Phytochemical and Pharmacological Study of the Eysenhardtia Genus
Abstract
1. Introduction
2. Taxonomic Classification
2.1. Description of the Eysenhardtia Genus
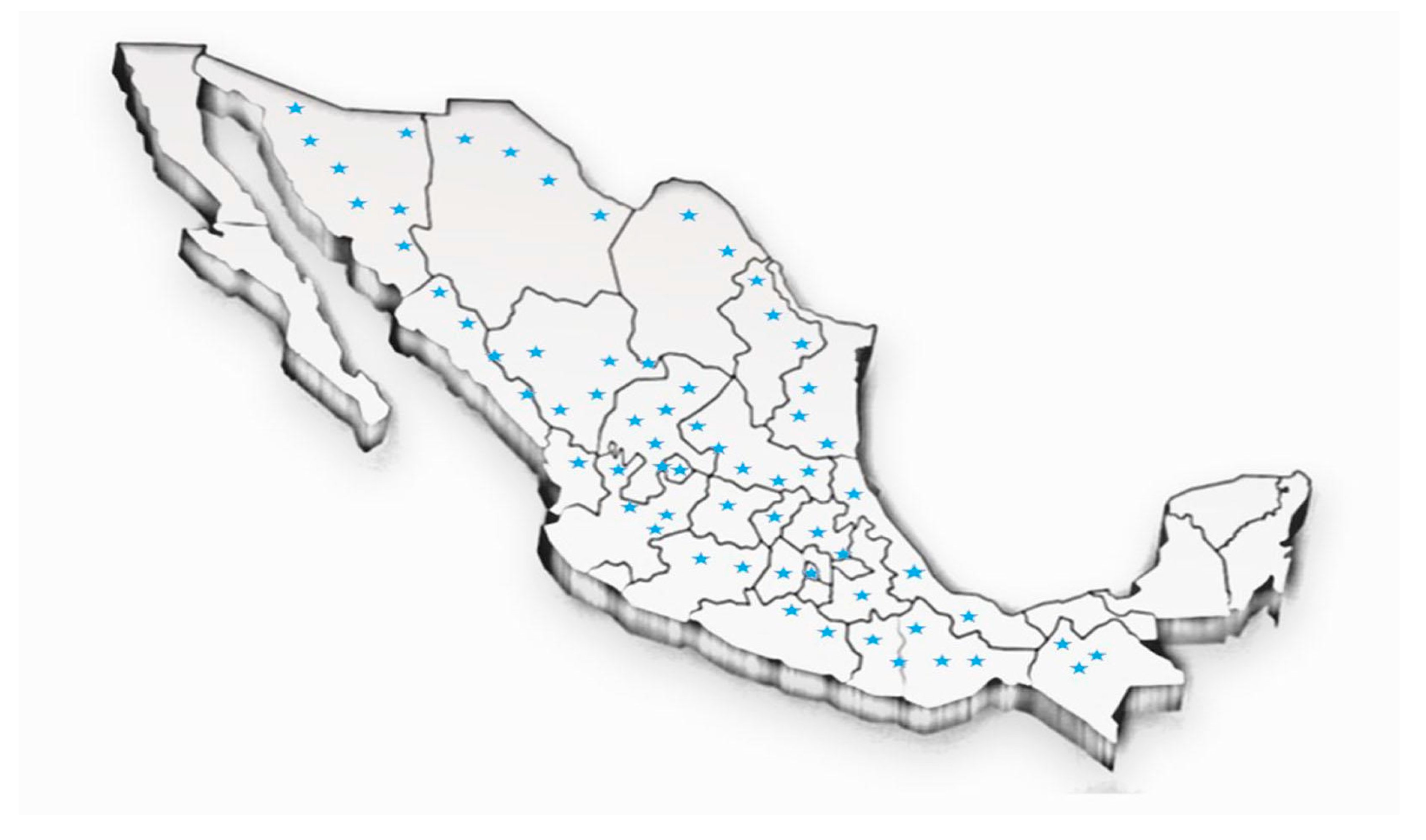
2.2. Geographical Distribution of the Eysenhardtia Genus
3. Secondary Metabolites Isolated from the Eysenhardtia Genus
4. Pharmacological Activities
4.1. Urinary Disorders
4.2. Antimicrobial Activity
4.3. Antidiabetic Activity
4.4. Anti-Inflammatory Activity
4.5. Antinociceptive Activity
4.6. Antidiarrheal
4.7. Muscle Relaxant
4.8. Cytotoxic Activity
4.9. Nanoparticles and Eysenhardtia
4.10. Other Applications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petrovska, B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed. Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Dar, R.A.; Shahnawaz, M.; Qazi, P.H. General overview of medicinal plants: A review. J. Phytopharm. 2017, 6, 349–351. [Google Scholar]
- Heinrich, M.; Ankli, A.; Frei, B.; Weimann, C.; Sticher, O. Medicinal Plants in Mexico: Healers’ Consensus and Cultural Importance. Soc. Sci. Med. 1998, 47, 1859–1871. [Google Scholar] [CrossRef]
- Esquivel, R.G.; Pérez, E.C.; Ochoa, A.Z.; García, M.E.P. Ethnomedicinal plants used for the treatment of dermatological affections on the Purépecha Plateau, Michoacán, Mexico. Acta Bot. Mex. 2018, 125, 95–132. [Google Scholar] [CrossRef]
- Monardes, N.B. Historia Medicinal de las Cosas que se Traen de Nuestras Indias Occidentales que Sirven en Medicina; Padilla Libros: Sevilla, Spain, 1565; pp. 1–276. [Google Scholar]
- Acuña, A.U.; Amat-Guerri, F. Early History of Solution Fluorescence: The Lignum nephriticum of Nicolás Monardes. Springer Ser. Fluoresc. 2008, 4, 3–20. [Google Scholar]
- Alvarez, L.; Rios, M.Y.; Esquivel, C.; Chavez, M.I.; Delgado, G.; Aguilar, M.I.; Villarreal, M.L.; Navarro, V. Cytotoxic isoflavans from Eysenhardtia polystachya. J. Nat. Prod. 1998, 61, 767–770. [Google Scholar] [CrossRef]
- Muyskens, M.; Vitz, E. The Fluorescence of Lignum nephriticum: A Flash Back to the Past and a Simple Demonstration of Natural Substance Fluorescence. J. Chem. Educ. 2009, 83, 765–768. [Google Scholar] [CrossRef]
- Torres, P.A.A.; Lomeli, M.G.R.; Lopez, F.D.; Fuentes, F.J.T.; Richter, H.G.; Silva, J.A.G. Natural decay resistance of Eysenhardtia polystachya (Ortega) Sarg. Int. Wood Prod. J. 2010, 1, 81–84. [Google Scholar]
- Alarcon-Aguilar, F.J.; Roman-Ramos, R.; Pérez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Zapata-Morales, J.R.; Arana-Argáez, V.; Torres-Romero, J.C.; Ramirez-Villanueva, E.; Pérez-Medina, S.E.; Ramirez-Morales, M.A.; Juarez-Mendez, M.A.; Infante-Barrios, Y.P.; Marinez-Gutierrez, F.; et al. Pharmacological and toxicological study of a chemical-standardized ethanol extract of the branches and leaves from Eysenhardtia polystachya (Ortega) Sarg. (Fabaceae). J. Ethnopharmacol. 2018, 224, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, E.; De Bernardi, M.; Fronza, G. Coatline A and B, two C-Glucosyl-α-hydroxydihydrochalcones from Eysenhardtia polystachya. Phytochemistry 1982, 21, 2931–2933. [Google Scholar] [CrossRef]
- Pérez-Gutierrez, R.; Garcia-Baez, E. Evaluation of antidiabetic, antioxidant and antiglycating activities of the Eysenhardtia polystachya. Pharmacogn. Mag. 2014, 10, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gutierrez, R.M. Evaluation of anti-inflammatory activity of the bark of Eysenhardtia polystachya in experimental animal models. Afr. J. Pharm. Pharmacol. 2015, 9, 230–236. [Google Scholar]
- Pablo-Pérez, S.S.; Parada-Cruz, B.; Barbier, O.C.; Meléndez-Camargo, M.E. The ethanolic extract of Eysenhardtia polystachya (Ort.) Sarg. Bark and its fractions delay the progression of rheumatoid arthritis and show antinociceptive activity in murine models. Iran J. Pharm. Res. 2018, 17, 236–248. [Google Scholar] [PubMed]
- Camacho-Morfin, F. Germinación de semillas de palo dulce (Eysenhardtia polystachya). Rev. Cienc. For. 1987, 62, 3–13. [Google Scholar]
- Rivas-Morales, C.; Oranday-Cardenas, M.A.; Verde-Star, M.J.; Morales-Rubio, M.E.; Garza-González, E. Activity of extracts from two Eysenhardtia species against microorganisms related to urinary tract infections. Br. J. Biomed. Sci. 2009, 66, 102–106. [Google Scholar] [CrossRef]
- Sarg, O. Eysenhardtia polystachya. Silva N. Am. 1892, 3, 1–4. Available online: http://www.conabio.gob.mx/conocimiento/info_especies/arboles/doctos/28-legum18m.pdf (accessed on 13 July 2020).
- CONAFOR. Fichas técnicas sobre características tecnológicas y usos de maderas comercializadas en México. Tomo II. SEMARNAT 2007, 1, 83–84. [Google Scholar]
- Lang, J.M. Eysenhardtia (Leguminosae): Taxonomic Revision and Relationships. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1972. Available online: https://lib.dr.iastate.edu/rtd/4750 (accessed on 13 July 2020).
- Gutierrez, L.; Sumano, H.; Rivero, F.; Alcala, Y. Ixodicide activity of Eysenhardtia polystachya against Rhipicephalus (Boophilus) microplus. J. Anim. Sci. 2015, 93, 1980–1986. [Google Scholar] [CrossRef]
- Contu, S. Eysenhardtia polystachya. IUCN Red List Threat. Species 2012, 1. [Google Scholar] [CrossRef]
- Burns, D.T.; Dalgarno, B.G.; Gargan, P.E.; Grimshaw, J. An isoflavone and a coumestan from Eysenhardtia polystachya-Robert Boyle’s fluorescent acid-base indicator. Phytochemistry 1984, 23, 167–169. [Google Scholar] [CrossRef]
- Alvarez, L.; Delgado, G. C-and O-glycosyl-α-hydroxydihydrochalcones from Eysenhardtia polystachya. Phytochemistry 1999, 50, 681–687. [Google Scholar] [CrossRef]
- Hoffmann, J.J.; Wachter, G.A.; Gutterman, J.U. Antibacterial and Antifungal Flavonones from Eysenhardtia texana. U.S. Patent 6,136,849, 24 October 2000. [Google Scholar]
- Wächter, G.A.; Hoffmann, J.J.; Furbacher, T.; Blake, M.E.; Timmermanm, B.N. Antibacterial and antifungal flavanones from Eysenhardtia texana. Phytochemistry 1999, 52, 1469–1471. [Google Scholar] [CrossRef]
- Pérez, R.M.G.; Vargas, S.R.; Perez, G.S.; Zavala, M.S.; Perez, C.G. Antiurolithiatic activity of 7-hydroxy-2′,4′,5′-trimethoxyisoflavone and 7-hydroxy-4′- methoxyisoflavone from Eysenhardtia polystachya. J. Herbs. Spices Med. Plants 2000, 7, 27–34. [Google Scholar] [CrossRef]
- Pérez, R.M.G.; Vargas, R.S.; García, L.M.D.; Dávila, L.B. Efecto de isoflavonas aisladas de la corteza de Eysenhardtia polystachya sobre el crecimiento de cristales de oxalato y fosfato de calcio urinario. Bol. Col. Mex. Urol. 2002, 17, 134–139. [Google Scholar]
- Narváez-Mastache, J.M.; Garduño-Ramírez, M.L.; Alvarez, L.; Delgado, G. Antihyperglycemic activity and chemical constituents of Eysenhardtia platycarpa. J. Nat. Prod. 2006, 69, 1687–1691. [Google Scholar] [CrossRef]
- Narváez, J.M.; Soto, C.; Delgado, G. Antioxidant evaluation of Eysenhardtia species (Fabaceae): Relay synthesis of 3-O-Acetyl-11α,12α-epoxy-oleanan-28,13β-olide isolated from E. platycarpa and its protective effect in experimental diabetes. Biol. Pharm. Bull. 2007, 30, 1503–1510. [Google Scholar] [CrossRef]
- Narvaez, J.; Novillo, F.; Delgado, G. Antioxidant aryl-prenylcoumarin, flavan-3-ols and flavonoids from Eysenhardtia subcoriacea. Phytochemistry 2008, 69, 451–456. [Google Scholar] [CrossRef]
- Narváez, J.M.; Soto, C.; Delgado, G. Hypoglycemic and antioxidant effects of Subcoriacin in normal and streptozotocin-induced diabetic rats. J. Mex. Chem. Soc. 2010, 54, 240–244. [Google Scholar]
- Pérez, R.M.G.; Garcia, A.H.C.; Muñiz, A.R. Properties of Flavonoids Isolated from the Bark of Eysenhardtia polystachya and their effect on oxidative stress in streptozotocin-induced diabetes mellitus in mice. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Pérez, R.M.G.; Garcia, A.H.C.; Mota, J.M.F. Dihydrochalcones from the Bark of Eysenhardtia polystachya inhibits formation of advanced glycation end products at multiple stages in vitro studies. J. Pharm. Pharmacol. 2017, 1, 3–23. [Google Scholar]
- Pérez, R.M.G.; García, A.H.C.; Paredes, S.P.C.; Muñiz, A.R.; Mota, J.M.F.; Flores-Valle, S.O. 3′-O-β-d-glucopyranosyl-α,4,2′,4′,6′-pentahydroxy-dihydrochalcone, from bark of Eysenhardtia polystachya prevents diabetic nephropathy via inhibiting protein glycation in STZ-nicotinamide induced diabetic mice. Molecules 2019, 24, 1214. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.M.G.; Vargas, R.S.; Perez, S.G.; Zavala, M.S. Antiurolithiatic activity of Eysenhardtia polystachya aqueous extract on rats. Phyther. Res. 1998, 12, 144–145. [Google Scholar] [CrossRef]
- Pablo, S.S.P.; Estévez, M.M.C.; Meléndez, M.E.C. Diuretic activity of the bark of Eysenhardtia polystachya. Bangladesh J. Pharmacol. 2016, 11, 212–217. [Google Scholar] [CrossRef]
- Aggarwal, A.; Singla, S.K.; Tandon, C. Urolithiasis: Phytotherapy as an adjunct therapy. Indian J. Exp. Biol. 2014, 53, 103–111. [Google Scholar]
- Zavala, M.A.S.; Perez, S.G.; Perez, R.M.G. Antimicrobial screening of some medicinal plants. Phyther. Res. 1997, 11, 368–371. [Google Scholar] [CrossRef]
- Rosas-Piñon, Y.; Mejia, A.; Diaz-Ruiz, G.; Aguilar, M.I.; Sanchez-Nieto, S.; Rivero-Cruz, J.F. Ethnobotanical survey and antibacterial activity of plants used in the Altiplane region of Mexico for the treatment of oral cavity infections. J. Ethnopharmacol. 2012, 141, 860–865. [Google Scholar] [CrossRef]
- Pérez-Medina, S.E.; Alonso-Castro, A.J. Determinación del efecto analgésico de D-pinitol, un compuesto obtenido de Eysenhardtia polystachya (Ortega) Sarg. (Fabaceae). Jovenes Ciencia. Rev. Divulg. Cient. 2018, 4, 1197–1201. [Google Scholar]
- Cortés-Arroyo, A.R.; Lara-Chacón, B.; Aoki-Maki, K. Screening and selection of plants by positive pharmacologic effect on jejunum muscular contractility. Pharm. Biol. 2004, 42, 24–29. [Google Scholar] [CrossRef][Green Version]
- Pérez, R.M.G.; Martinez, F.F.J.; Garcia, A.H.C.; Hoyo, C.V. Silver nanoparticles synthesized using Eysenhardtia polystachya and assessment of the inhibition of glycation in multiple stages in vitro and in the Zebrafish Model. J. Clust. Sci. 2018, 29, 1291–1303. [Google Scholar]
- Garcia, A.H.C.; Martinez, F.F.J.; Perez, R.M.G.; Muñiz, A.R. Silver nanoparticles synthesized with a fraction from the bark of Eysenhardtia polystachya with high chalcone and dihydrochalcone content effectively inhibit oxidative stress in the zebrafish embryo model. Nanomed. J. 2018, 5, 152–162. [Google Scholar]
- Garcia, A.H.C.; Perez, R.M.G.; Manriquez, G.A.; Muñiz, A.R. Protection of silver nanoparticles using Eysenhardtia polystachya in peroxide-induced pancreatic β-cell damage and their antidiabetic properties in zebrafish. Int. J. Nanomed. 2018, 13, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Hernandez-Martinez, A.R.; Pool, H.; Molina, G.; Cruz-Sosa, M.; Luna-Barcenas, G.; Estevez, M. Synthesis and functionalization of silica-based nanoparticles with fluorescent biocompounds extracted from Eysenhardtia polystachya for biological applications. Mater. Sci. Eng. C 2015, 57, 49–57. [Google Scholar] [CrossRef]
- Bernabé-Antonio, A.; Maldonado-Magaña, A.; Ramírez-López, C.B.; Salcedo-Lopez, E.; Meza-Contreras, J.C.; Gonzalez-Garcia, Y.; Lopez-Dellamary, F.A.T.; Cruz-Sosa, F. Establishment of callus and cell suspension cultures of Eysenhardtia polystachya (Ortega) and fungistatic activity of their extracts. S. Afr. J. Bot. 2017, 112, 40–47. [Google Scholar] [CrossRef]
- Hernandez-Martinez, Á.R.; Molina, G.A.; Rodríguez-Torres, A.; Ledesma-Mendoza, B.; Del-Real, A.; Barroso-Flores, J.; Estevez, M. Fluorescence decay rate of selected compounds from Eysenhardtia polystachya extracts and their viability as biosensors. Mater. Sci. Eng. C 2019, 104, 109978. [Google Scholar] [CrossRef]

| Phenolic Compounds | |||||
|---|---|---|---|---|---|
| Number | Compound | Structure | Species | Part Used (Type of Extract) | Reference(s) |
| 1 | coatline A (3′-C-β-glucopyranosyl-α,2′,4′,4-tetrahydroxydihydrochalcone) | 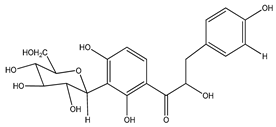 | E. polystachya | Wood (aqueous) | [13] |
| 2 | coatline B (3′-C-β-glucopyranosyl-α,2′,4′,3,4-pentahydroxydihydrochalcone) | 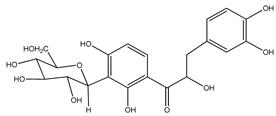 | E. polystachya | Wood (aqueous) | [13] |
| 3 | matlaline | 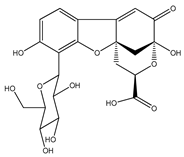 | E. polystachya | Wood (aqueous) | [7] |
| 4 | octa-acetyl coatline A | 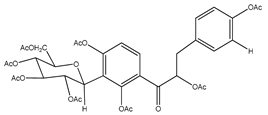 | E. polystachya | Wood (aqueous) | [13] |
| 5 | nona-acetyl coatline B | 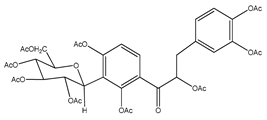 | E. polystachya | Wood (aqueous) | [13] |
| 6 | nona-acetyldihydrocoatline B | 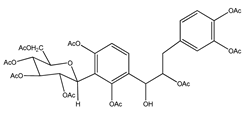 | E. polystachya | Wood (aqueous) | [13] |
| 7 | 3,4-dimethoxy-8,9-methylenedioxypterocarpan | 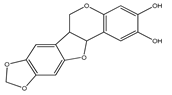 | E. polystachya | Heartwood (methanol) Bark (CHCl3-MeOH) | [8,24] |
| 8 | dehydrorotenone | 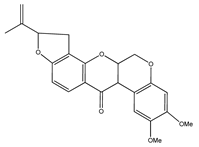 | E. polystachya | Heartwood (methanol) | [24] |
| 9 | angustlegor-retoside | 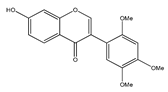 | E. polystachya | Heartwood (Methanol) | [24] |
| 10 | (3S)-7-hydroxy-2′,3′,4′,5′,8-pentamethoxyisoflavan | 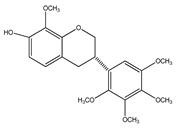 | E. polystachya | Bark (CHCl3-MeOH) | [8] |
| 11 | (3S)-3′,7-dihydroxy-2′,4′,5′,8-tetramethoxyisoflavan |  | E. polystachya | Bark (CHCl3-MeOH) | [8] |
| 12 | (3S)-2′,3′,4′,5′,8-pentamethoxy-7-O-acetylisoflavan |  | E. polystachya | Bark (CHCl3-MeOH) | [8] |
| 13 | (3S)-2′,4′,5′,8-tetramethoxy-3′,7-O-diacetylisoflavan | 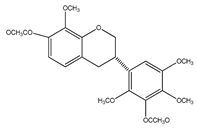 | E. polystachya | Bark (CHCl3-MeOH) | [8] |
| 14 | isoduartin (2′,7-dihydroxy-3′,4′,8-trimethoxyisoflavan) | 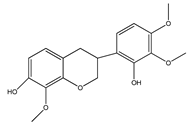 | E. polystachya | Bark (CHCl3-MeOH) | [8] |
| 15 | cuneatin (7-hydroxy-2′-methoxy-4′,5′-(methylendioxy)isoflavone) |  | E. polystachya | Bark (CHCl3-MeOH) | [8] |
| 16 | 7-hydroxy-2′,4′,5′-trimethoxyisoflavone | 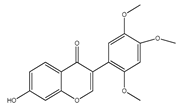 | E. polystachya | Bark (CHCl3-MeOH) Heartwood (Aqueous) | [8] |
| 17 | (α-R)-α,3,4,2′,4′-pentahydroxydihydrochalcone | 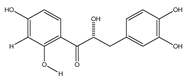 | E. polystachya | Bark (CHCl3-MeOH) | [25] |
| 18 | (αR)-3′-C-β-D-xylopyranosyl-α,3,4,2′,4′-pentahydroxydihydrochalcone | 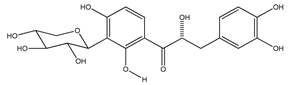 | E. polystachya | Bark (CHCl3-MeOH) | [25] |
| 19 | (αR)-3′-O-β-D-xylopyranosyl-α,3,4,2′,4′-pentahydroxydihydrochalcone | 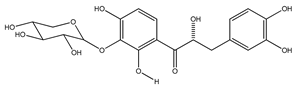 | E. polystachya | Bark (CHCl3-MeOH) | [25] |
| 20 | 4′,5,7-trihydroxy-8-methyl-6-(3-methyl-[2-butenyl])-(2S)-flavanone | 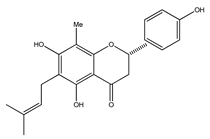 | E. texana | Aerial parts (CH2Cl2-MeOH) | [26,27] |
| 21 | 4′,5,7-trihydroxy-6-methyl-8-(3-methyl-[2-butenyl])-(2S)-flavanone | 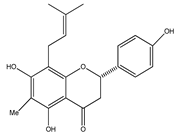 | E. texana | Aerial parts (CH2Cl2-MeOH) | [26,27] |
| 22 | 4′,5-dihydroxy-7-methoxy-6-(3-methyl-[2-butenyl])-(2S)-flavanone | 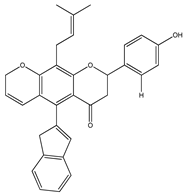 | E. texana | Aerial parts (CH2Cl2-MeOH) | [26,27] |
| 23 | 7-hydroxy-4′-methoxyisoflavone | 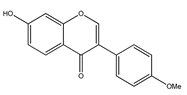 | E. polystachya | Heartwood (Aqueous) | [28,29] |
| 24 | 4′-O-methyl-8-prenylnaringenin | 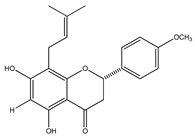 | E. platycarpa | Leaves (Methanolic) | [30] |
| 25 | 5,4′,1″-trihydroxy-6,7-(3″,3″-dimethylchroman)flavone | 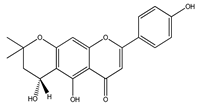 | E. platycarpa | Branches (Methanolic) | [30] |
| 26 | (2S)-4′-O-methyl-6-methyl-8-prenylnaringenin | 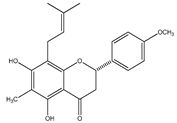 | E. platycarpa | Branches and leaves (Methanolic) | [30] |
| 27 | 5,7-dihydroxy-6-methyl-8-prenylflavanone | 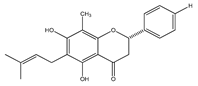 | E. platycarpa | Branches and leaves (Methanolic) | [30] |
| 28 | 5,7-dihydroxy-8-methyl-6-prenylflavanone | 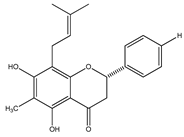 | E. platycarpa | Branches and leaves (Methanolic) | [30] |
| 29 | 5,7-dihydroxy-6-prenylflavanone | 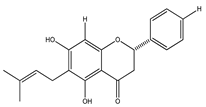 | E. platycarpa | Branches (Methanolic) | [30] |
| 30 | 5-hydroxy-7-methoxy-8-prenylflavanone | 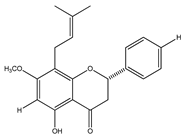 | E. platycarpa | Leaves (Methanolic) | [30] |
| 31 | 5,7-dihydroxy-8-prenylflavanone | 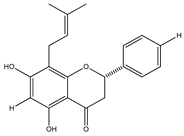 | E. platycarpa | Branches (Methanolic) | [30] |
| 32 | subcoriacin (3-(2′-hydroxy-4′,5′-methylendioxyphenyl)-6-(3″-hydroxymethyl-4″-hydroxybut-2″-enyl)-7-hydroxycoumarin) | 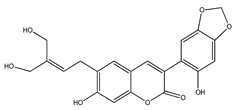 | E. subcoriacea | Bark (Methanolic) | [31,32] |
| 33 | (+)-cathechin | 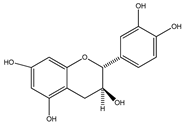 | E. platycarpa, E. subcoriacea | Bark (Methanolic) | [30,31,33] |
| 34 | (−)-epicatechin | 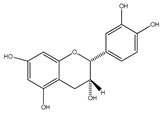 | E. platycarpa, E. subcoriacea | Bark (Methanolic) | [30,31,33] |
| 35 | (+)-afzelechin | 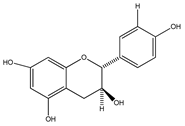 | E. platycarpa, E. subcoriacea | Bark (Methanolic) | [30,31,33] |
| 36 | eriodictyol |  | E. platycarpa, E. subcoriacea | Bark (Methanolic) | [30,31,33] |
| 37 | (+)-catechin-3-O-β-D-galactopyranoside | 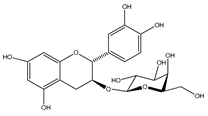 | E. platycarpa, E. subcoriacea | Bark (Methanolic) | [30,31,33] |
| 38 | quercetin-3-O-β-D-galactopyranoside |  | E. platycarpa, E. subcoriacea | Bark (Methanolic) | [30,31,33] |
| 39 | 2′,4′-dihydroxychalcone-6′-O-β-D-glucopyranoside | 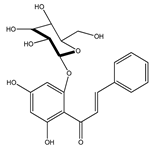 | E. polystachya | Bark (Water/MeOH) | [34] |
| 40 | α,3,2′,4′-tetrahydroxy-4-methoxy-dihydrochalcone-3′-C-β-glucopyranosy-6′-O-β-D-glucopyranoside | 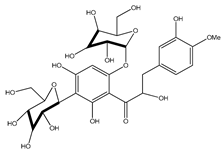 | E. polystachya | Bark (Water/MeOH) | [34] |
| 41 | 7-hydroxy-5,8′-dimethoxy-6′α-L-rhamnopyranosyl-8-(3-phenyl-transacryloyl)-1-benzopyran-2-one | 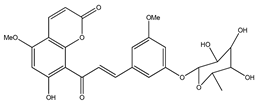 | E. polystachya | Bark (Water/MeOH) | [34] |
| 42 | 6′,7-dihydroxy-5,8-dimethoxy-8(3-phenyl-trans-acryloyl)-1-benzopyran-2-one |  | E. polystachya | Bark (Water/MeOH) | [34] |
| 43 | 9-hydroxy-3,8-dimethoxy-4-prenylpterocarpan | 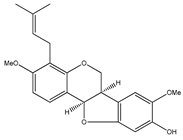 | E. polystachya | Bark (Water/MeOH) | [34] |
| 44 | 5,4′-dihydroxy-7,2′-dimethoxyl-isoflavone | 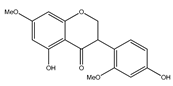 | E. polystachya | Bark (Water/MeOH) | [34] |
| 45 | α,4,4′-trihydroxy-dihydrochalcone-2′-O-β-D-glucopyranoside | 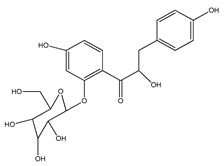 | E. polystachya | Bark (Water/MeOH) | [34] |
| 46 | (3R)-5,7-2′,4′-tetrahydroxyl-3′-methoxyl-isoflavanone | 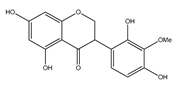 | E. polystachya | Bark (Water/MeOH) | [34] |
| 47 | flemichapparin C (9-methoxy-2,3-methylenedioxycoumestan) | 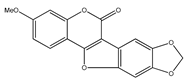 | E. polystachya | Bark (Water/MeOH) | [34] |
| 48 | neohesperidin-dihydrochalcone | 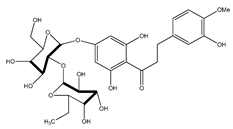 | E. polystachya | Bark (Water/MeOH) | [34] |
| 49 | hesperetin dihydrochalcone glucoside |  | E. polystachya | Bark (Water/MeOH) | [34] |
| 50 | aspalathin | 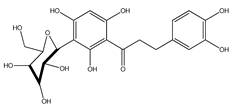 | E. polystachya | Bark (Water/MeOH) | [34] |
| 51 | sandwicensin | 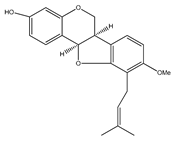 | E. polystachya | Bark (Water/MeOH) | [34] |
| 52 | 2′-O-α-L-rhamnopyranosyl- α,6′-dihydroxy-4′-acetyl-4-methoxydihydrochalcone | 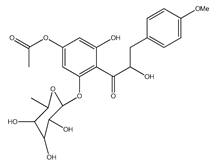 | E. polystachya | Bark (Water/MeOH) | [35] |
| 53 | 6′methoxy-sieboldin | 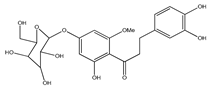 | E. polystachya | Bark (Water/MeOH) | [35] |
| 54 | 2′-O-β-D-glucopyranosyl-4′-methoxy-4-hydroxy-3-isoprenyldihydrochalcone | 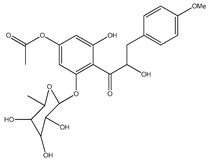 | E. polystachya | Bark (Water/MeOH) | [35] |
| 55 | 2′,4′,6′-trihydroxy-4,5-dimethoxy-3-isoprenyldihydrochalcone | 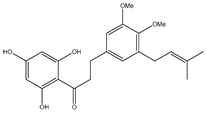 | E. polystachya | Bark (Water/MeOH) | [35] |
| 56 | 3′-C-β-glucopyranosyl-α, 2′,4′,6′-trihydroxy-4-methoxydihydrochalcone | 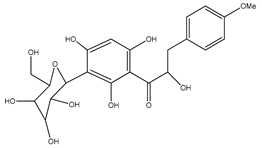 | E. polystachya | Bark (Water/MeOH) | [35] |
| 57 | 3′-C-β-glucopyranosyl-α, 2′,4-trihydroxy-4′,6′-dimethoxydihydrochalcone | 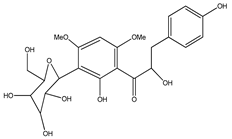 | E. polystachya | Bark (Water/MeOH) | [35] |
| 58 | 3-hydroxyphloretin-4′-O-β-D-glucopyranoside | 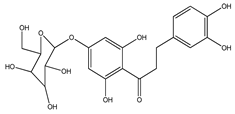 | E. polystachya | Bark (Water/MeOH) | [35] |
| 59 | 3,4′-dihydroxy-2,4,6-trimethoxydihydrochalcone |  | E. polystachya | Bark (Water/MeOH) | [35] |
| 60 | 2′,4′,4-trihydroxy-3′-methoxydihydrochalcone | 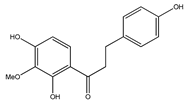 | E. polystachya | Bark (Water/MeOH) | [35] |
| 61 | 2′,4′,6′,4-tetrahydroxy-3,5-diisoprenyldihydrochalcone | 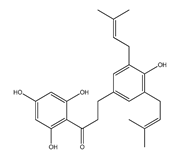 | E. polystachya | Bark (Water/MeOH) | [35] |
| 62 | 3′-C-β-glucopyranosyl-α,2′,4′,3,4-pentahydroxydihydroxychalcone | 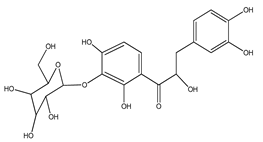 | E. polystachya | Bark (Water/MeOH) | [35] |
| 63 | 3′-O-β-D-glucopyranosyl-α,4,2′,4′,6′-pentahydroxydihydrochalcone | 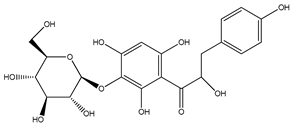 | E. polystachya | Bark (Water/MeOH) | [36] |
| Sterols and terpenoids | |||||
| 64 | oleanolic acid | 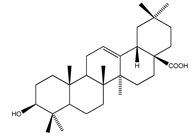 | E. platycarpa | Branches (Methanolic) | [30] |
| 65 | β-sitosterol |  | E. platycarpa | Branches (methanolic) | [30] |
| 66 | β-sitosteryl β-D-glucopyranoside | 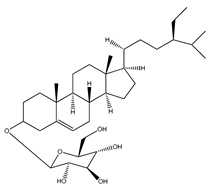 | E. platycarpa | Branches (Methanolic) | [30] |
| 67 | β-sitosteryl palmitate | 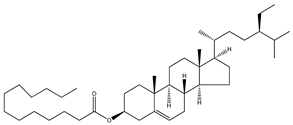 | E. platycarpa | Branches (Methanolic) | [30] |
| 68 | stigmasterol | 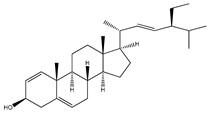 | E. polystachya | Bark (CHCl3-MeOH) | [8] |
| 69 | 3-O-acetyl-11α,12α-epoxy-oleanan-28,13β-olide | 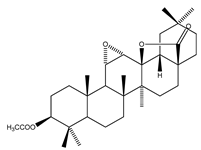 | E. platycarpa | Branches (Methanolic) | [30,33] |
| 70 | lupeol | 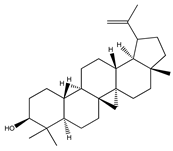 | E. platycarpa | Branches (Methanolic) | [30] |
| 71 | betulinic acid | 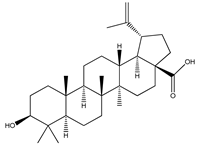 | E. platycarpa | Branches (Methanolic) | [30] |
| 72 | 3-O-acetyloleanolic acid | 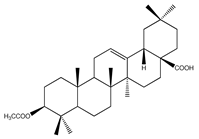 | E. platycarpa | Branches and leaves (Methanolic) | [30] |
| Fatty acids | |||||
| 73 | stearic acid | 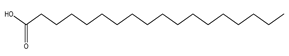 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 74 | arachidic acid | 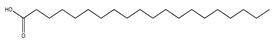 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 75 | palmitic acid | 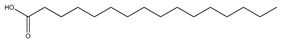 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 76 | linoleic acid | 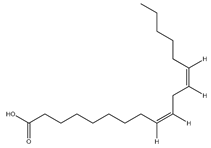 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| Hydroxybenzene | |||||
| 77 | syringol | 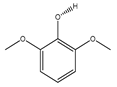 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 78 | catechol | 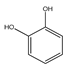 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| Cyclohexane | |||||
| 79 | 3-O-methyl-myo-inositol | 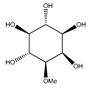 | E. platycarpa | Branches (Methanolic) | [30] |
| 80 | D-pinitol | 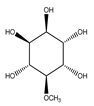 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 81 | lactic acid | 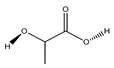 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 82 | 2-furoic acid | 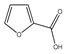 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 83 | succinic acid | 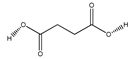 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 84 | fumaric acid | 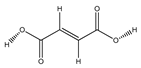 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 85 | sinapic acid | 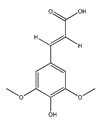 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 86 | N,N-diethyl carbamate | 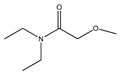 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| Carbohydrates or saccharides | |||||
| 87 | D-erythrose | 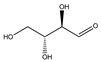 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 88 | D-mannose | 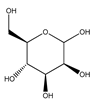 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 89 | D-arabinose |  | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 90 | xilose |  | E. polystachya | Branches and leaves (Ethanolic) | [12] |
| 91 | trehalose | 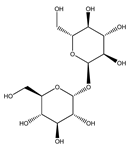 | E. polystachya | Branches and leaves (Ethanolic) | [12] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Campoy, A.; Garcia, E.; Muñiz-Ramirez, A. Phytochemical and Pharmacological Study of the Eysenhardtia Genus. Plants 2020, 9, 1124. https://doi.org/10.3390/plants9091124
Garcia-Campoy A, Garcia E, Muñiz-Ramirez A. Phytochemical and Pharmacological Study of the Eysenhardtia Genus. Plants. 2020; 9(9):1124. https://doi.org/10.3390/plants9091124
Chicago/Turabian StyleGarcia-Campoy, Abraham, Efrén Garcia, and Alethia Muñiz-Ramirez. 2020. "Phytochemical and Pharmacological Study of the Eysenhardtia Genus" Plants 9, no. 9: 1124. https://doi.org/10.3390/plants9091124
APA StyleGarcia-Campoy, A., Garcia, E., & Muñiz-Ramirez, A. (2020). Phytochemical and Pharmacological Study of the Eysenhardtia Genus. Plants, 9(9), 1124. https://doi.org/10.3390/plants9091124