Survey on Fungi in Antarctica and High Arctic Regions, and Their Impact on Climate Change
Abstract
1. Introduction
2. History of Fungal Research in the Antarctic Region and Near Syowa Station
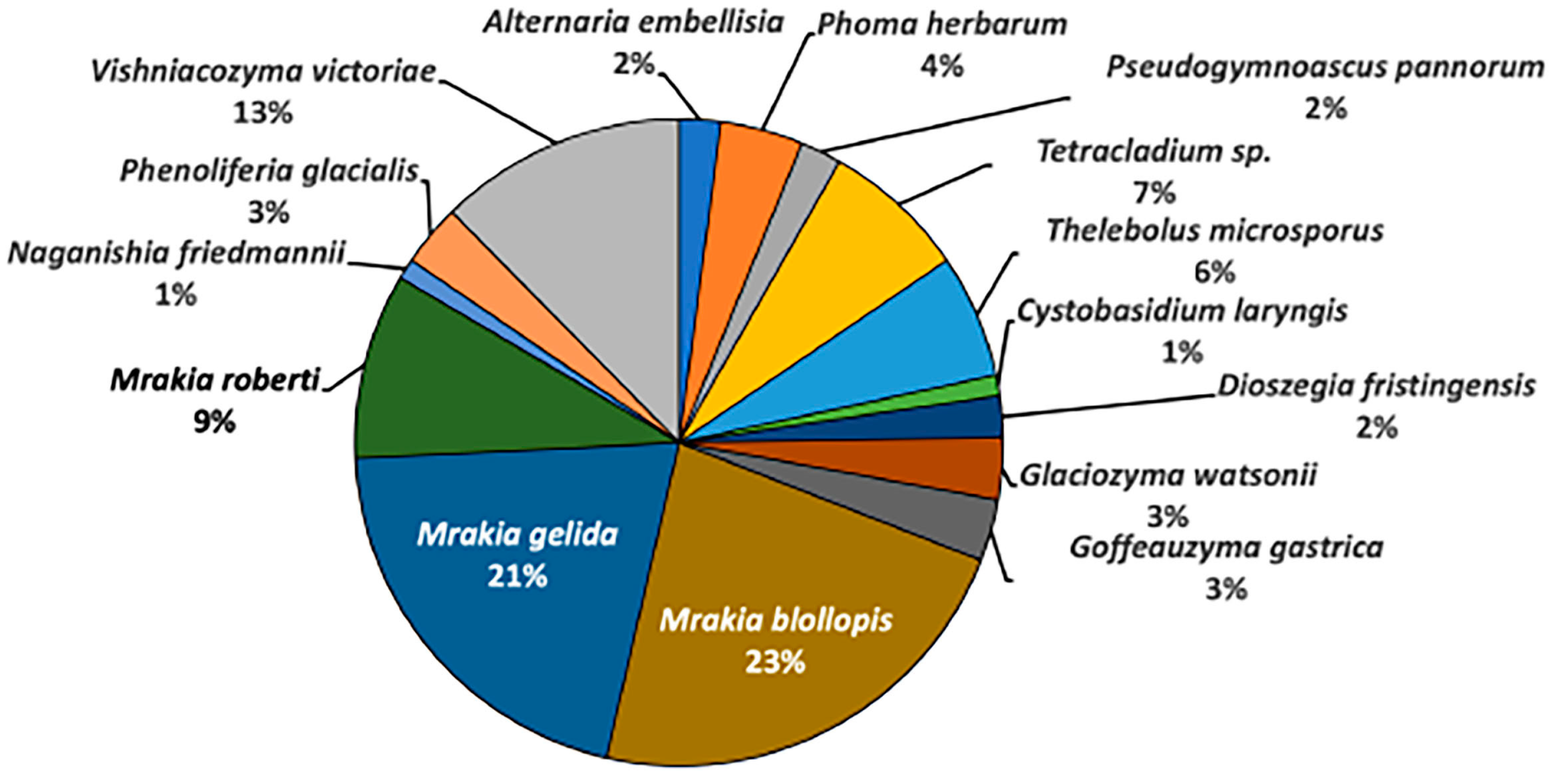
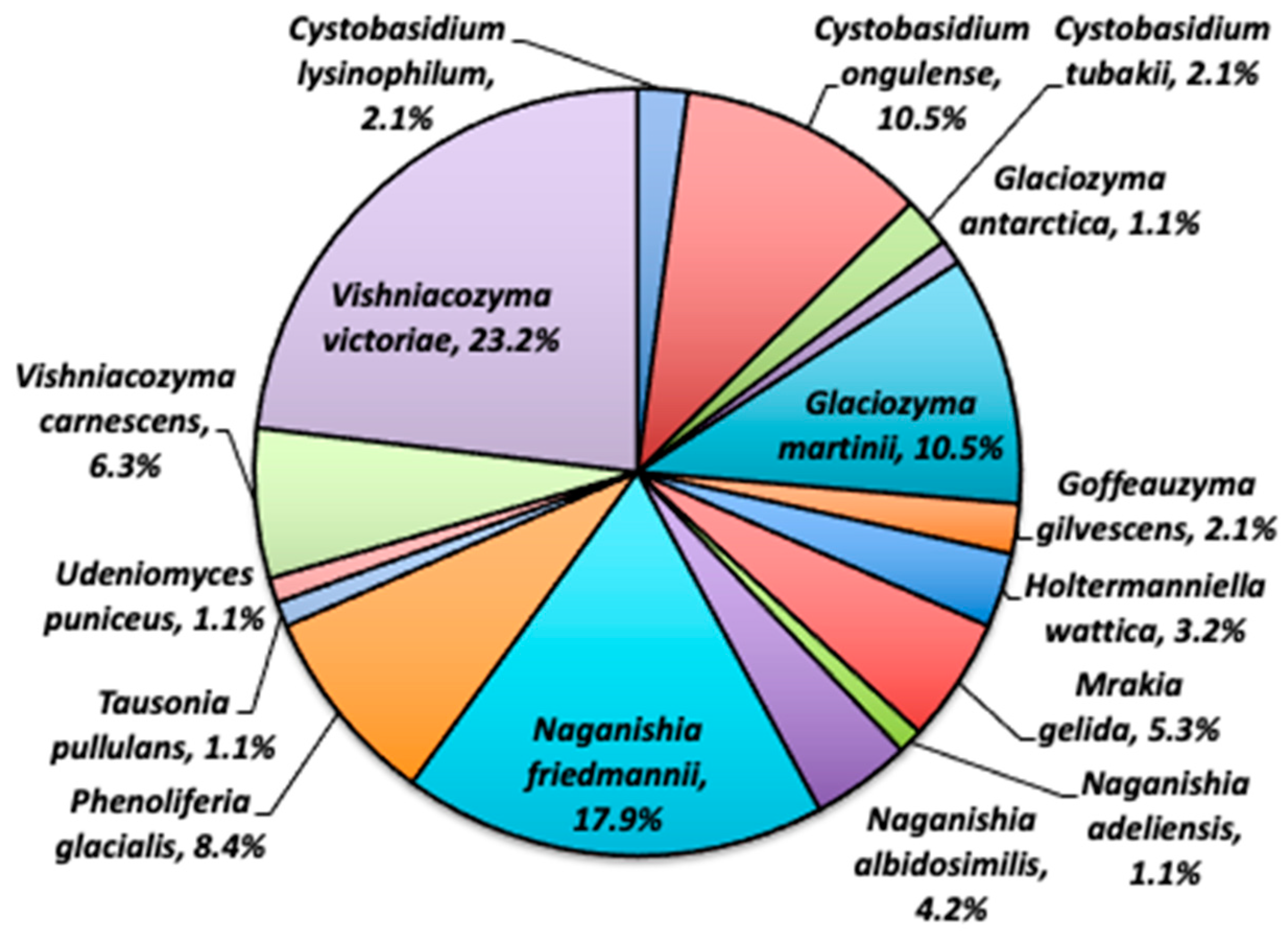
3. Fungal Diversity Research near Syowa Station
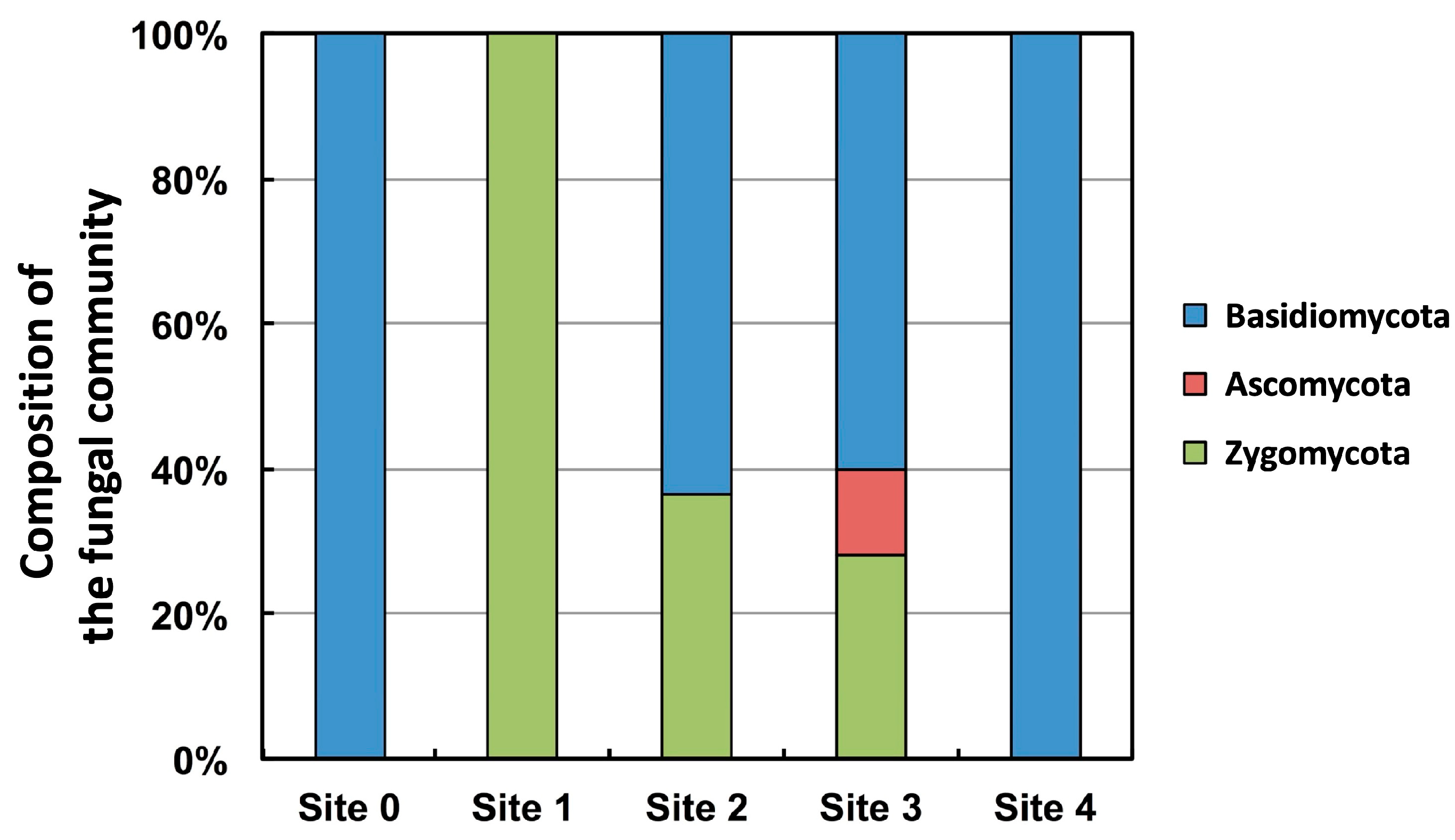
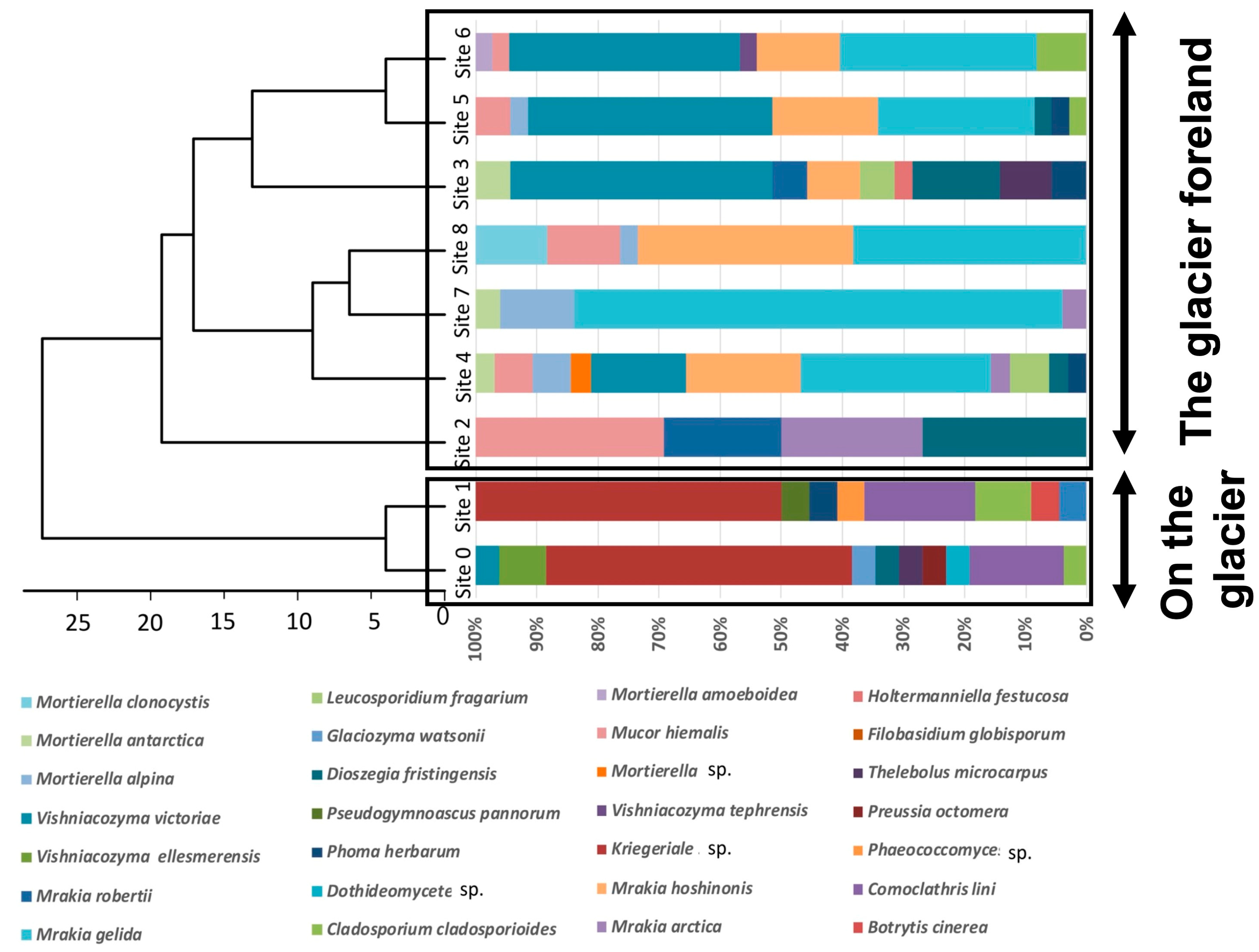
4. Fungal Diversity Research in the Glacier Retreat Area of Svalbard, High Arctic
- Immediately after the glacier retreated and exposed the ground, Mortierella spp. and Mucor spp., both of which belong to Zygomycota, colonized the area (Site 1).
- Mrakia sp. colonized the area by utilizing the nutrients produced by these zygomycetes (Site 2).
- To colonize the area, ascomycete and basidiomycete yeasts (except for Mrakia sp.) utilized the nutrients accumulated by Mrakia sp. (Site 3).
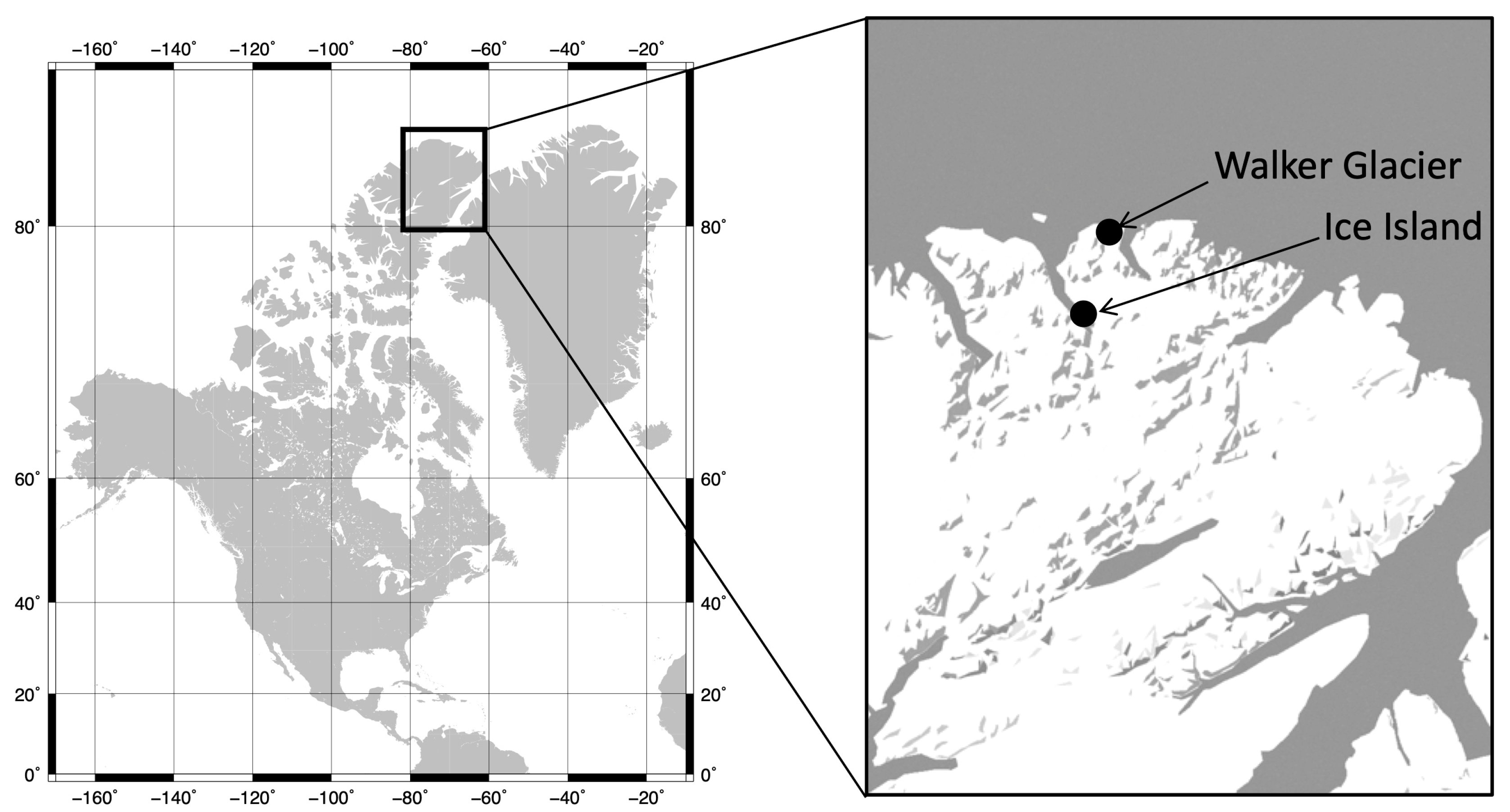
5. Fungal Survey in the Ellesmere Island, Canadian High Arctic
6. Growth and Enzyme Activities at Sub-Zero Temperatures
7. Future Research Prospects
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onofri, S.; Zucconi, L.; Tosi, S. Continental Antarctic Fungi; IHW Verlag: München, Germany, 2007. [Google Scholar]
- Tsuji, M.; Kudoh, S. Soil Yeasts in the Vicinity of Syowa Station, East Antarctica: Their Diversity and Extracellular Enzymes, Cold Adaptation Strategies, and Secondary Metabolites. Sustainability 2020, 12, 4518. [Google Scholar] [CrossRef]
- Armstrong, T. Bellingshausen and the discovery of Antarctica. Polar Rec. 1971, 15, 887–889. [Google Scholar] [CrossRef]
- Boomer, E.; Rousseau, M. Note préliminaire sur les champignons recueillis par l’Expedition Antarctique Belge. Bull. De L’académie R. Des Sci. De Belg. Cl. Des Sci. 1900, 8, 640–646. [Google Scholar]
- Fell, J.W.; Statzell, A.C.; Hunter, I.L.; Phaff, H.J. Leucosporidium gen. n., the heterobasidiomycetous stage of several yeasts of the genus Candida. Antonie Van Leeuwenhoek 1969, 35, 433–462. [Google Scholar] [CrossRef] [PubMed]
- di Menna, M.E. Three new yeasts from Antarctica soils: Candida nivalis, Candida gelida and Candida frigida spp. n. Antonie Van Leeuenwoek 1966, 32, 25–28. [Google Scholar] [CrossRef] [PubMed]
- di Menna, M.E. Yeasts in Antarctic soil. Antonie Van Leeuwenhoek 1966, 32, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Bridge, P.D.; Spooner, B.M. Non-lichenized Antarctic fungi: Transient visitors or members of a cryptic ecosystem? Fungal Ecol. 2012, 5, 381–394. [Google Scholar] [CrossRef]
- Tubaki, K. On some fungi isolated from the Antarctic materials. Biol. Results Jpn. Antarct. Res. Exped. 1961, 14, 1–9. [Google Scholar] [CrossRef][Green Version]
- Tubaki, K. Note on some fungi and yeasts from Antarctica. Antarct. Rec. 1961, 11, 161–162. [Google Scholar]
- Soneda, M. On some yeasts from the Antarctic region. Biol. Results Jpn. Antarct. Res. Exped. 1961, 15, 3–10. [Google Scholar] [CrossRef]
- Tubaki, K.; Asano, I. Additional species of fungi isolated from the Antarctic materials. JARE Sci. Rep. Ser. E Biol. 1965, 27, 1–27. [Google Scholar]
- Goto, S.; Sugiyama, J.; Iizuka, H. A Taxonomic Study of Antarctic Yeasts. Mycologia 1969, 61, 748–774. [Google Scholar] [CrossRef]
- Kitamoto, D.; Yanagishita, H.; Shinbo, T.; Nakane, T.; Kamisawa, C.; Nakahara, T. Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J. Biotechnol. 1993, 29, 91–96. [Google Scholar] [CrossRef]
- Kitamoto, D.; Ikegami, T.; Suzuki, G.T.; Sasaki, A.; Takeyama, Y.; Idemoto, Y.; Koura, N.; Yanagishita, H. Microbial conversion of n-alkanes into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma (Candida Antarctica). Biotechnol. Lett. 2001, 23, 1709–1714. [Google Scholar] [CrossRef]
- Tsuji, M.; Fujiu, S.; Xiao, N.; Hanada, Y.; Kudoh, S.; Kondo, H.; Tsuda, S.; Hoshino, T. Cold adaptation of fungi obtained from soil and lake sediment in the Skarvsnes ice-free area, Antarctic. FEMS Microbiol. Lett. 2013, 346, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hirose, D.; Tanabe, Y.; Uchida, M.; Kudoh, S.; Osono, T. Microfungi associated with withering willow wood in ground contact near Syowa Station, East Antarctica for 40 years. Polar Biol. 2013, 36, 919–924. [Google Scholar] [CrossRef]
- Tsuji, M. A catalog of fungi recorded from the vicinity of Syowa Station. Mycoscience 2018, 59, 319–324. [Google Scholar] [CrossRef]
- Tsuji, M. Genetic diversity of yeasts from East Ongul Island, East Antarctica and their extracellular enzymes secretion. Polar Biol. 2018, 41, 249–258. [Google Scholar] [CrossRef]
- Tsuji, M.; Tsujimoto, M.; Imura, S. Cystobasidium tubakii and Cystobasidium ongulense, new basidiomycetous yeast species isolated from East Ongul Island, East Antarctica. Mycoscience 2017, 58, 103–107. [Google Scholar] [CrossRef]
- Tsuji, M. An index of non-lichenized fungi recorded in the vicinity of Syowa Station, East Antarctica. In Fungi in Polar Regions; Tsuji, M., Hoshino, T., Eds.; CRC Press: Oxford, UK, 2019; pp. 1–16. [Google Scholar]
- Nowak, A.; Hodson, A. Changes in meltwater chemistry over a 20-year period following a thermal regime switch from polythermal to cold-based glaciation at Austre Brøggerbreen, Svalbard. Polar Res. 2014, 33, 22779. [Google Scholar] [CrossRef]
- Tsuji, M.; Uetake, J.; Tanabe, Y. Changes in the fungal community of Austre Brøggerbreen deglaciation area, Ny-Ålesund, Svalbard, High Arctic. Mycoscience 2016, 57, 448–451. [Google Scholar] [CrossRef]
- Tsuji, M.; Vincent, W.F.; Tanabe, Y.; Uchida, M. Glacier Retreat Results in Loss of Fungal Diversity. Sustainability 2022, 14, 1617. [Google Scholar] [CrossRef]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Mrakia arctica sp. nov., a new psychrophilic yeast isolated from an ice island in the Canadian High Arctic. Mycoscience 2018, 59, 54–58. [Google Scholar] [CrossRef]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Gelidatrema psychrophila sp. nov., a novel yeast species isolated from an ice island in the Canadian High Arctic. Mycoscience 2018, 59, 67–70. [Google Scholar] [CrossRef]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Vishniacozyma ellesmerensis sp. nov., a new psychrophilic yeast isolated from a retreating glacier in the Canadian High Arctic. Int. J. Syst. Evol. Microbiol. 2019, 69, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Mrakia hoshinonis sp. nov., a novel psychrophilic yeast isolated from a retreating glacier on Ellesmere Island in the Canadian High Arctic. Int. J. Syst. Evol. Microbiol. 2019, 69, 944–948. [Google Scholar] [CrossRef]
- Tsuji, M.; Kudoh, S.; Hoshino, T. Draft Genome Sequence of Cryophilic basidiomycetous yeast Mrakia blollopis SK-4 isolated from an algal mat of Naga-ike Lake in Skarvsnes ice-free area, East Antarctica. Genome Announc. 2015, 3, e01454-14. [Google Scholar] [CrossRef]
- Tsuji, M.; Ishihara, J.; Gotoh, Y.; Hayashi, T.; Takahashi, H. Draft Genome Sequences of Five Cystobasidium ongulense Strains Isolated from Areas near Syowa Station, East Antarctica. Microbiol. Resour. Announc. 2022, 11, e0022422. [Google Scholar] [CrossRef]
- Tsuji, M.; Ishihara, J.; Toyoda, A.; Takahashi, H. High-quality Genome Sequence of Cystobasidium tubakii JCM 31526T isolated from East Ongul Island, Antarctica. Microbiol. Resour. Announc. 2022, 11, e0074122. [Google Scholar] [CrossRef]
- Tsuji, M.; Ishihara, J.; Toyoda, A.; Takahashi, H.; Kudoh, S. Genome Sequence of Basidiomycetous yeast Mrakia gelida MGH-2 isolated from Skarvsnes ice-free area, East Antarctica. Microbiol. Resour. Announc. 2023, 12, e01064-22. [Google Scholar] [CrossRef]

| Through 2012 | 2013–2022 | Total | |
|---|---|---|---|
| Chytridiomycota | 0 | 0 | 0 |
| Zygomycota | 0 | 0 | 0 |
| Ascomycota | 12 | 49 | 61 |
| Basidiomycota | 4 | 12 | 16 |
| Total | 16 | 61 | 77 |
| Species | Habitat | Growth at −3 °C | Optimum Growth Temperature | Maximum Growth Temperature |
|---|---|---|---|---|
| Cystobasidium lysinophilum | East Ongul Island | + | 25 °C | 30 °C |
| Cystobasidium ongulense | East Ongul Island | + | 20 °C | 30 °C |
| Cystobasidium tubakii | East Ongul Island | + | 15–17 °C | 25 °C |
| Glaciozyma Antarctica | East Ongul Island | + | 10 °C | 15 °C |
| Glaciozyma martinii | East Ongul Island | + | 15 °C | 17 °C |
| Goffeauzyma gilvescens | East Ongul Island | + | 20 °C | 25 °C |
| Holtermanniella wattica | East Ongul Island | + | 15 °C | 25 °C |
| Mrakia arctica | Ellesmere Island | + | 15 °C | 20 °C |
| Mrakia gelida | East Ongul Island | + | 15 °C | 20 °C |
| Mrakia hoshinonis | Ellesmere Island | + | 15 °C | 20 °C |
| Naganishia adeliensis | East Ongul Island | + | 25 °C | 30 °C |
| Naganishia albidosimilis | East Ongul Island | + | 25 °C | 30 °C |
| Naganishia friedmannii | East Ongul Island | + | 20 °C | 25 °C |
| Phenoliferia glacialis | East Ongul Island | + | 15 °C | 17 °C |
| Tausonia pullulans | East Ongul Island | + | 15 °C | 25 °C |
| Udeniomyces puniceus | East Ongul Island | + | 20 °C | 25 °C |
| Vishniacozyma carnescens | East Ongul Island | + | 20 °C | 25 °C |
| Vishniacozyma ellesmerensis | Ellesmere Island | + | 15–17 °C | 20 °C |
| Vishniacozyma victoriae | East Ongul Island | + | 17 °C | 25 °C |
| Species | Habitat | Lipase | Cellulase | Protease |
|---|---|---|---|---|
| Cystobasidium lysinophilum | East Ongul Island | - | - | - |
| Cystobasidium ongulense | East Ongul Island | 0.43 ± 0.05 | 0.17 ± 0.06 | - |
| Cystobasidium tubakii | East Ongul Island | 0.19 ± 0.07 | 0.13 ± 0.01 | - |
| Glaciozyma Antarctica | East Ongul Island | 0.41 ± 0.13 | - | - |
| Glaciozyma martinii | East Ongul Island | - | - | - |
| Goffeauzyma gilvescens | East Ongul Island | 2.50 ± 0.20 | - | - |
| Holtermanniella wattica | East Ongul Island | 2.27 ± 0.09 | - | - |
| Mrakia arctica | Ellesmere Island | 6.15 ± 0.68 | 5.34 ± 0.78 | 0.75 ± 0.12 |
| Mrakia gelida | East Ongul Island | - | 0.35 ± 0.07 | - |
| Mrakia hoshinonis | Ellesmere Island | 4.29 ± 0.34 | 2.55 ± 0.20 | 1.53 ± 0.05 |
| Naganishia adeliensis | East Ongul Island | 1.23 ± 0.41 | - | 0.40 ± 0.12 |
| Naganishia albidosimilis | East Ongul Island | 0.84 ± 0.16 | - | - |
| Naganishia friedmannii | East Ongul Island | - | - | 1.11 ± 0.08 |
| Phenoliferia glacialis | East Ongul Island | - | - | - |
| Tausonia pullulans | East Ongul Island | 2.60 ± 0.33 | 1.88 ± 0.21 | - |
| Udeniomyces puniceus | East Ongul Island | - | 2.92 ± 0.37 | 0.86 ± 0.35 |
| Vishniacozyma carnescens | East Ongul Island | 0.56 ± 0.31 | 0.49 ± 0.22 | - |
| Vishniacozyma ellesmerensis | Ellesmere Island | 1.56 ± 0.16 | - | - |
| Vishniacozyma victoriae | East Ongul Island | 0.62 ± 0.03 | - | 0.49 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, M. Survey on Fungi in Antarctica and High Arctic Regions, and Their Impact on Climate Change. Climate 2023, 11, 195. https://doi.org/10.3390/cli11090195
Tsuji M. Survey on Fungi in Antarctica and High Arctic Regions, and Their Impact on Climate Change. Climate. 2023; 11(9):195. https://doi.org/10.3390/cli11090195
Chicago/Turabian StyleTsuji, Masaharu. 2023. "Survey on Fungi in Antarctica and High Arctic Regions, and Their Impact on Climate Change" Climate 11, no. 9: 195. https://doi.org/10.3390/cli11090195
APA StyleTsuji, M. (2023). Survey on Fungi in Antarctica and High Arctic Regions, and Their Impact on Climate Change. Climate, 11(9), 195. https://doi.org/10.3390/cli11090195







