Analysis of the Antimicrobial, Cytotoxic, and Antioxidant Activities of Cnidium officinale Extracts
Abstract
1. Introduction
2. Results
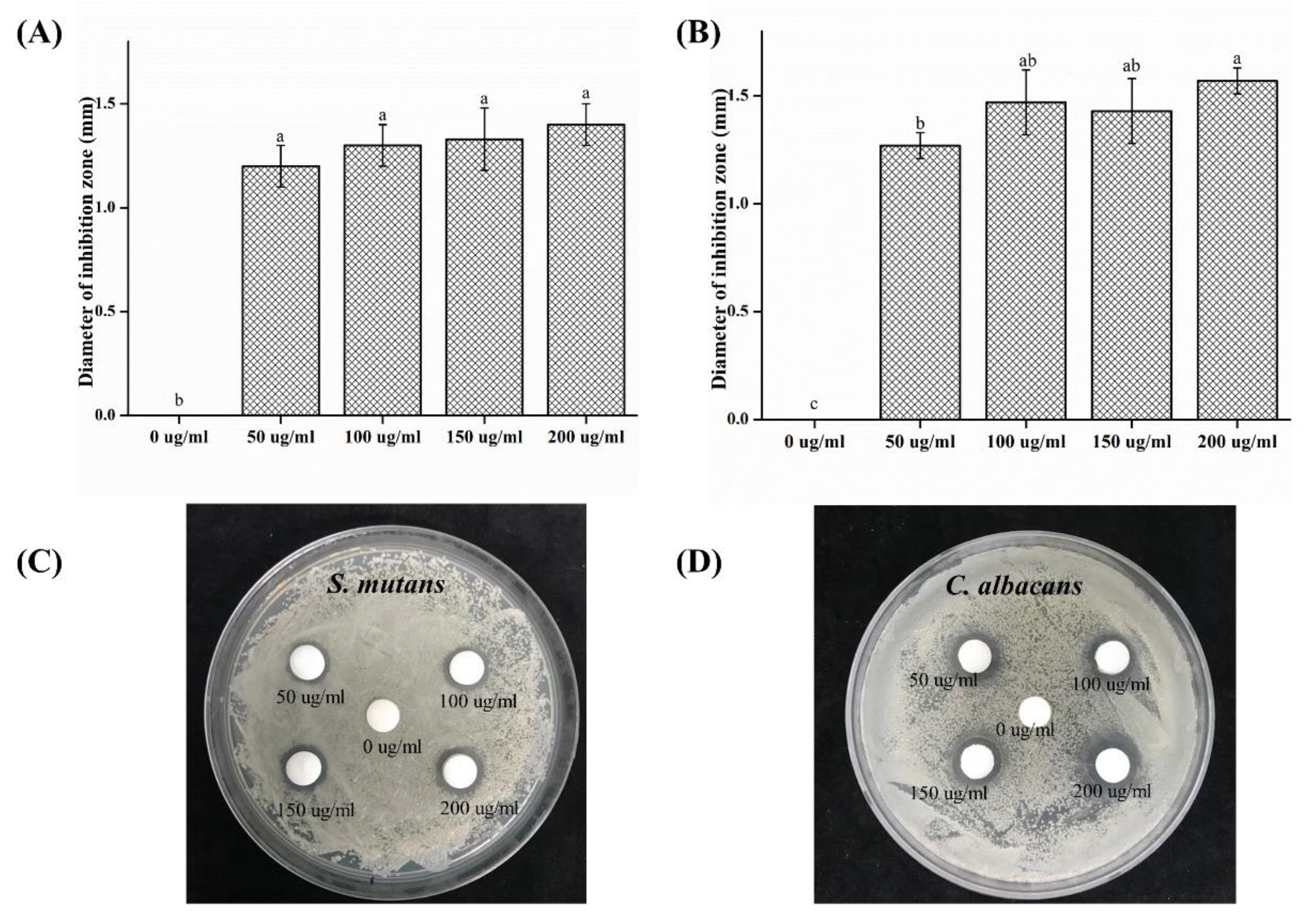
2.1. Inhibition Zone Test
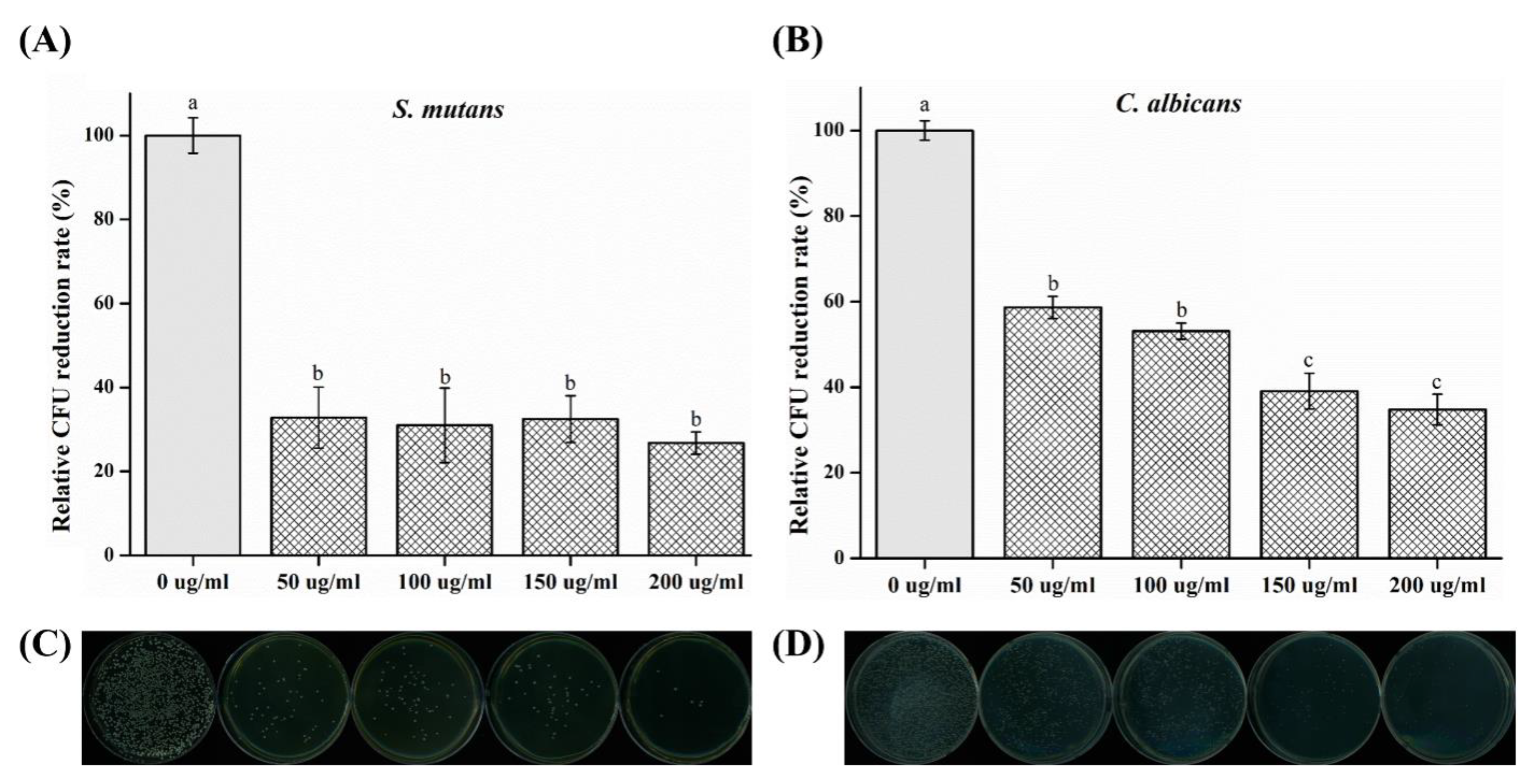
2.2. Colony-Forming Unit (CFU) Counts
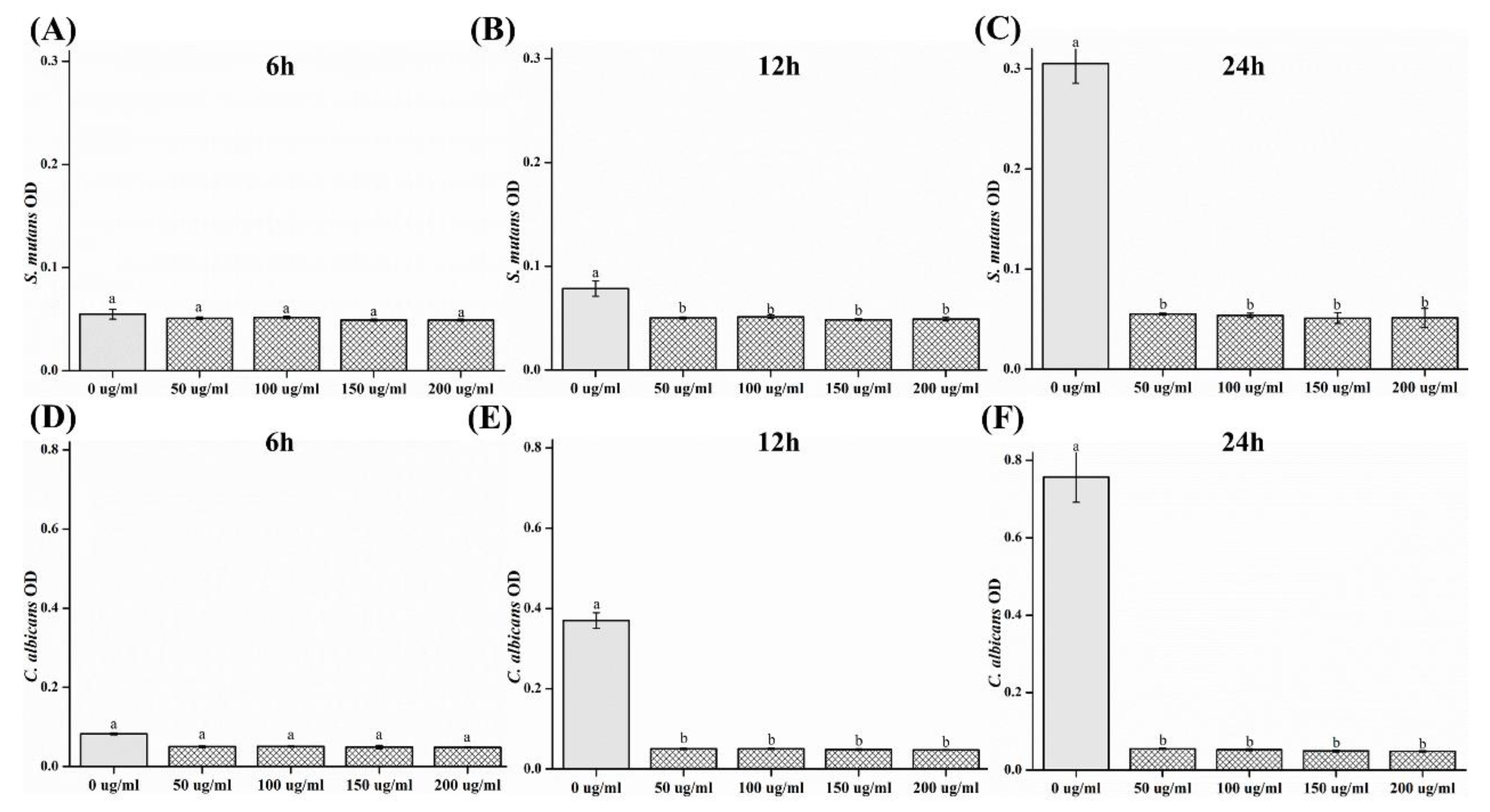
2.3. Optical Density (OD)
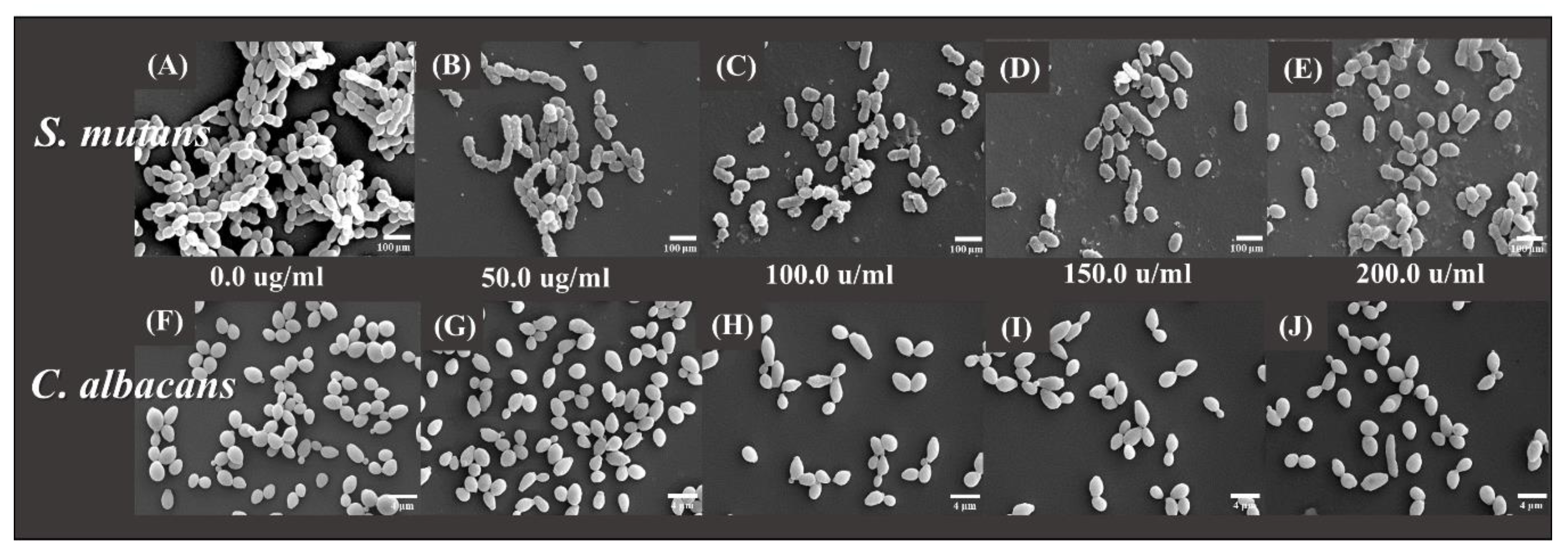
2.4. Scanning Electron Microscopy (SEM)
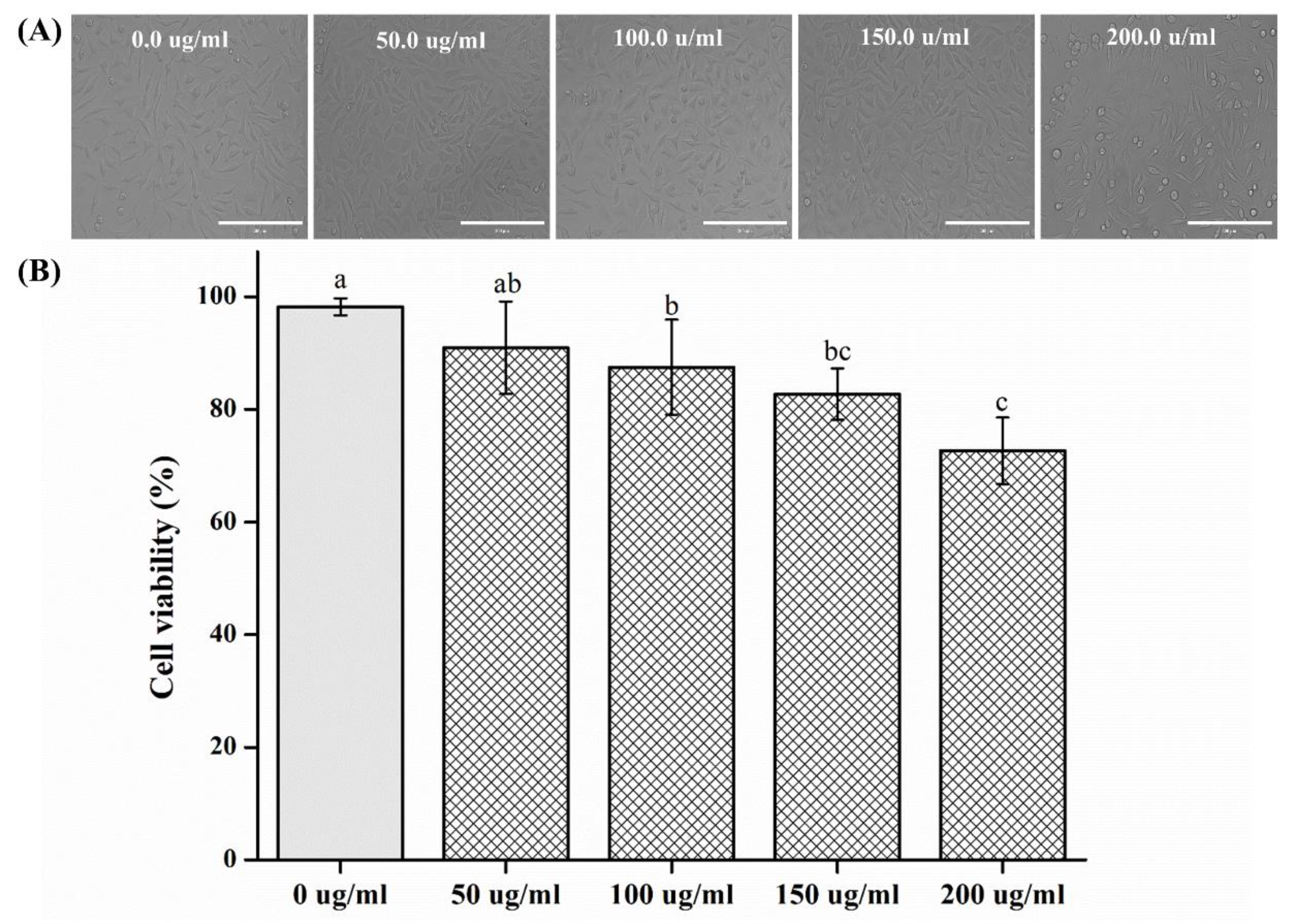
2.5. Biological Properties
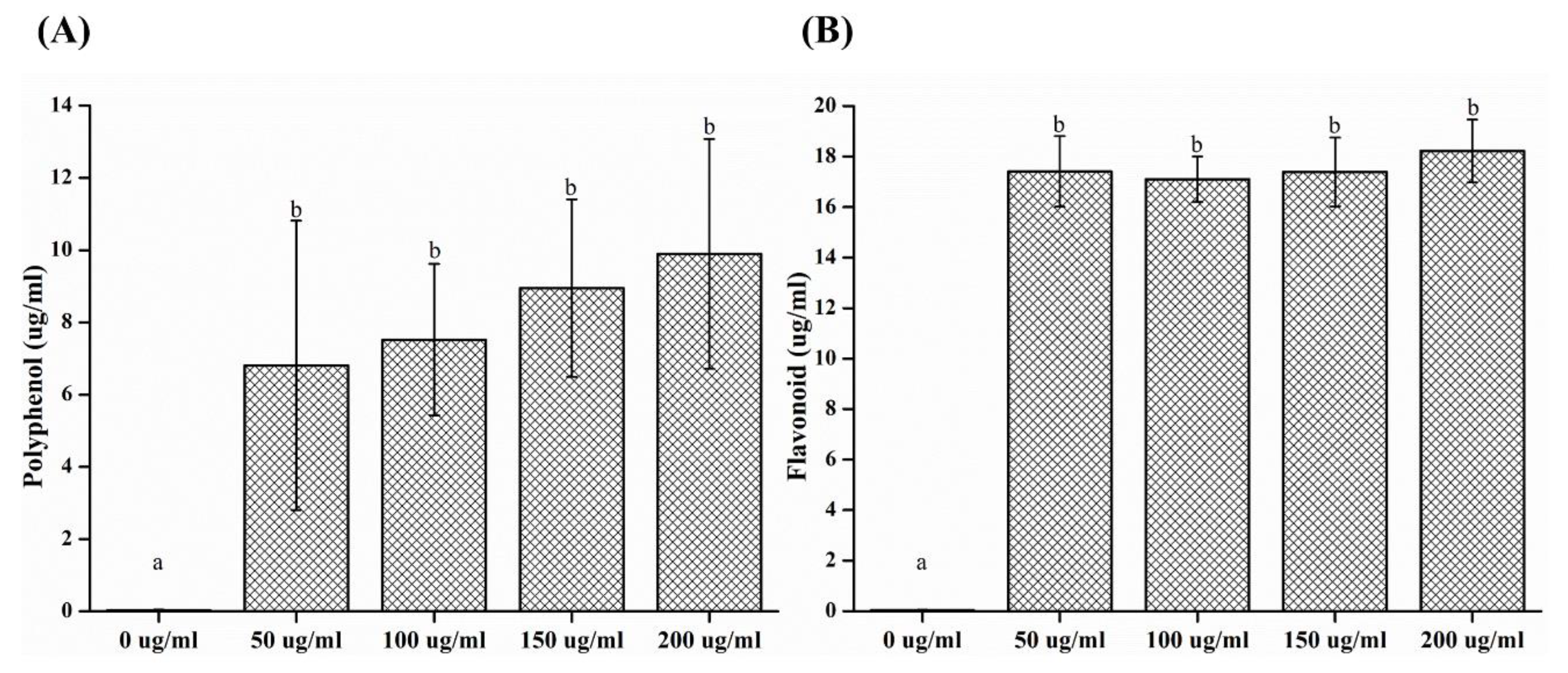
2.6. Measurement of Polyphenol and Flavonoid Contents
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Microbial Preparation
4.3. Inhibition Zone Test
4.4. OD
4.5. CFUs
4.6. SEM
4.7. Biological Properties
4.8. Measurement of Polyphenol and Flavonoid Contents
4.9. Statistical Analyses
5. Conclusions
- In the inhibition zone test, the growth inhibition zone was observed to increase; the inhibition zones of both strains of microbes showed significant differences as compared to that of the control (p < 0.05) group.
- CFU measurement showed a reduction in the number of colonies; both the strains of microbes demonstrated a significant difference in CFU reduction as compared to that of the control (p < 0.05) group.
- The OD values decreased, such that both the strains of microbes showed a significant difference in OD as compared to that of the control group after 12 and 24 h (p < 0.05).
- SEM analysis demonstrated a tendency for both the microbial numbers to decrease as compared to that of the control group.
- In cell viability studies, no significant differences were observed in the control group and the 50 μg/mL solution (p > 0.05); significant differences in cell viability were observed in 100, 150, and 200 μg/mL groups as compared to that of the control (p < 0.05) group.
- In the analysis of polyphenol and flavonoid contents, significant differences were observed between the control and experimental groups (p < 0.05).
Author Contributions
Funding
Conflicts of Interest
References
- Lee, M.J.; Kwon, J.S.; Kim, J.Y.; Ryu, J.H.; Seo, J.Y.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dent. Mater. 2019, 35, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, M.J.; Oh, S.H.; Kwon, J.S. Novel dental poly (methyl methacrylate) containing phytoncide for antifungal effect and inhibition of oral multispecies biofilm. Materials (Basel) 2020, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.L.; Kirchberg, M.; Sarembe, S.; Kiesow, A.; Sculean, A.; Mäder, K.; Buchholz, M.; Eick, S. In vitro evaluation of antimicrobial activity of minocycline formulations for topical application in periodontal therapy. Pharmaceutics 2020, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Noh, M.H.; Jang, E.Y.; Yang, S.B.; Kang, S.W.; Kwack, K.H.; Ryu, J.I.; Lee, J.Y. Effects of sodium tripolyphosphate on oral commensal and pathogenic bacteria. Pol. J. Microbiol. 2019, 68, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Kadri, S.S.; Dekker, J.P.; Danner, R.L.; Chen, H.C.; Fram, D.; Zhang, F.; Wang, R.; Klompas, M.; CDC Prevention Epicenters Program. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw. Open 2020, 3, e202899. [Google Scholar] [CrossRef]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential oils of aromatic plants with antibacterial, anti-biofilm and anti-quorum sensing activities against pathogenic bacteria. Antibiotics (Basel) 2020, 9, 147. [Google Scholar] [CrossRef]
- She, W.; Ye, W.; Shi, Y.; Zhou, L.; Zhang, Z.; Chen, F.; Qian, P.Y. A novel chresdihydrochalcone from streptomyces chrestomyceticus exhibiting activity against gram-positive bacteria. J. Antibiot. (Tokyo) 2020, 73, 429–434. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Ceylan, O.; Fru, G.C.; Ozturk, M.; Duru, M.E.; Shaheen, F. Antibiofilm, antiquorum sensing and antioxidant activity of secondary metabolites from seeds of annona senegalensis, persoon. Microb. Pathog. 2020, 144, 104191. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef]
- Aghbash, B.N.; Pouresmaeil, M.; Dehghan, G.; Nojadeh, M.S.; Mobaiyen, H.; Maggi, F. Chemical composition, antibacterial and radical scavenging activity of essential oils from Satureja macrantha C.A.Mey. at different growth stages. Foods 2020, 9, 494. [Google Scholar] [CrossRef]
- Al-Thobity, A.M.; Al-Khalifa, K.S.; Gad, M.M.; Al-Hariri, M.; Ali, A.A.; Alnassar, T. In vitro evaluation of the inhibitory activity of thymoquinone in combatting Candida albicans in denture stomatitis prevention. Int. J. Environ. Res. Public Health 2017, 14, 743. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, D.B.; Kim, J.E.; Moon, S.H.; Son, J.Y.; Lee, E.Y.; Kwon, J.S. Mechanical properties and antibacterial effects on streptococcus mutans of composite resins containing phytoncide. J. Korean Soc. Dent. Hyg. 2019, 19, 467–477. [Google Scholar]
- Kim, J.E.; Kim, H.E.; Hwang, J.K.; Lee, H.J.; Kwon, H.K.; Kim, B.I. Antibacterial characteristics of curcuma xanthorrhiza extract on streptococcus mutans biofilm. J. Microbiol. 2008, 46, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, J.G.; Lee, J.; Lee, C.; Shim, J.; Kim, Y.T. Analgesic effects of cnidium officinale extracts on postoperative, neuropathic, and menopausal pain in rat models. Evid.-Based Complement. Alternat. Med. 2019, 2019, 9698727. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lee, Y.R.; Kim, C.S.; Jo, K.; Sohn, E.; Kim, J.S.; Kim, J. Cnidium officinale extract and butylidenephthalide inhibits retinal neovascularization in vitro and in vivo. BMC Complement. Altern. Med. 2016, 16, 231. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, Y.R.; Kim, J.S.; Kim, Y.H.; Kim, J. Cinidium officinale and its bioactive compound, butylidenephthalide, inhibit laser-induced choroidal neovascularization in a rat model. Molecules 2015, 20, 20699–20708. [Google Scholar] [CrossRef]
- Hop, H.T.; Arayan, L.T.; Reyes, A.W.B.; Huy, T.X.N.; Baek, E.J.; Min, W.; Lee, H.J.; Lee, C.H.; Rhee, M.H.; Kim, S. Inhibitory effect of the ethanol extract of a rice bran mixture comprising angelica gigas, cnidium officinale, artemisia princeps, and camellia sinensis on brucella abortus uptake by professional and nonprofessional phagocytes. J. Microbiol. Biotechnol. 2017, 27, 1885–1891. [Google Scholar] [CrossRef]
- Tran, H.N.K.; Cao, T.Q.; Kim, J.A.; Youn, U.J.; Kim, S.; Woo, M.H.; Min, B.S. Anti-inflammatory activity of compounds from the rhizome of cnidium officinale. Arch. Pharm. Res. 2018, 41, 977–985. [Google Scholar] [CrossRef]
- de la Cruz, J.; Kim, D.H.; Hwang, S.G. Anti cancer effects of cnidium officinale makino extract mediated through apoptosis and cell cycle arrest in the HT-29 human colorectal cancer cell line. Asian Pac. J. Cancer Prev. 2014, 15, 5117–5121. [Google Scholar]
- Lee, S.H.; Lee, J.H.; Oh, E.Y.; Kim, G.Y.; Choi, B.T.; Kim, C.; Choi, Y.H. Ethanol extract of cnidium officinale exhibits anti-inflammatory effects in BV2 microglial cells by suppressing NF-KappaB nuclear translocation and the activation of the PI3K/Akt signaling pathway. Int. J. Mol. Med. 2013, 32, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kwon, J.S.; Jiang, H.B.; Choi, E.H.; Park, G.; Kim, K.M. The antibacterial effect of non-thermal atmospheric pressure plasma treatment of titanium surfaces according to the bacterial wall structure. Sci. Rep. 2019, 9, 1938. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Lee, M.J.; Kim, J.Y.; Kim, D.; Ryu, J.H.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Novel anti-biofouling light-curable fluoride varnish containing 2-methacryloyloxyethyl phosphorylcholine to prevent enamel demineralization. Sci. Rep. 2019, 9, 1432. [Google Scholar] [CrossRef] [PubMed]
- Khoury, Z.H.; Vila, T.; Puthran, T.R.; Sultan, A.S.; Montelongo-Jauregui, D.; Melo, M.A.S.; Jabra-Rizk, M.A. The role of Candida albicans secreted polysaccharides in augmenting streptococcus mutans adherence and mixed biofilm formation: In vitro and in vivo studies. Front. Microbiol. 2020, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.S.; Lee, N.H. Antimicrobial activity of the aerial part (leaf and stem) extracts of cnidium officinale Makino, a Korean medicinal herb. Korean J. Microbiol. Biotechnol. 2007, 35, 30–35. [Google Scholar]
- Loffredo, L.; Perri, L.; Nocella, C.; Violi, F. Antioxidant and antiplatelet activity by polyphenol-rich nutrients: Focus on extra virgin olive oil and cocoa. Br. J. Clin. Pharmacol. 2017, 83, 96–102. [Google Scholar] [CrossRef]
- Lee, I.C.; Kim, M.K. Antioxidant, antimicrobial and anti-inflammatory of mixed medicinal herb extract. Korean J. Herbol. 2015, 30, 51–58. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-J.; Kang, M.-K. Analysis of the Antimicrobial, Cytotoxic, and Antioxidant Activities of Cnidium officinale Extracts. Plants 2020, 9, 988. https://doi.org/10.3390/plants9080988
Lee M-J, Kang M-K. Analysis of the Antimicrobial, Cytotoxic, and Antioxidant Activities of Cnidium officinale Extracts. Plants. 2020; 9(8):988. https://doi.org/10.3390/plants9080988
Chicago/Turabian StyleLee, Myung-Jin, and Min-Kyung Kang. 2020. "Analysis of the Antimicrobial, Cytotoxic, and Antioxidant Activities of Cnidium officinale Extracts" Plants 9, no. 8: 988. https://doi.org/10.3390/plants9080988
APA StyleLee, M.-J., & Kang, M.-K. (2020). Analysis of the Antimicrobial, Cytotoxic, and Antioxidant Activities of Cnidium officinale Extracts. Plants, 9(8), 988. https://doi.org/10.3390/plants9080988





