographical Position | Altitude (m) | PC Average, 10 Years | PCC Average, 2030–2061 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tmin | Tmax | PCP | Tmin | Tmax | PCP | ||||
| 1 | Beni (609) (Myagdi) | 28°21′ 83°34′ | 835 | 14.38 | 25.69 | 144.29 | 14.82 | 26.33 | 152.13 |
| 2 | Dumkauli (706) (Nawalparasi) | 27°41′ 84°13′ | 154 | 18.67 | 30.64 | 208.68 | 19.77 | 31.51 | 192.53 |
| 3 | Kanchikot (715)(Argakhachi) | 27°56′ 83°09′ | 1760 | 12.79 | 20.22 | 162.77 | 15.40 | 24.00 | 156.22 |
| 4 | Nuwakot (1004) (Nuwakot) | 27°55′ 85°10′ | 1003 | 16.32 | 26.77 | 167.92 | 15.14 | 26.14 | 196.03 |
| 5 | Rampur (902) (Chitwan) | 27°37′ 84°25′ | 256 | 17.95 | 30.98 | 161.07 | 18.95 | 31.06 | 178.55 |
| 6 | Tamghas (725) (Gulmi) | 28°04′ 83°15′ | 1530 | 12.42 | 22.3 | 167.68 | 15.49 | 25.37 | 165.87 |
| Parameter | Species | ||
|---|---|---|---|
| Adina cordifolia | Alnusnepalensis | Bombaxceiba | |
| Geographic origin of seed | 27.1024, 85.5720 | 27.2593, 85.2930 | 26.55.3185.59.68 |
| Habitat | |||
| Soil texture | Sandy/clay 6 | Sandy 5 | Sandy loam 9 |
| Seedfall Julian date (days) | 180 6 | 90 7 | 180 9 |
| Rooting zone depth (m) 1 | 0.10 | 0.10 | 0.10 |
| Coarse fragment (%) 1 | 0.30 | 0.10 | 0.30 |
| Probalistic Germination Functions thresholds of polynomial regression for germination based on GDD | |||
| Minimum GDD threshold (days) | 130 | 130 | 10 |
| Maximum GDD threshold (days) | 560 | 530 | 170 |
| Minimum temperature (°C) | 15 | 15 | 15 |
| Maximum temperature (°C) | 35 | 35 8 | 35 |
| b0 | −0.0415 | −0.0353 | −0.0004 |
| b1 | 0.0004 | 0.0004 | 0.0017 |
| b2 | −5.7E-07 | −5.3E-07 | −1.8E-05 |
| b3 | 4.6E-08 | ||
| Other Germination Parameters | |||
| Germination moisture threshold (MPa) | −2 | −2 | −2 |
| Physiological Base Temperature (°C) | 5 2 | 5 2 | 5 2 |
| Establishment Parameters | |||
| GDD minimum | 4000 3 | 3500 3 | 4500 3 |
| GDD maximum | 7000 3 | 6000 3 | 7000 3 |
| Frost tolerance (0–1) | 0 3,4 | 0.1 3,4 | 0.1 2,3,4 |
| Frost season length (days) | 0-10 3,6 | 30 3 | 30 3 |
| Heat moisture index (dimensionless) 4 | 47.5 2,3,4 | 34 2,3,4 | 50.66 3,4 |
| Drought tolerance | 0.30 | 0.55 3 | 0.30 2,4 |
| Minimum temperature | 0 2 | −1 2 | −3 2 |
| Species | Temperature | Wald |
|---|---|---|
| Adina cordifolia | * (p = 0.018) | 8.004 |
| Alnusnepalensis | * (p = 0.023) | 7.506 |
| Bombaxcieba | ns (p =0.335) | 2.189 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chhetri, S.B.; Rawal, D.S. Germination Phenological Response Identifies Flora Risk to Climate Change. Climate 2017, 5, 73. https://doi.org/10.3390/cli5030073
Chhetri SB, Rawal DS. Germination Phenological Response Identifies Flora Risk to Climate Change. Climate. 2017; 5(3):73. https://doi.org/10.3390/cli5030073
Chicago/Turabian StyleChhetri, Sarala Budhathoki, and Deepa Shree Rawal. 2017. "Germination Phenological Response Identifies Flora Risk to Climate Change" Climate 5, no. 3: 73. https://doi.org/10.3390/cli5030073
APA StyleChhetri, S. B., & Rawal, D. S. (2017). Germination Phenological Response Identifies Flora Risk to Climate Change. Climate, 5(3), 73. https://doi.org/10.3390/cli5030073




