Tea Tree Oil Induces Systemic Resistance against Fusarium wilt in Banana and Xanthomonas Infection in Tomato Plants
Abstract
:1. Introduction
2. Results
2.1. Efficacy of TTO Compared to Chemical Fungicides in Field-Grown Banana Plants
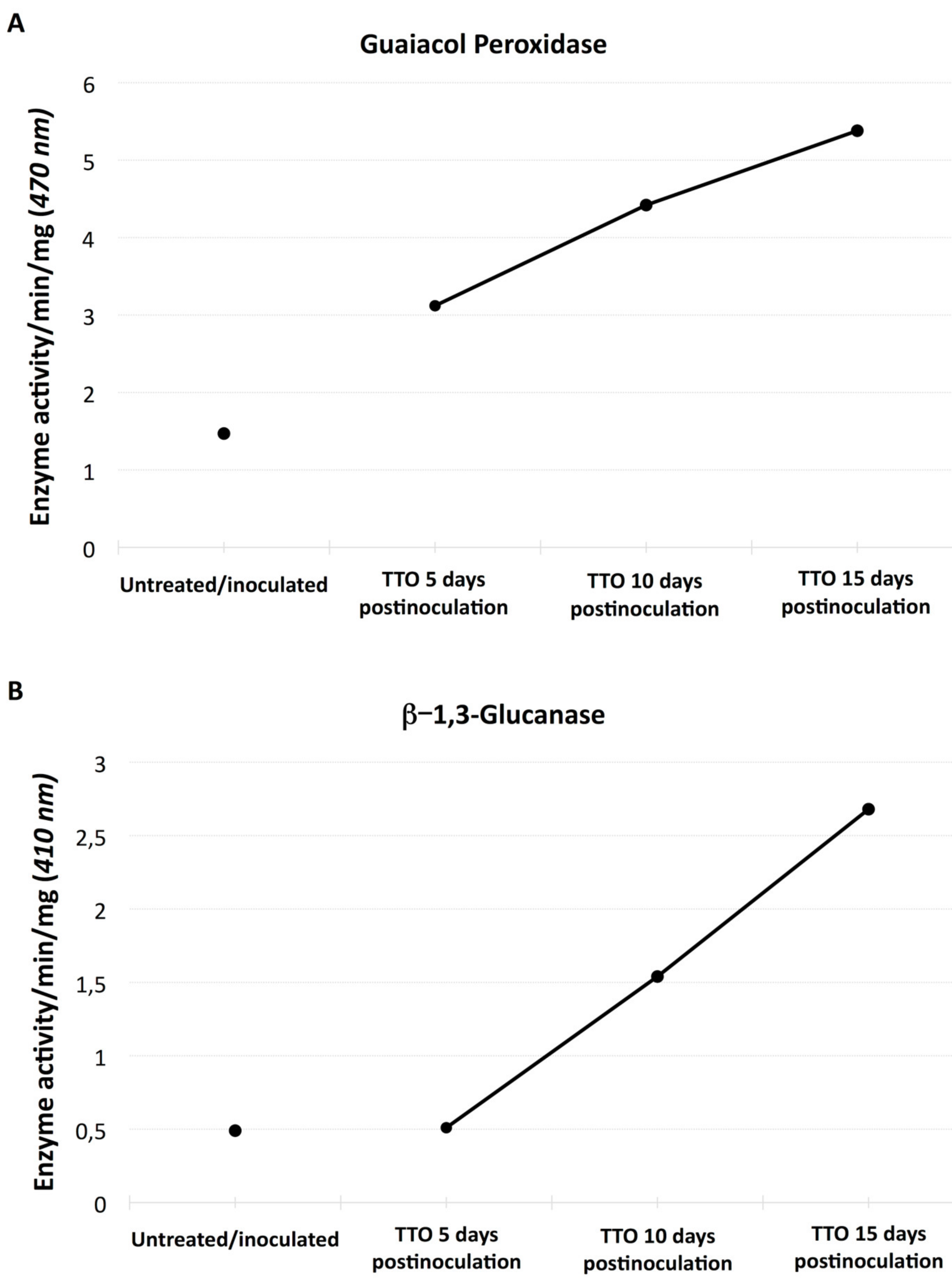
2.2. Induction of Enzyme Activities in Banana Plants
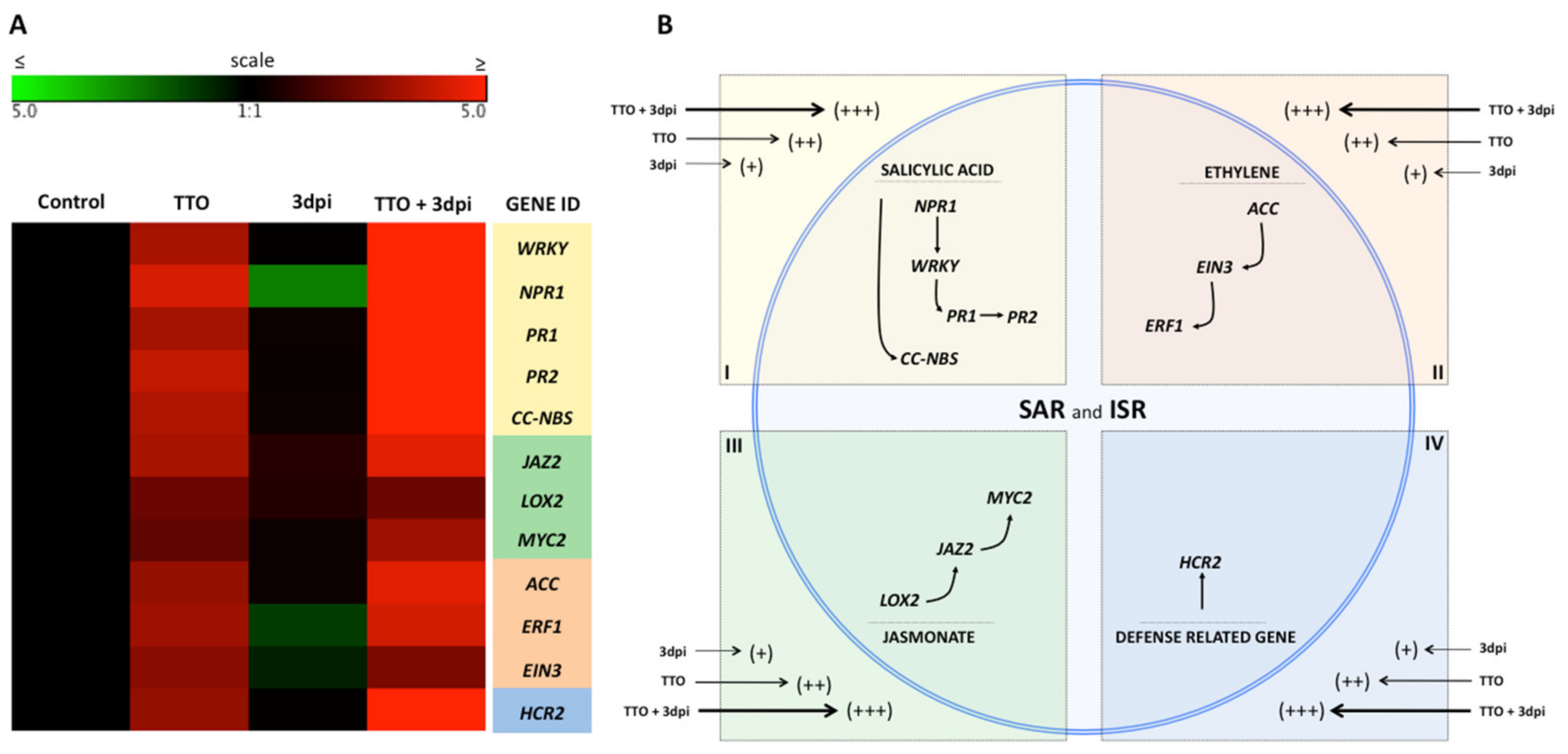
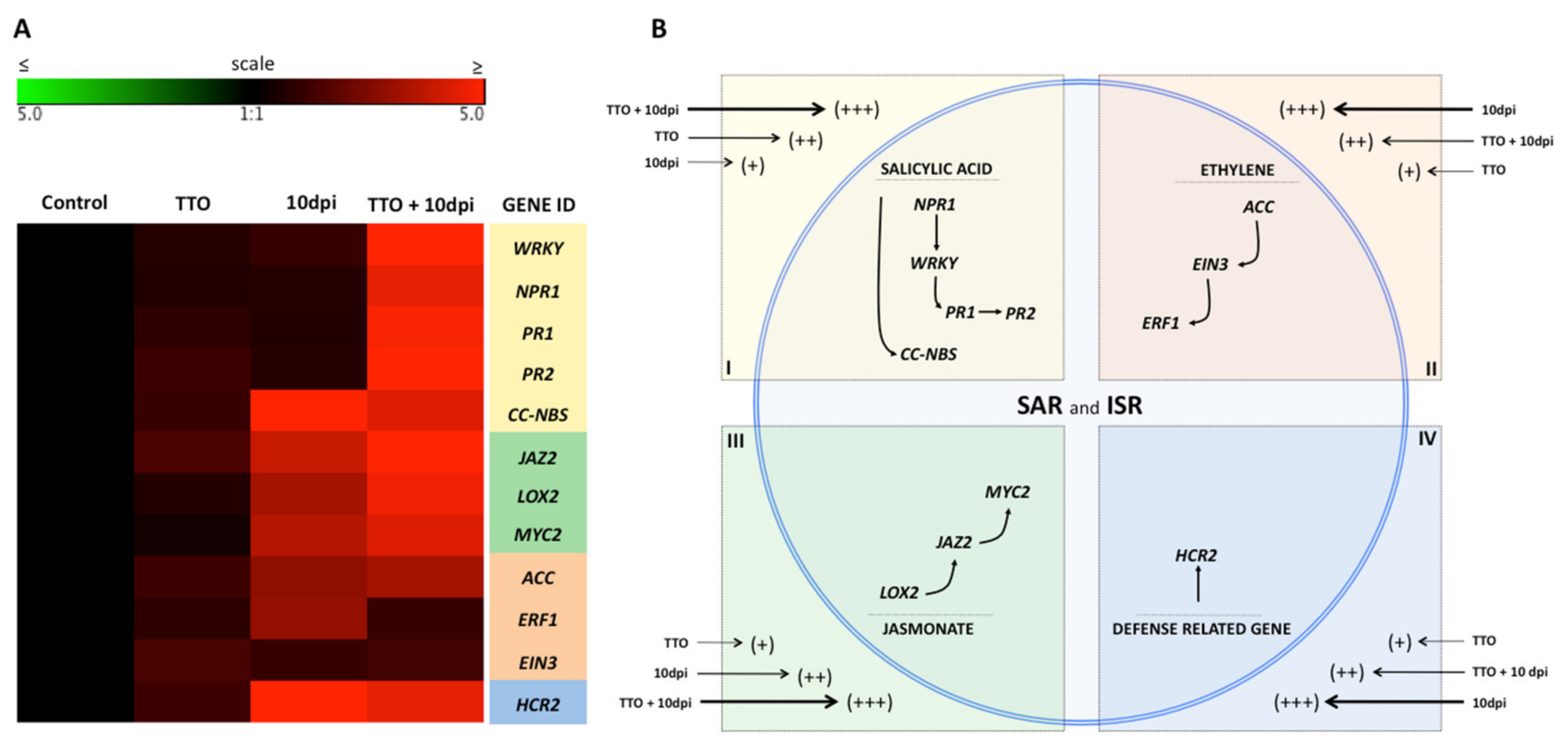
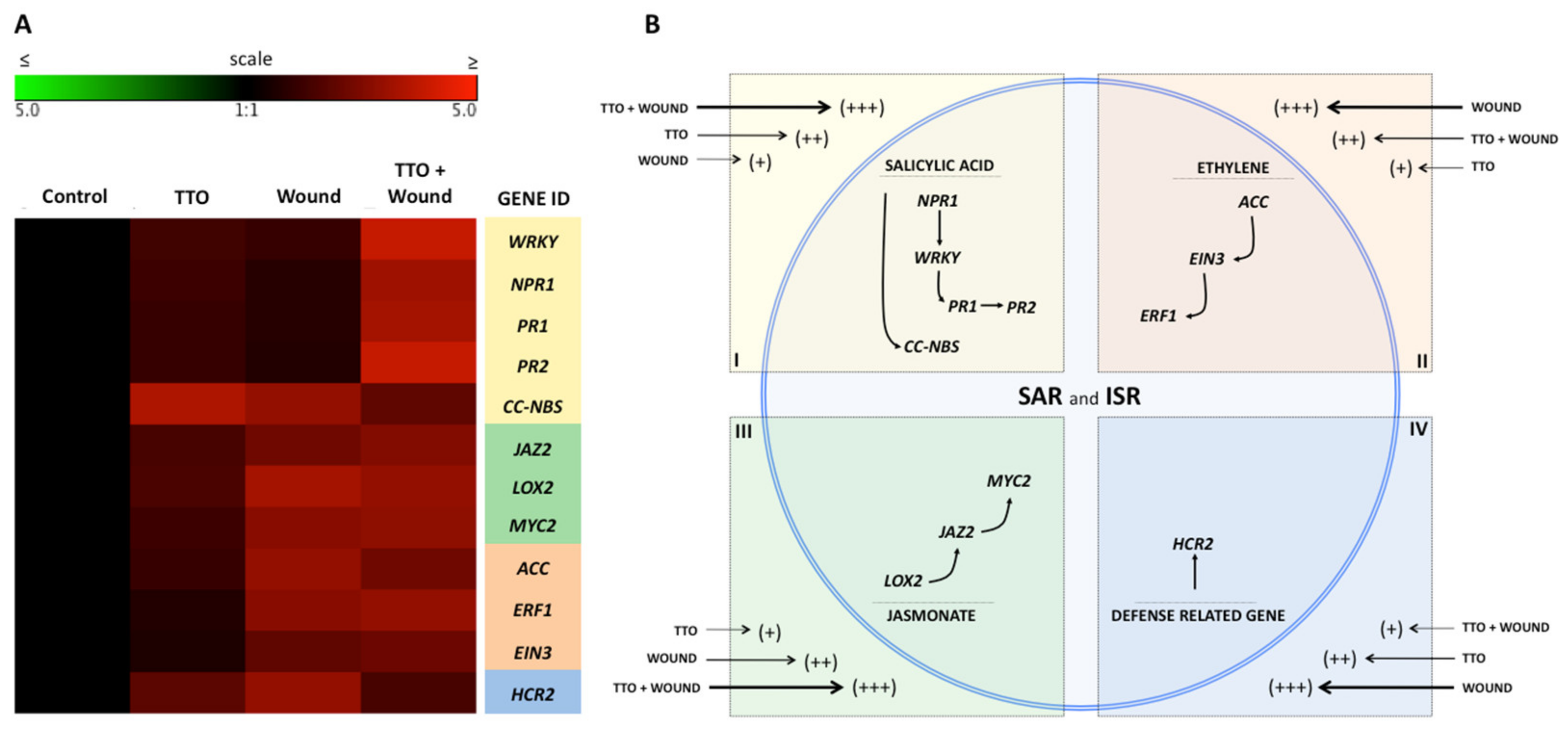
2.3. Expression of SAR and ISR Defense-Related Genes in Banana
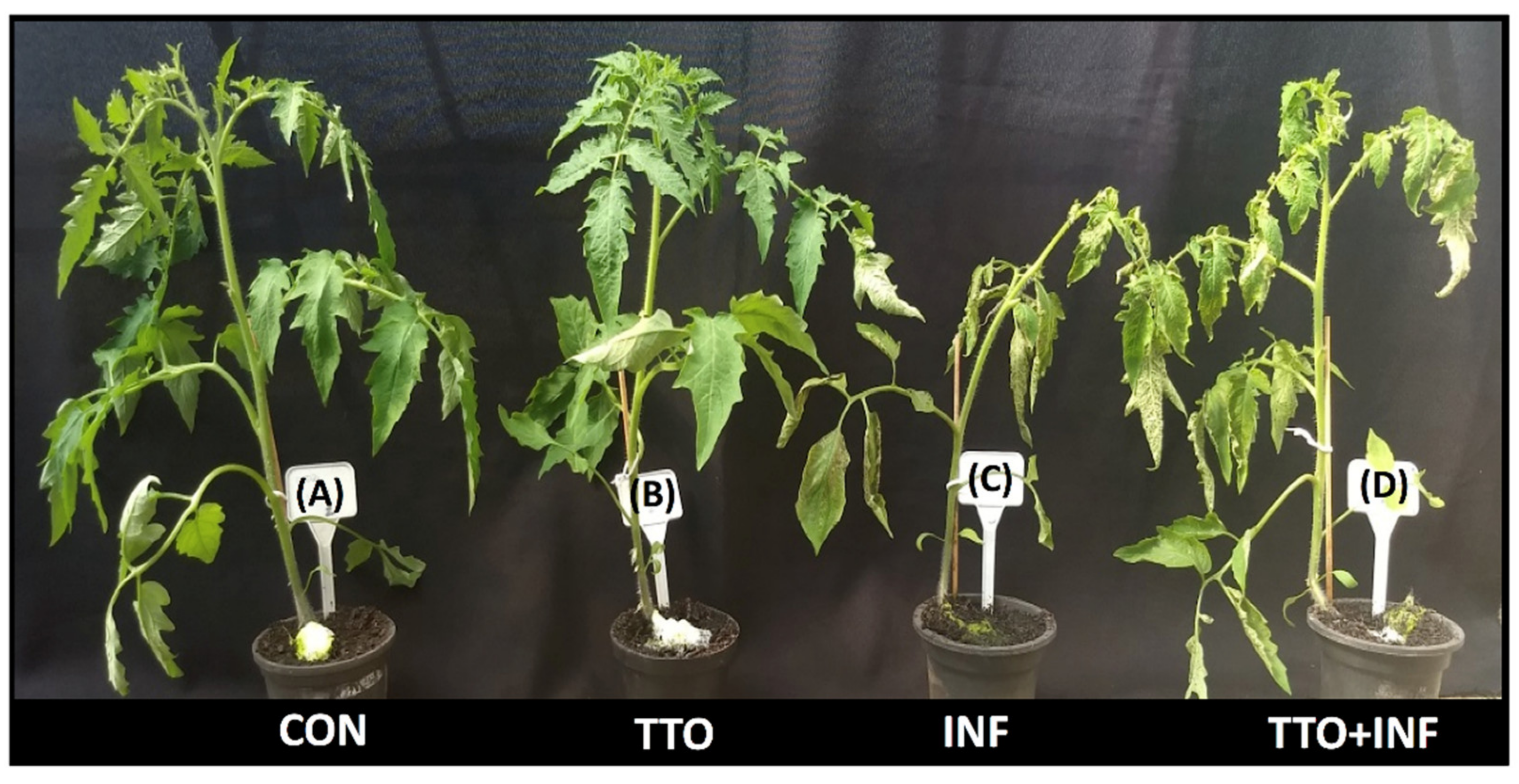
2.4. Induction of Resistance in Tomatoes vs X. Campestris pv. Vesicatoria
2.5. TTO Primes Tomato Plants to Have a Defense Reaction to a Subsequent Challenge
3. Discussion
4. Materials and Methods
4.1. Banana vs F. oxysporum
4.2. Induction of Systemic Resistance in Field-Grown Banana Plants
4.3. Analysis of PR Proteins Activity in Banana Plants
4.4. Activities PR Proteins in Banana Root Samples
4.5. Expression of SAR and ISR Defense-Related Genes in Banana
4.6. Induction of Resistance in Tomatoes vs X. campestris pv. vesicatoria
4.6.1. Plants
4.6.2. Pathogen and Inoculation
4.6.3. Experimental Design
4.7. RNA Extraction and Reverse Transcription
4.7.1. RNA Extraction and Reverse Transcription of Banana Plants
4.7.2. RNA Extraction and Reverse Transcription of Tomato Plants
4.8. Gene Expression Analysis
4.9. Analysis of Housekeeping Reference Genes
4.10. Gene Array
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janeway, C.A.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrios, G. Plant Pathology, 5th ed.; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Dalio, R.J.D.; Magalhães, D.M.; Rodrigues, C.M.; Arena, G.D.; Oliveira, T.S.; Souza-Neto, R.R.; Picchi, S.C.; Martins, P.M.M.; Santos, P.J.C.; Maximo, H.J.; et al. PAMPs, PRRs, effectors and R-genes associated with citrus–pathogen interactions. Ann. Bot. 2017, 119, 749–774. [Google Scholar]
- Liu, C.; Zhao, C.; Pan, H.-H.; Kang, J.; Yu, X.-T.; Wang, H.-Q.; Li, B.-M.; Xie, Y.-Z.; Chen, R.-Y. Chemical constituents from Inonotus obliquus and their biological activities. J. Nat. Prod. 2013, 77, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations Rome. Available online: http://www.fao.org/3/ca5625en/ca5625en.pdf (accessed on 2 September 2020).
- O’Donnell, K.; Gueidan, C.; Sink, S.; Johnston, P.R.; Crous, P.W.; Glenn, A.E.; Riley, R.; Zitomer, N.C.; Colyer, P.; Waalwijk, C.; et al. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet. Biol. 2009, 46, 936–948. [Google Scholar] [CrossRef]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Kema, G.H.J.; Ma, L.-J. Impact of diseases on export and smallholder production of banana. Annu. Rev. Phytopathol. 2015, 53, 269–288. [Google Scholar] [CrossRef]
- Obradovic, A.; Jones, J.B.; Momol, M.T.; Balogh, B.; Olson, S.M. Management of tomato bacterial spot in the field by foliar applications of bacteriophages and SAR inducers. Plant Dis. 2004, 88, 736–740. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B.; Jones, J.P.; Stall, R.E.; Zitter, T.A. Compendium of Tomato Diseases; APS Press: St. Paul, MN, USA, 1991. [Google Scholar]
- Vallad, G.E.; Subbarao, K.V. Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology 2008, 98, 871–885. [Google Scholar] [CrossRef] [Green Version]
- Metraux, J.-P.; Nawrath, C.; Genoud, T. Systemic acquired resistance. Euphytica 2002, 124, 237–243. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhage, A.; van Wees, S.C.; Pieterse, C.M. Plant immunity: It’s the hormones talking, but what do they say? Plant Physiol. 2010, 154, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-W.; Kaimoyo, E.; Kumar, D.; Mosher, S.; Klessig, D.F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 2007, 318, 113–116. [Google Scholar] [CrossRef]
- Pascholati, S.F.; Blumer, S.; Rezende, D.C.; Brand, S.C. Systemic acquired resistance (SAR) x induced systemic resistance (ISR). In NEFIT. Indução de Resistência—Novos Conceitos e Aplicações; UFLA: Lavras, Brazil, 2010; pp. 29–40. [Google Scholar]
- Shah, J.; Zeier, J. Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 2013, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z. Molecular basis for jasmonate and ethylene signal interactions in Arabidopsis. J. Exp. Bot. 2014, 65, 5743–5748. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Dalio, R.J.D.; Fleischmann, F.; Humez, M.; Osswald, W. Phosphite Protects Fagus sylvatica seedlings towards Phytophthora plurivora via local toxicity, priming and facilitation of pathogen recognition. PLoS ONE 2014, 9, e87860. [Google Scholar] [CrossRef] [Green Version]
- Havko, N.E.; Major, I.T.; Jewell, J.B.; Attaran, E.; Browse, J.; Howe, G.A. Control of carbon assimilation and partitioning by jasmonate: An accounting of growth–defense tradeoffs. Plants 2016, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinas, N.A.; Saze, H.; Saijo, Y. Epigenetic control of defense signaling and priming in plants. Front. Plant Sci. 2016, 7, 1201. [Google Scholar] [CrossRef]
- Carson, C.; Riley, T.V. Antimicrobial activity of the essential oil of Melaleuca alternifolia. Lett. Appl. Microbiol. 1993, 16, 49–55. [Google Scholar] [CrossRef]
- Carson, C.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the antimicrobial actions of tea tree oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Cheng, S.; Wang, H.; Yu, D.; Mungai, C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 2013, 114, 1642–1649. [Google Scholar] [CrossRef]
- Shao, X.; Wang, H.; Xu, F.; Cheng, S. Effects and possible mechanisms of tea tree oil vapor treatment on the main disease in postharvest strawberry fruit. Postharvest Biol. Technol. 2013, 77, 94–101. [Google Scholar] [CrossRef]
- Reuveni, M.; Barbier, M.; Viti, A.J. Essential tea tree oil as a tool to combat black Sigatoka in banana. Outlooks Pest Manag. 2020. [Google Scholar] [CrossRef]
- Reuveni, M.; Neifeld, D.; Dayan, D.; Kotzer, Y. BM-608—A novel organic product based on essential tea tree oil for the control of fungal diseases in tomato. Acta Hortic. 2009, 808, 129–132. [Google Scholar] [CrossRef]
- Martillo, E.E.; Reuveni, M. A new potent bio-fungicide for the control of Banana Black Sigatoka. Phytopathology 2009, 99, S80. [Google Scholar]
- Vardi, Y.; Reuveni, M. Antifungal activity of a new broad spectrum biocide in the controlling of plant diseases. Phytopathology 2009, 99, 5134. [Google Scholar]
- Carson, C.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (Tea Tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuveni, M.; Sanches, E.; Barbier, M.; Ramirez, F. Mode of activity of Timorex Gold against black Sigatoka (Mycosphaerella fijiensis) on banana leaves. In Proceedings of the 4th International Banana Congress, San Jose, Costa Rica, 20–24 February 2010. [Google Scholar]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Nimchuk, Z.; Eulgem, T.; Iii, B.F.H.; Dangl, J.L. Recognition and response in the plant immune system. Annu. Rev. Genet. 2003, 37, 579–609. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U.; Pieterse, C.M.; Mauch-Mani, B. Priming in plant–pathogen interactions. Trends Plant Sci. 2002, 7, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Acharya, K.; Chandra, S.; Chakraborty, N.; Acharya, R. Nitric oxide functions as a signal in induced systemic resistance. Arch. Phytopathol. Plant Prot. 2011, 44, 1335–1342. [Google Scholar] [CrossRef]
- Gullner, G.; Zechmann, B.; Künstler, A.; Király, L. The signaling roles of glutathione in plant disease resistance. In Glutathione in Plant Growth, Development, and Stress Tolerance; Hossain, M.A., Mostofa, M.G., Vivancos, P.D., Burritt, D.J., Fujita, M., Tran, L.S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 331–357. [Google Scholar]
- Tanou, G.; Fotopoulos, V.; Molassiotis, A. Priming against environmental challenges and proteomics in plants: Update and agricultural perspectives. Front. Plant Sci. 2012, 3, 216. [Google Scholar] [CrossRef] [Green Version]
- Máximo, H.J.; Dalio, R.J.D.; Dias, R.O.; Litholdo, C.G.; Felizatti, H.L.; Machado, M.A. PpCRN7 and PpCRN20 of Phythophthora parasitica regulate plant cell death leading to enhancement of host susceptibility. BMC Plant Biol. 2019, 19, 544. [Google Scholar] [CrossRef]
- Rodrguez-Garca, C.; Peraza-Echeverra, L.; Islas-Flores, I.; Canto-Canch, B.; Grijalva-Arango, R. Isolation of retro-transcribed RNA from in vitro Mycosphaerella fijiensis-infected banana leaves. Genet. Mol. Res. 2010, 9, 1460–1468. [Google Scholar] [CrossRef]
- Dalio, R.J.D.; Maáximo, H.J.; Oliveira, T.S.; Azevedo, T.M.; Felizatti, H.L.; Camopos, M.A.; Machado, M.A. Molecular basis of Citrus sunki susceptibility and Poncirus trifoliate resistance upon Phytophthora parasitica attack. Mol. Plant-Microbe Interact. 2018, 31, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Máximo, H.J.; Dalio, R.J.D.; Rodrigues, C.M.; Breton, M.C.; Machado, M.A. Reference genes for RT-qPCR analysis in Citrus and Poncirus infected by zoospores of Phytophthora parasitica. Trop. Plant Pathol. 2017, 42, 76–85. [Google Scholar] [CrossRef]
- Liva, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fernald, R.D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, H.W.; Wallis, A.W. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]


| Lycopersicon esculentum mill | |||
| ID_GENE | primerF | primerR | amplicon |
| NPR-1 | GGTCAGTGTGCTCGCCTATT | CACAGCTGGCCTACAAGCTAC | 109 |
| PR-1 | GGATCGGACAACGTCCTTAC | GCAACATCAAAAGGGAAATAAT | 124 |
| PR-2 | TATAGCCGTTGGAAACGAAG | TGATACTTTGGCCTCTGGTC | 95 |
| PR-3 | CAATTCGTTTCCAGGTTTTG | ACTTTCCGCTGCAGTATTTG | 88 |
| BSMT1 | GTTTAACGAGGCCGTTGATG | TCGTCAAGGAAACTGTCACG | 200 |
| ERF1 | GGGGTCCTTGGTCTCTACTCA | GTAGCTTTTAAAACAGCAGCTGGA | 112 |
| ACC | AGCTACGTCAATGGCAGCAC | AGGAAGGGTGGGGACTTCTG | 79 |
| MYC2 | ACCACATGAAAACAAAGCTGGAC | TCTCCGCCTCTACGTGGTTT | 95 |
| WRKY | ATCCTCGCCAGCAGTTAGCA | TCGTGGAGCTTTGCAAGGTAG | 145 |
| CC-NBS | TTCTGCAGAGTGTTCAATGGCAGC | CACAAACCCTTCAGCAACCCACAA | 185 |
| JAZ2 | CCCCACCACCACTCAGACTAA | TATGGCGCTCTAGCCGTGT | 117 |
| LOX2 | ACTGGTAGACCACCAACACGA | ACGCTCGTCTCTCGGTACAT | 93 |
| EIN3 | CAGAAGTTCGACTAGAAACGGCTAT | TCCTCTGCTCTCAAGGATACAACA | 147 |
| HCR2 | GCATGCAAGGACTGGTATGGA | TCTCGAGAAAAGGGAGGGATGA | 124 |
| Musa paradisiaca | |||
| ID_GENE | primerF | primerR | amplicon |
| NPR-1 | GGAGATCCACAAGTAGGTGAAGC | AGTCTTGCCAGAGCAACTCG | 104 |
| PR-1 | TCCGGCCTTATTTCACATTC | GCCATCTTCATCATCTGCAA | 126 |
| PR-2 | TCGCTGGGCTGTGGTAAGT | TCGCTGGGCTGTGGTAAGT | 82 |
| PR-3 | GTCACCACCAACATCATCAA | CCAGCAAGTCGCAGTACCTC | 150 |
| BSMT1 | GTTTAACGAGGCCGTTGATG | TCGTCAAGGAAACTGTCACG | 200 |
| ERF1 | CCCAAATGTTGGTCCGTTTC | TCGCTGTCTTCCACGATTCA | 79 |
| ACC | GATGCTGCACATCGGCTAGT | GCCACCTGAATACGGCAGAC | 123 |
| MYC2 | CGGATCTACCGACGTGGTCT | AGCGTCCGGAGAGCTAAAGT | 82 |
| housekeeping genes | |||
| ID_GENE | primerF | primerR | amplicon |
| GAPC2 | TCTTGCCTGCTTTGAATGGA | TGTGAGGTCAACCACTGCGACAT | 80 |
| DIM1 | CGAAACCTGTATGCAGATGG | ACGGTTGAGGGATCGTAAAG | 138 |
| CYP | CGGATCTCAGTTCTTCGTCTG | ACTTTCTCGATGGCCTTGAC | 111 |
| FBOX | TTGGAAACTCTTTCGCCACT | CAGCAACAAAATACCCGTCT | 112 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalio, R.J.D.; Maximo, H.J.; Roma-Almeida, R.; Barretta, J.N.; José, E.M.; Vitti, A.J.; Blachinsky, D.; Reuveni, M.; Pascholati, S.F. Tea Tree Oil Induces Systemic Resistance against Fusarium wilt in Banana and Xanthomonas Infection in Tomato Plants. Plants 2020, 9, 1137. https://doi.org/10.3390/plants9091137
Dalio RJD, Maximo HJ, Roma-Almeida R, Barretta JN, José EM, Vitti AJ, Blachinsky D, Reuveni M, Pascholati SF. Tea Tree Oil Induces Systemic Resistance against Fusarium wilt in Banana and Xanthomonas Infection in Tomato Plants. Plants. 2020; 9(9):1137. https://doi.org/10.3390/plants9091137
Chicago/Turabian StyleDalio, Ronaldo J. D., Heros J. Maximo, Rafaela Roma-Almeida, Janaína N. Barretta, Eric M. José, Agnelo J. Vitti, Daphna Blachinsky, Moshe Reuveni, and Sérgio F. Pascholati. 2020. "Tea Tree Oil Induces Systemic Resistance against Fusarium wilt in Banana and Xanthomonas Infection in Tomato Plants" Plants 9, no. 9: 1137. https://doi.org/10.3390/plants9091137






