Molecularly Imprinted Polymer-Based Optical Sensor for Isopropanol Vapor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of IPA–MIP
2.3. Sensor Fabrication
3. Experimental
4. Material Characterization
4.1. FTIR Analysis
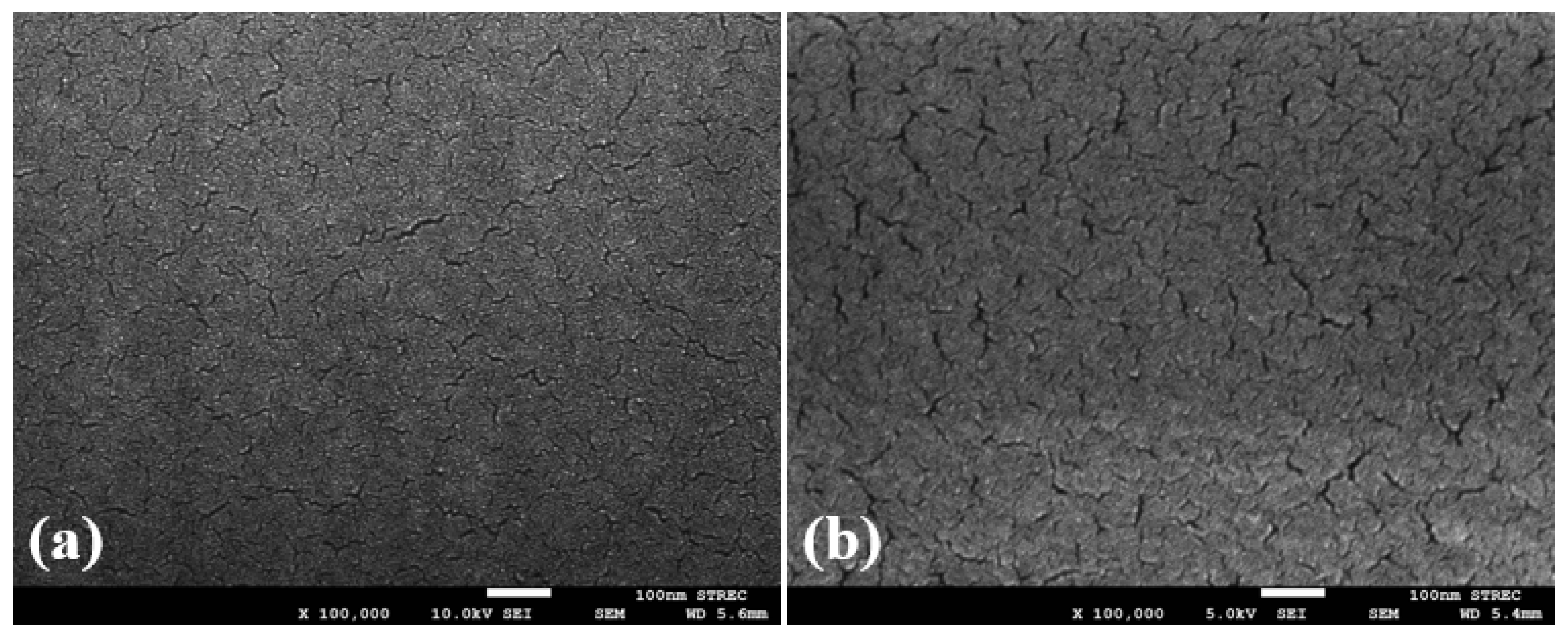
4.2. Morphological Study of the IPA–MIP and NIP
5. Results and Discussion
5.1. Saturation Time
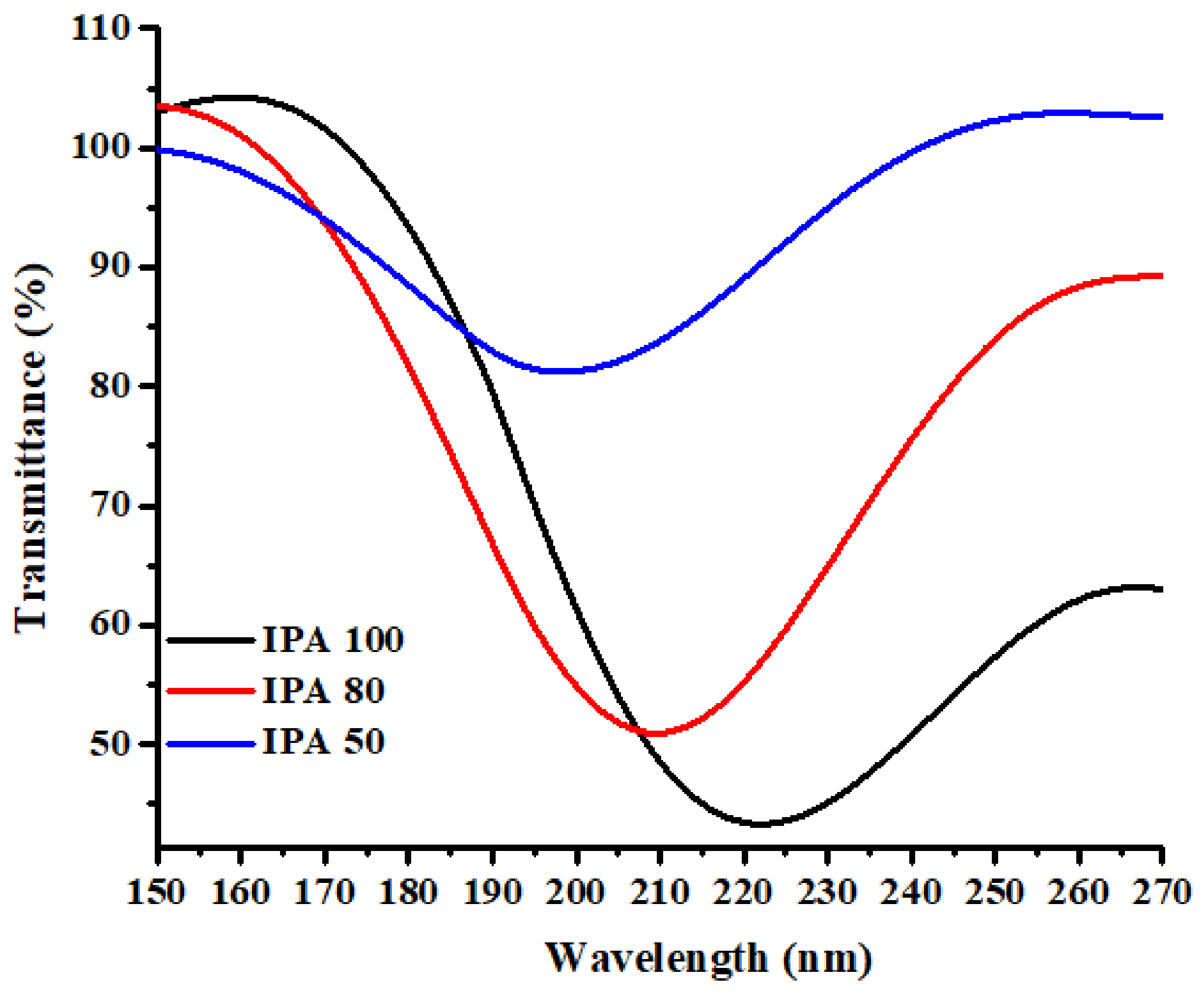
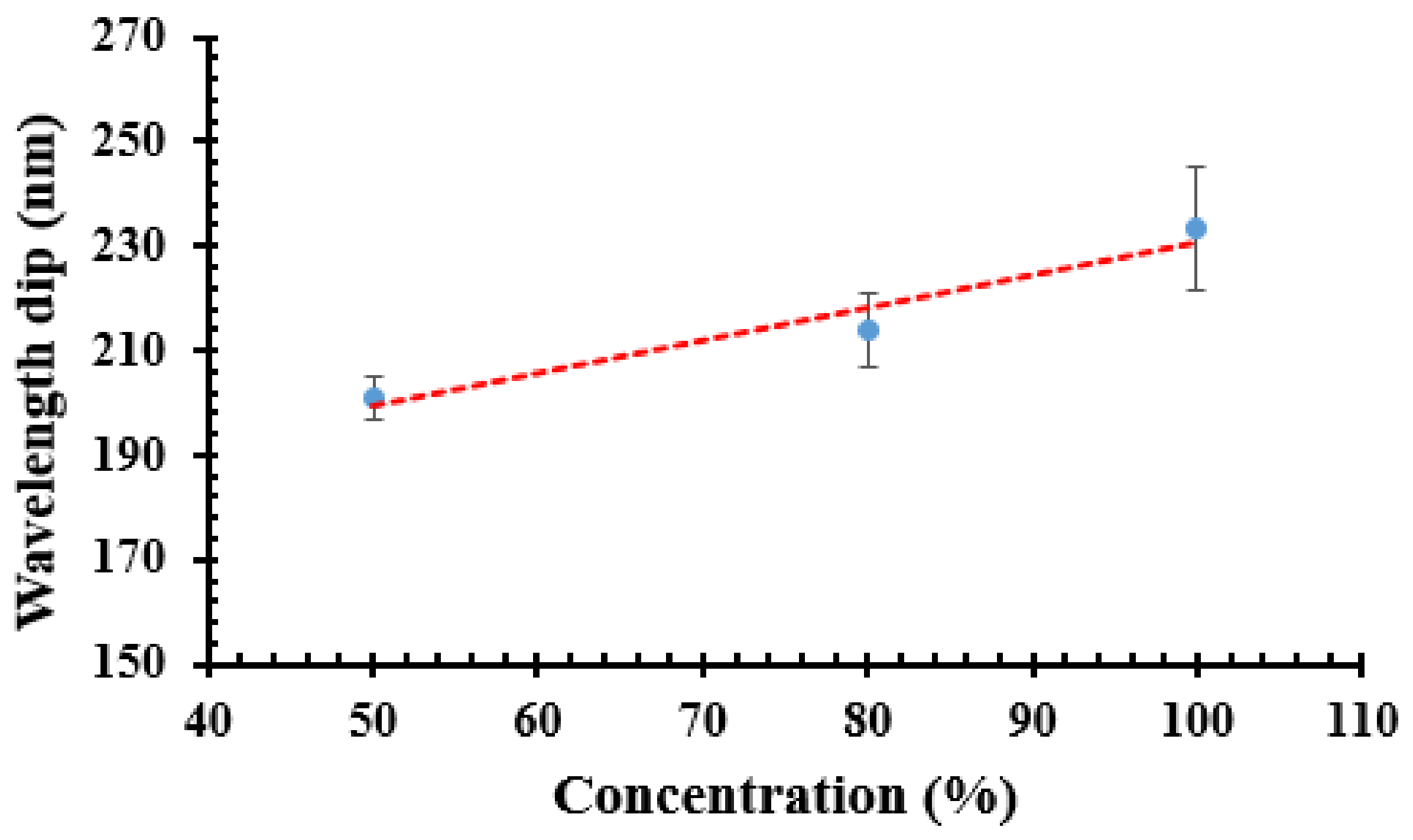
5.2. Sensitivity
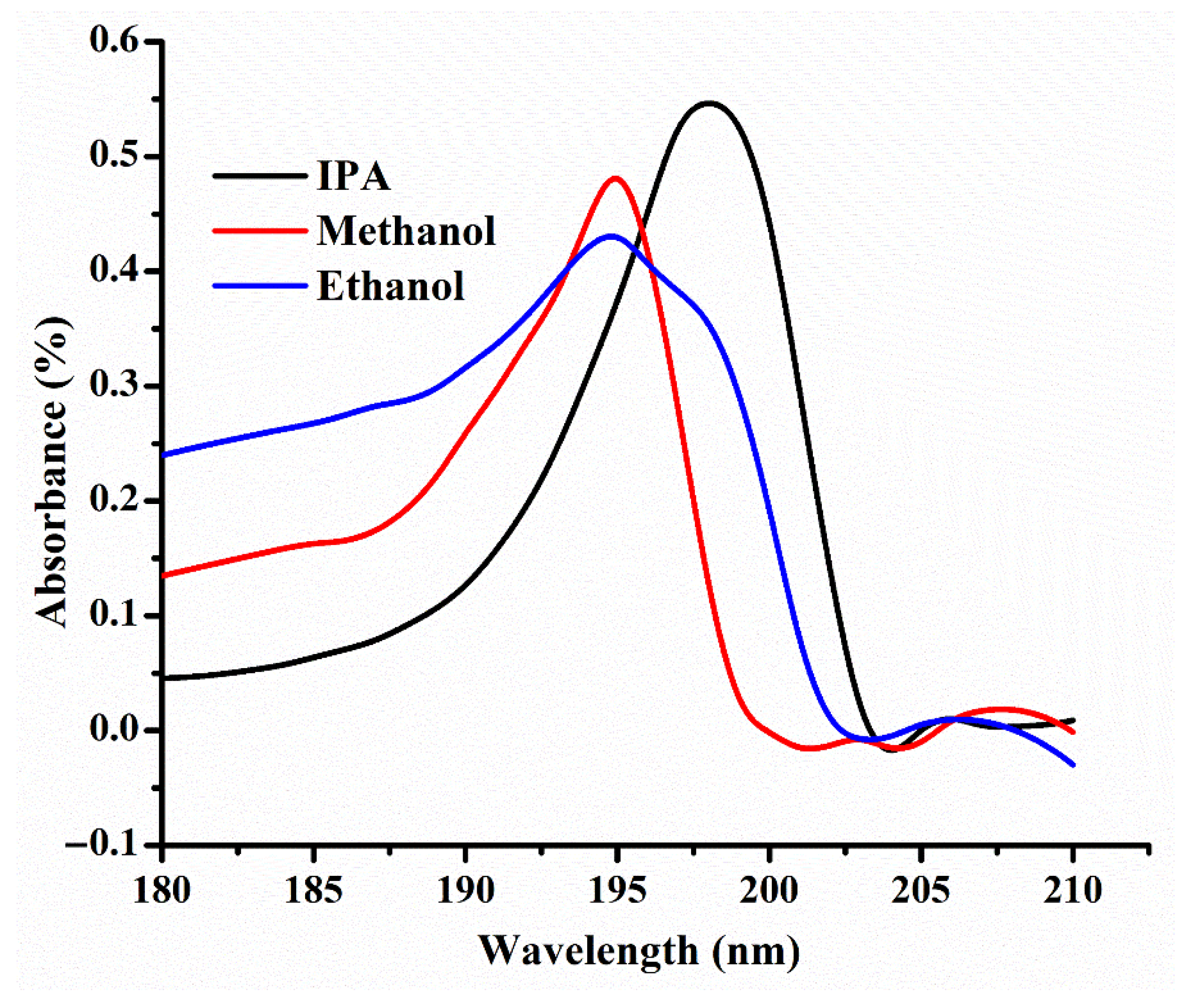
5.3. Selectivity
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, M. Breath Tests in Medicine. Sci. Am. 1992, 267, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Crofford, O.B.; Mallard, R.E.; Winton, R.E.; Rogers, N.L.; Jackson, J.C. Acetone in breath and blood. Trans. Am. Clin. Climatol. Assoc. 1977, 88, 128–139. [Google Scholar] [PubMed]
- Leopold, J.H.; van Hooijdonk, R.T.; Sterk, P.J.; Abu-Hanna, A.; Schultz, M.J.; Bos, L.D. Glucose prediction by analysis of exhaled metabolites—A systematic review. BMC Anesthesiol. 2014, 14, 46. [Google Scholar] [CrossRef]
- Machado, R.F.; Laskowski, D.; Deffenderfer, O.; Burch, T.; Zheng, S.; Mazzone, P.J.; Mekhail, T.; Jennings, C.; Stoller, J.K.; Pyle, J.; et al. Detection of Lung Cancer by Sensor Array Analyses of Exhaled Breath. Am. J. Respir. Crit. Care Med. 2005, 171, 1286–1291. [Google Scholar] [CrossRef]
- Barker, M.; Hengst, M.; Schmid, J.; Buers, H.-J.; Mittermaier, B.; Klemp, D.; Koppmann, R. Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur. Respir. J. 2006, 27, 929–936. [Google Scholar] [CrossRef]
- Dragonieri, S.; Schot, R.; Mertens, B.J.A.; le Cessie, S.; Gauw, S.A.; Spanevello, A.; Resta, O.; Willard, N.P.; Vink, T.J.; Rabe, K.F.; et al. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clin. Immunol. 2007, 120, 856–862. [Google Scholar] [CrossRef]
- Molina, D.K. A Characterization of Sources of Isopropanol Detected on Postmortem Toxicologic Analysis. J. Forensic Sci. 2010, 55, 998–1002. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Liu, Y.; Cheng, S.; Duan, Y. Exhaled isopropanol: New potential biomarker in diabetic breathomics and its metabolic correlations with acetone. RSC Adv. 2017, 7, 17480–17488. [Google Scholar] [CrossRef]
- Pathak, A.K.; Viphavakit, C. VOC biomarker monitoring for diabetes through exhaled breath using Ag/P-TiO2 composite plasmonic sensor. IEEE Sens. J. 2021, 21, 22631–22637. [Google Scholar] [CrossRef]
- Lerner, B.M.; Gilman, J.B.; Aikin, K.C.; Atlas, E.L.; Goldan, P.D.; Graus, M.; Hendershot, R.; Isaacman-VanWertz, G.A.; Koss, A.; Kuster, W.C.; et al. An improved, automated whole air sampler and gas chromatography mass spectrometry analysis system for volatile organic compounds in the atmosphere. Atmos. Meas. Tech. 2017, 10, 291–313. [Google Scholar] [CrossRef]
- Endo, T.; Yanagida, Y.; Hatsuzawa, T. Colorimetric detection of volatile organic compounds using a colloidal crystal-based chemical sensor for environmental application. Sens. Actuators B Chem. 2007, 125, 589–595. [Google Scholar] [CrossRef]
- Viphavakit, C.; Patchoo, W.; Boonruang, S.; Themistos, C.; Komodromos, M.; Mohammed, W.S.; Rahman, B.M.A. Demonstration of Polarization-Independent Surface Plasmon Resonance Polymer Waveguide for Refractive Index Sensing. J. Light. Technol. 2017, 35, 3012–3019. [Google Scholar] [CrossRef][Green Version]
- Viphavakit, C.; Atthi, N.; Boonruang, S.; Themistos, C.; Komodromos, M.; Mohammed, W.S.; Rahman, B.M.A. Realization of a polymer nanowire optical transducer by using the nanoimprint technique. Appl. Opt. 2014, 53, 7487. [Google Scholar] [CrossRef]
- Vu, D.L.; Lin, T.-F.; Lin, T.-H.; Wu, M.-C. Highly-Sensitive Detection of Volatile Organic Compound Vapors by Electrospun PANI/P3TI/PMMA Fibers. Polymers 2020, 12, 455. [Google Scholar] [CrossRef]
- Herdes, C.; Sarkisov, L. Computer Simulation of Volatile Organic Compound Adsorption in Atomistic Models of Molecularly Imprinted Polymers. Langmuir 2009, 25, 5352–5359. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Pathak, A.K.; Viphavakit, C. A Review on All-optical Fiber-based VOC sensors: Heading Towards the Development of Promising Technology. Sens. Actuators A Phys. 2022, 338, 113455. [Google Scholar] [CrossRef]
- Swargiary, K.; Jolivot, R.; Mohammed, W.S. Demonstration of a Polymer-Based Single Step Waveguide by 3D Printing Digital Light Processing Technology for Isopropanol Alcohol-Concentration Sensor. Photonic Sens. 2022, 12, 10–12. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Bai, H.; Wang, G.; Hu, X.; Kumar, S.; Min, R. Biocompatible and Biodegradable Polymer Optical Fiber for Biomedical Application: A Review. Biosensors 2021, 11, 472. [Google Scholar] [CrossRef]
- Leal-Junior, A.; Frizera, A.; Marques, C. A fiber Bragg gratings pair embedded in a polyurethane diaphragm: Towards a temperature-insensitive pressure sensor. Opt. Laser Technol. 2020, 131, 106440. [Google Scholar] [CrossRef]
- Leal-Junior, A.G.; Frizera, A.; Marques, C. Thermal and Mechanical Analyses of Fiber Bragg Gratings-Embedded Polymer Diaphragms. IEEE Photonics Technol. Lett. 2020, 32, 623–626. [Google Scholar] [CrossRef]
- Pandey, P.S.; Raghuwanshi, S.K.; Kumar, S. Recent Advances in Two-Dimensional Materials-Based Kretschmann Configuration for SPR Sensors: A Review. IEEE Sens. J. 2022, 22, 1069–1080. [Google Scholar] [CrossRef]
- Nadeem, M.D.; Raghuwanshi, S.K.; Kumar, S. Recent Advancement of Phase Shifted Fiber Bragg Grating Sensor for Ultrasonic Wave Application: A Review. IEEE Sens. J. 2022, 22, 7463–7474. [Google Scholar] [CrossRef]
- Pathak, A.K.; Singh, V.K. A wide range and highly sensitive optical fiber pH sensor using polyacrylamide hydrogel. Opt. Fiber Technol. 2017, 39, 43–48. [Google Scholar] [CrossRef]
- Gangwar, R.K.; Bhardwaj, V.; Singh, V.K. Magnetic field sensor based on selectively magnetic fluid infiltrated dual-core photonic crystal fiber. Opt. Eng. 2016, 55, 026111. [Google Scholar] [CrossRef]
- Pesavento, M.; Zeni, L.; de Maria, L.; Alberti, G.; Cennamo, N. SPR-Optical Fiber-Molecularly Imprinted Polymer Sensor for the Detection of Furfural in Wine. Biosensors 2021, 11, 72. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, B.D. Fiber optic SPR sensor for the detection of 3-pyridinecarboxamide (vitamin B3) using molecularly imprinted hydrogel. Sens. Actuators B Chem. 2013, 177, 279–285. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, B.D. Optical fiber sensor for the detection of tetracycline using surface plasmon resonance and molecular imprinting. Analyst 2013, 138, 7254. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Pesavento, M.; Zeni, L. High selectivity and sensitivity sensor based on MIP and SPR in tapered plastic optical fibers for the detection of l-nicotine. Sens. Actuators B Chem. 2014, 191, 529–536. [Google Scholar] [CrossRef]
- Koster, E.H.M.; Crescenzi, C.; den Hoedt, W.; Ensing, K.; de Jong, G.J. Fibers Coated with Molecularly Imprinted Polymers for Solid-Phase Microextraction. Anal. Chem. 2001, 73, 3140–3145. [Google Scholar] [CrossRef]
- Lépinay, S.; Ianoul, A.; Albert, J. Molecular imprinted polymer-coated optical fiber sensor for the identification of low molecular weight molecules. Talanta 2014, 128, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, H.Y.; Bottaro, C.S. Analysis of thiophenes in seawater: Molecularly imprinted polymer thin-film extraction with desorption electrospray ionization mass spectrometry. Int. J. Mass Spectrom. 2019, 443, 9–15. [Google Scholar] [CrossRef]
- Liu, L.; Hao, F.; Morgan, S.P.; Correia, R.; Norris, A.; Korposh, S. A reflection-mode fibre-optic sensor for breath carbon dioxide measurement in healthcare. Sens. Bio-Sens. Res. 2019, 22, 100254. [Google Scholar] [CrossRef]
- Wiebe, R.; Gaddy, V.L. The Solubility of Carbon Dioxide in Water at Various Temperatures from 12 to 40° and at Pressures to 500 Atmospheres. Critical Phenomena. J. Am. Chem. Soc. 1940, 62, 815–817. [Google Scholar] [CrossRef]
- Bell, J.; Nel, P.; Stuart, B. Non-invasive identification of polymers in cultural heritage collections: Evaluation, optimisation and application of portable FTIR (ATR and external reflectance) spectroscopy to three-dimensional polymer-based objects. Herit. Sci. 2019, 7, 95. [Google Scholar] [CrossRef]
- Asman, S.; Mohamad, S.; Sarih, N. Exploiting β-Cyclodextrin in Molecular Imprinting for Achieving Recognition of Benzylparaben in Aqueous Media. Int. J. Mol. Sci. 2015, 16, 3656. [Google Scholar] [CrossRef]
- Chatterjee, T.N.; Roy, R.B.; Tudu, B.; Pramanik, P.; Deka, H.; Tamuly, P.; Bandyopadhyay, R. Detection of theaflavins in black tea using a molecular imprinted polyacrylamide-graphite nanocomposite electrode. Sens. Actuators B Chem. 2017, 246, 840–847. [Google Scholar] [CrossRef]
- Mane, S.; Ponrathnam, S.; Chavan, N. Design and synthesis of cauliflower-shaped hydroxyl functionalized core-shell polymer. Des. Monomers Polym. 2015, 18, 723–733. [Google Scholar] [CrossRef]
- Pathak, A.K.; Singh, V.K. Theoretical assessment of D-shaped optical fiber chemical sensor associated with nanoscale silver strip operating in near-infrared region. Opt. Quantum Electron. 2020, 52, 199. [Google Scholar] [CrossRef]
- Mishra, G.P.; Kumar, D.; Chaudhary, V.S.; Kumar, S. Design and Sensitivity Improvement of Microstructured-Core Photonic Crystal Fiber Based Sensor for Methane and Hydrogen Fluoride Detection. IEEE Sens. J. 2022, 22, 1265–1272. [Google Scholar] [CrossRef]
- Pathak, A.K.; Rahman, B.M.A.; Viphavakit, C. Nanowire Embedded Micro-Drilled Dual-Channel Approach to Develop Highly Sensitive Biosensor. IEEE Photonics Technol. Lett. 2022, 34, 707–710. [Google Scholar] [CrossRef]
- Nwosu, F.O.; Saliu, O.D.; Oyinlola, K.A.; Ojo, E.O. Colorimetric Based Polysorbate Crosslinked Cellulose-Ag–Cu Nanohybrid Sensor for Urea Sensing Applications. J. Polym. Environ. 2020, 28, 1475–1483. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathak, A.K.; Limprapassorn, P.; Kongruttanachok, N.; Viphavakit, C. Molecularly Imprinted Polymer-Based Optical Sensor for Isopropanol Vapor. J. Sens. Actuator Netw. 2022, 11, 46. https://doi.org/10.3390/jsan11030046
Pathak AK, Limprapassorn P, Kongruttanachok N, Viphavakit C. Molecularly Imprinted Polymer-Based Optical Sensor for Isopropanol Vapor. Journal of Sensor and Actuator Networks. 2022; 11(3):46. https://doi.org/10.3390/jsan11030046
Chicago/Turabian StylePathak, A. K., P. Limprapassorn, N. Kongruttanachok, and C. Viphavakit. 2022. "Molecularly Imprinted Polymer-Based Optical Sensor for Isopropanol Vapor" Journal of Sensor and Actuator Networks 11, no. 3: 46. https://doi.org/10.3390/jsan11030046
APA StylePathak, A. K., Limprapassorn, P., Kongruttanachok, N., & Viphavakit, C. (2022). Molecularly Imprinted Polymer-Based Optical Sensor for Isopropanol Vapor. Journal of Sensor and Actuator Networks, 11(3), 46. https://doi.org/10.3390/jsan11030046







