Brassica tardarae (Brassicaceae), a New Species from a Noteworthy Biotope of South-Western Sicily (Italy)
Abstract
1. Introduction
2. Results
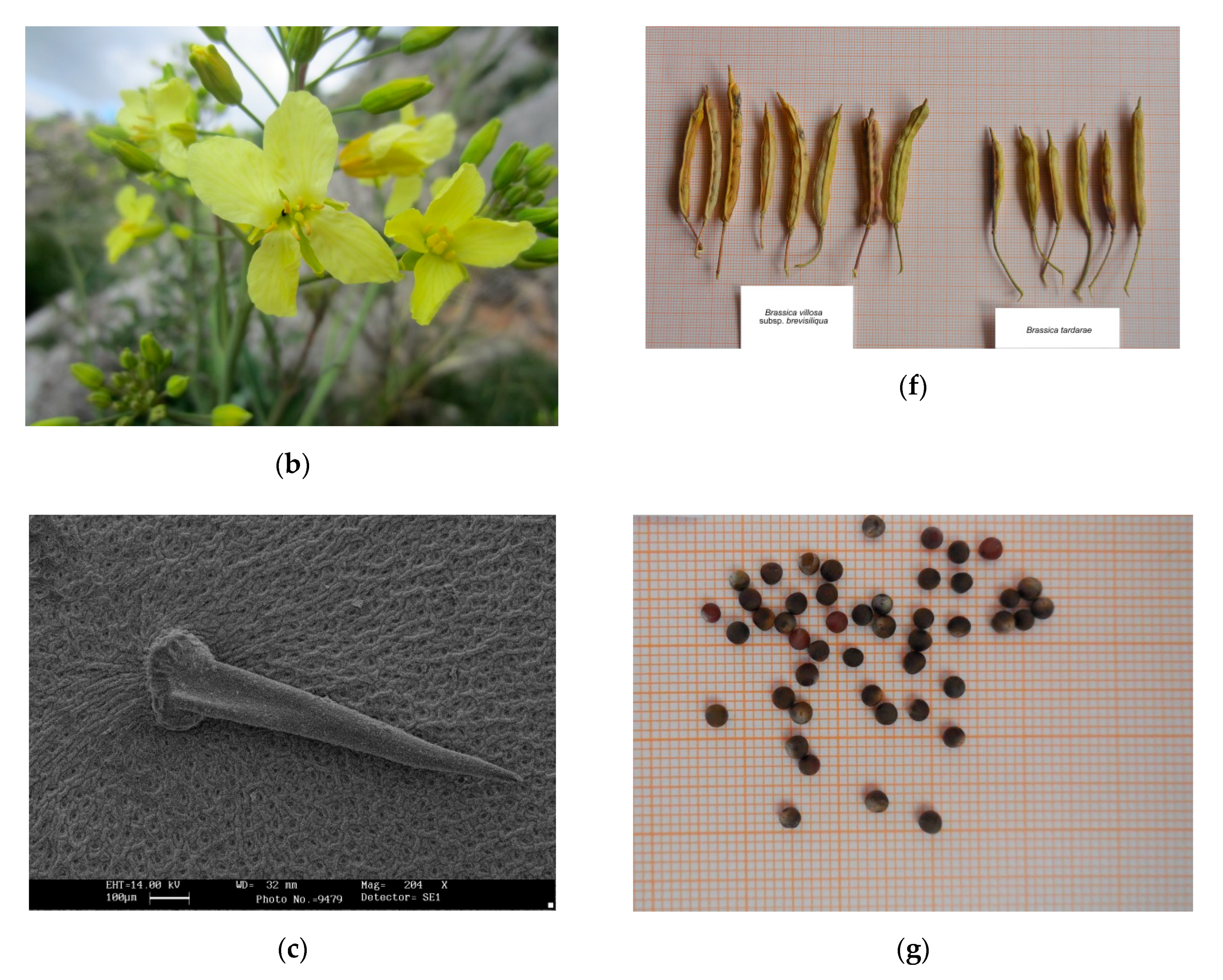
2.1. Morphological Analysis
2.2. Taxonomic Outcome
Brassica tardarae Ilardi, Geraci and Troia, sp. nov.
2.3. Analytical Key to the Taxa of Brassica sect. Brassica in Sicily
| 1. Leaves glabrous or hispid with bulbose, scattered hairs; | 2 |
| 1. Leaves villous or pubescent; | 7 |
| 2. Petals white; siliqua laterally compressed; | B. insularis |
| 2. Petals yellow; siliqua isodiametric or dorsally compressed; | 3 |
| 3. Leaf teeth obtuse; siliqua navicular isodiametric, 25–40 mm × 8–12 mm; valves thickened, spongy, dorsally smooth; rostrum widely conic, 10–15 mm, 1–2-seeded; | B. macrocarpa |
| 3. Leaf teeth acute; siliqua linear, 25–70 mm × 3–7 mm; valves thin, dorsally ribbed; rostrum subulate to narrowly conic, 4–11 mm, seedless; | 4 |
| 4. Petals pale yellow, 13–20 mm × 5–8 mm; siliqua 25–45(–50) mm × 5–7.5 mm; | 5 |
| 4. Petals yellow, 18–27 mm × 7–13 mm; siliqua 35–70 mm × 3–4.5 mm; | 6 |
| 5. Petals 14–20 mm × 5–7 mm; fruiting pedicels 10–18 mm, siliqua tetragonous, laterally compressed, 35–45(–50) mm × 6–7.5 mm; seed diameter 2.85–3.2 mm; | B. villosa subsp. brevisiliqua |
| 5. Petals 13–18 mm × 6.5–8 mm; fruiting pedicels 16–26 mm, siliqua almost isodiametric, slightly compressed, (20)–25–35(–40) mm × 5–6 mm; seed diameter 2.3–2.5 mm; | B. tardarae |
| 6. Leaves green, glabrous; leaf lamina ovate–lanceolate, acute, deeply incised, margin loosely dentate; | B. rupestris subsp. rupestris |
| 6. Leaves with hispid hairs, mainly in upper blade; leaf lamina ovate–elliptical, obtuse, lobed, margin minutely dentate; | B. rupestris subsp. hispida |
| 7. Ovary hairy; fruit corpus hairy, subglobose to ellipsoid, smooth, thickened, spongy, 8−18 × 8−11 mm; | B. trichocarpa |
| 7. Ovary glabrous; fruit corpus glabrous, linear–cylindrical, torulose, thin, 25−100 × 36.5 mm; | 8 |
| 8. Petiole auriculate at the base, up to 15(18) mm; | 9 |
| 8. Petiole not auriculate, up to 30 mm; | 10 |
| 9. Pedicels hairy, 14–35 mm; petals yellow; siliqua 60–100 mm × 3–5 mm, not ribbed dorsally; rostrum 10–20 mm; fruiting pedicels 25–35 mm; | B. incana subsp. incana |
| 9. Pedicels glabrous, 7–12 mm; petals white; siliqua 30–65 mm × 2.5–3 mm, ribbed dorsally; rostrum 4–10 mm; fruiting pedicels 14–20 mm; | B. incana subsp. raimondoi |
| 10. Leaf lamina lyrate, margin crispate–denticulate; petiole winged; siliqua 30–45 mm × 5–6.5 mm; | B. villosa subsp. drepanensis |
| 10. Leaf lamina lobed, margin dentate, petiole unwinged; siliqua 3–5 mm wide; | 11 |
| 11. Leaf lamina minutely dentate; siliqua laterally compressed, 25–35 × 4–5 mm; | B. villosa subsp. tineoi |
| 11. Leaf lamina broadly dentate; siliqua not laterally compressed, 30–75 mm × 3–5 mm; | 12 |
| 12. Leaf margin crenate–dentate; sepals 12–15 mm; petals 24–26mm; siliqua 30–60 mm × 4–4.5 mm, dorsally ribbed; | B. villosa subsp. villosa |
| 12. Leaf margin irregularly dentate; sepals 8–11 mm; petal 16–22 mm; siliqua 45–75 mm × (3–)3.5–3.8 mm, dorsally not ribbed. | B. villosa subsp. bivonana |
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Shehbaz, I.A. A generic and tribal synopsis of the Brassicaceae (Cruciferae). Taxon 2012, 61, 931–954. [Google Scholar] [CrossRef]
- Diederichsen, A. Cruciferae: Brassica. In Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops; Hanelt, P., Ed.; Institute of Plant Genetics and Crop Plant Research; Springer: Berlin/Heidelberg, Germany, 2001; Volume 3, pp. 1435–1465. [Google Scholar]
- Gómez-Campo, C. Taxonomy. In Biology of Brassica Coenospecies; Gómez-Campo, C., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 3–32. [Google Scholar] [CrossRef]
- Snogerup, S.; Gustafsson, M.; Bothmer von, R. Brassica sect. Brassica (Brassicaceae). I. Taxonomy and variation. Willdenowia 1990, 19, 271–365. [Google Scholar]
- Gustafsson, M.; Snogerup, S. A new subspecies of Brassica cretica from Peloponnisos, Greece. Bot. Chron. 1983, 3, 7–11. [Google Scholar]
- Mazzola, P.; Raimondo, F.M. A new species of Brassica from Sicily. Lagascalia 1988, 15, 249–251. [Google Scholar]
- Raimondo, F.M.; Mazzola, P. A new taxonomic arrangement in the Sicilian members of Brassica L. sect. Brassica. Lagascalia 1997, 19, 831–838. [Google Scholar]
- Giotta, C.; Piccitto, M.; Arrigoni, P.V. Un nuovo endemismo della Sardegna: Brassica tyrrhena sp. nov. (Brassicaceae). Webbia 2002, 57, 1–5. [Google Scholar] [CrossRef]
- Raimondo, F.M.; Geraci, A. A new taxonomic arrangement in Sicilian Brassica sect. Brassica (Cruciferae). Flora Medit. 2002, 12, 439–441. [Google Scholar]
- Sciandrello, S.; Brullo, C.; Brullo, S.; Giusso Del Galdo, G.; Minissale, P.; Salmeri, C. A new species of Brassica sect. Brassica (Brassicaceae) from Sicily. Plant Biosyst. An Int. J. Deal. All Asp. Plant Biol. 2013, 147, 812–820. [Google Scholar] [CrossRef]
- Brullo, C.; Brullo, S.; Giusso Del Galdo, G.; Ilardi, V. Brassica trichocarpa (Brassicaceae), a new species from Sicily. Phytotaxa 2013, 122, 45–60. [Google Scholar] [CrossRef][Green Version]
- Bothmer von, R.; Gustafsson, M.; Snogerup, S. Brassica sect. Brassica (Brassicaceae) II. Inter- and intraspecific crosses with cultivars of B. oleracea. Genet. Resour. Crop. Evol. 1995, 42, 165–178. [Google Scholar] [CrossRef]
- Maggioni, L. Domestication of Brassica oleracea L. Ph.D. Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2015. [Google Scholar]
- Pignatti, S.; La Rosa, M.; Guarino, R. Flora d’Italia, 2nd ed.; Edagricole-New Business Media: Milano-Bologna, Italy, 2017. [Google Scholar]
- Raimondo, F.M.; Mazzola, P.; Ottonello, D. On the taxonomy and distribution of Brassica sect. Brassica (Cruciferae) in Sicily. Flora Medit. 1991, 1, 63–86. [Google Scholar]
- Tatout, C.; Warwick, S.; Lenoir, A.; Deragon, J.-M. SINE insertions as clade markers for wild Crucifer species. Mol. Biol. Evol. 1999, 16, 1614–1621. [Google Scholar] [CrossRef]
- Branca, F.; Tribulato, A. Brassica macrocarpa. The IUCN Red List of Threatened Species 2011: E.T162139A5548195. Available online: http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T162139A5548195.en (accessed on 27 September 2019).
- Geraci, A.; Mazzola, P. Schede per una Lista Rossa della Flora vascolare e crittogamica Italiana. Brassica macrocarpa Guss. Ital. Bot. 2012, 44, 417–420. [Google Scholar]
- Rossi, G.; Montagnani, C.; Gargano, D.; Peruzzi, L.; Abeli, T.; Ravera, S.; Cogoni, A.; Fenu, G.; Magrini, S.; Gennai, M.; et al. Lista Rossa della Flora Italiana. 1. Policy Species e altre specie Minacciate; Comitato Italiano IUCN e Ministero dell’ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2013. [Google Scholar]
- Branca, F.; Donnini, D. Brassica rupestris. The IUCN Red List of Threatened Species 2011: E.T170114A6719025. Available online: http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T170114A6719025.en (accessed on 27 September 2019).
- Branca, F.; Donnini, D. Brassica villosa. The IUCN Red List of Threatened Species 2011: E.T170117A6720001. Available online: http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T170117A6720001.en (accessed on 27 September 2019).
- Conti, F.; Manzi, A.; Pedrotti, F. Liste Rosse Regionali delle Piante d’Italia; Associazione Italiana per il World Wildlife Fund & Società Botanica Italiana: Camerino, Italy, 1997. [Google Scholar]
- Scoppola, A.; Spampinato, G. Stato delle conoscenze sulla flora vascolare d’Italia—Atlante delle specie a rischio di estinzione. Versione 1.0. In Stato Delle Conoscenze Sulla Flora Vascolare d’Italia; Scoppola, A., Blasi, C., Eds.; Palombi Editori: Roma, Italy, 2005. [Google Scholar]
- Geraci, A. Studio della variazione inter- ed intra-specifica del popolamento di Brassica sect. Brassica in Sicilia e strategia di conservazione. Ph.D. Thesis, University of Palermo, Palermo, Italy, 1998. [Google Scholar]
- Geraci, A.; Divaret, I.; Raimondo, F.M.; Chèvre, A.-M. Genetic relationships between Sicilian wild populations of Brassica analysed with RAPD markers. Plant Breed. 2001, 120, 193–196. [Google Scholar] [CrossRef]
- Geraci, A.; Chèvre, A.-M.; Divaret, I.; Eber, F.; Raimondo, F.M. Isozyme analysis of genetic diversity in wild Sicilian populations of Brassica sect. Brassica in view of genetic resources management. Genet. Resour. Crop. Evol. 2004, 51, 137–146. [Google Scholar] [CrossRef]
- Geraci, A.; Inzerillo, S.; Oddo, E. Physio-morphological traits and drought stress responses in three wild Mediterranean taxa of Brassicaceae. Acta Physiol. Plant. 2019, 41, 106. [Google Scholar] [CrossRef]
- Branca, F.; Cartea, E. Brassica. In Wild Crop Relatives: Genomic and Breeding Resources, Oilseeds; Kole, C., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 17–36. [Google Scholar]
- Maggioni, L.; von Bothmer, R.; Poulsen, G.; Branca, F.; Bagger Jørgensen, R. Genetic diversity and population structure of leafy kale and Brassica rupestris Raf. in south Italy. Hereditas 2014, 151, 145–158. [Google Scholar] [CrossRef]
- Geraci, A.; Troia, A. Pollen features in Brassica sect. Brassica Sicilian populations. Manuscript in preparation.
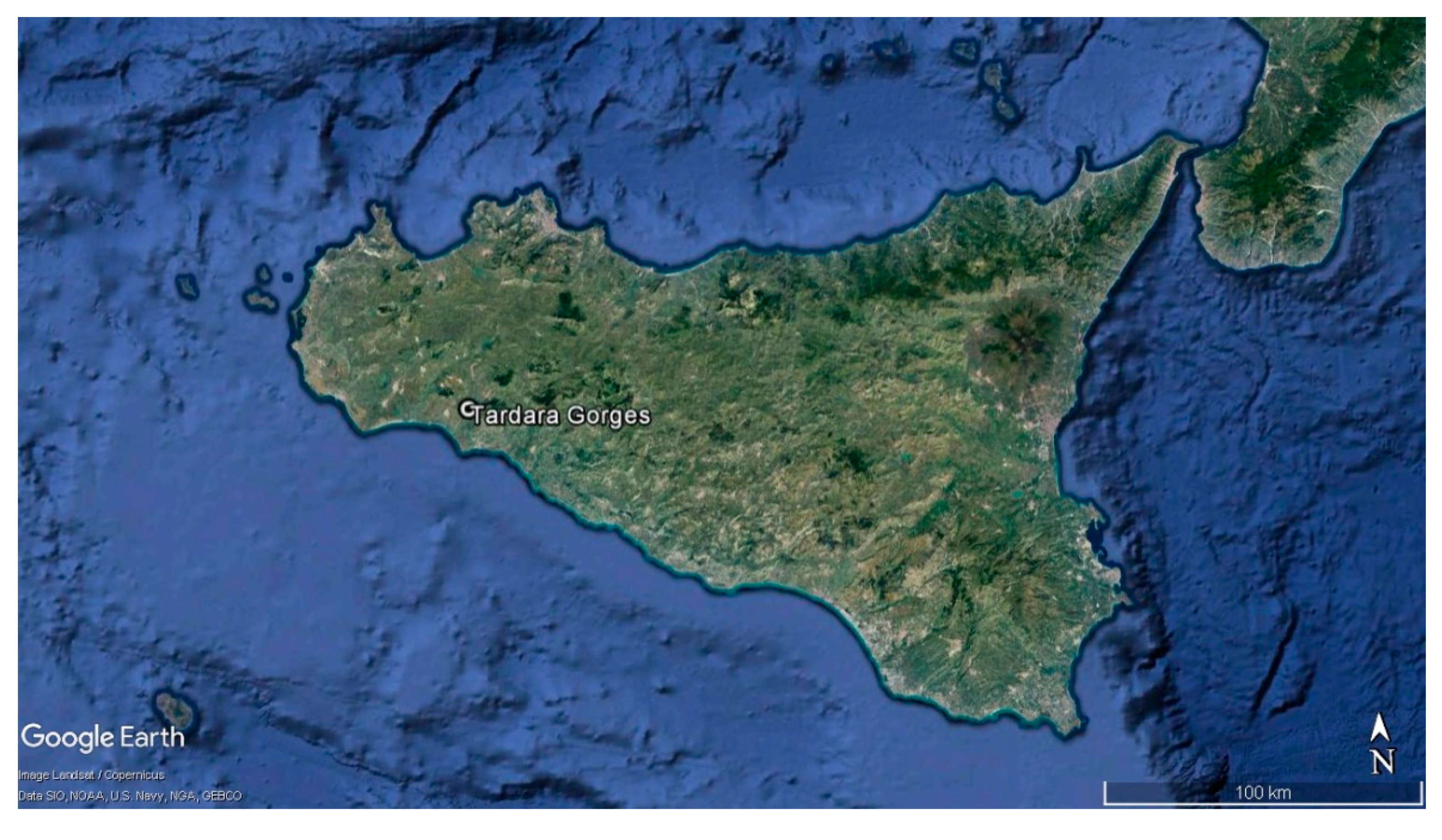
- Bazan, G.; Ilardi, V.; Raimondo, F.M. La vegetazione della Gola della Tardara (Sicilia sud-occidentale). Nat. Sicil. 2006, 30, 379–392. [Google Scholar]
- Raimondo, F.M.; Bancheva, S.T.; Ilardi, V. Centaurea saccensis (Asteraceae), a new species from SW-Sicily. Bocconea 2004, 17, 293–298. [Google Scholar]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN: Gland, Switzerland; Cambridge, UK, 2012. [Google Scholar]
- Hilpold, A.; Schönswetter, P.; Susanna, A.; Garcia-Jacas, N.; Vilatersana, R. Evolution of the central Mediterranean Centaurea cineraria group (Asteraceae): Evidence for relatively recent, allopatric diversification following transoceanic seed dispersal. Taxon 2011, 60, 528–538. [Google Scholar] [CrossRef]
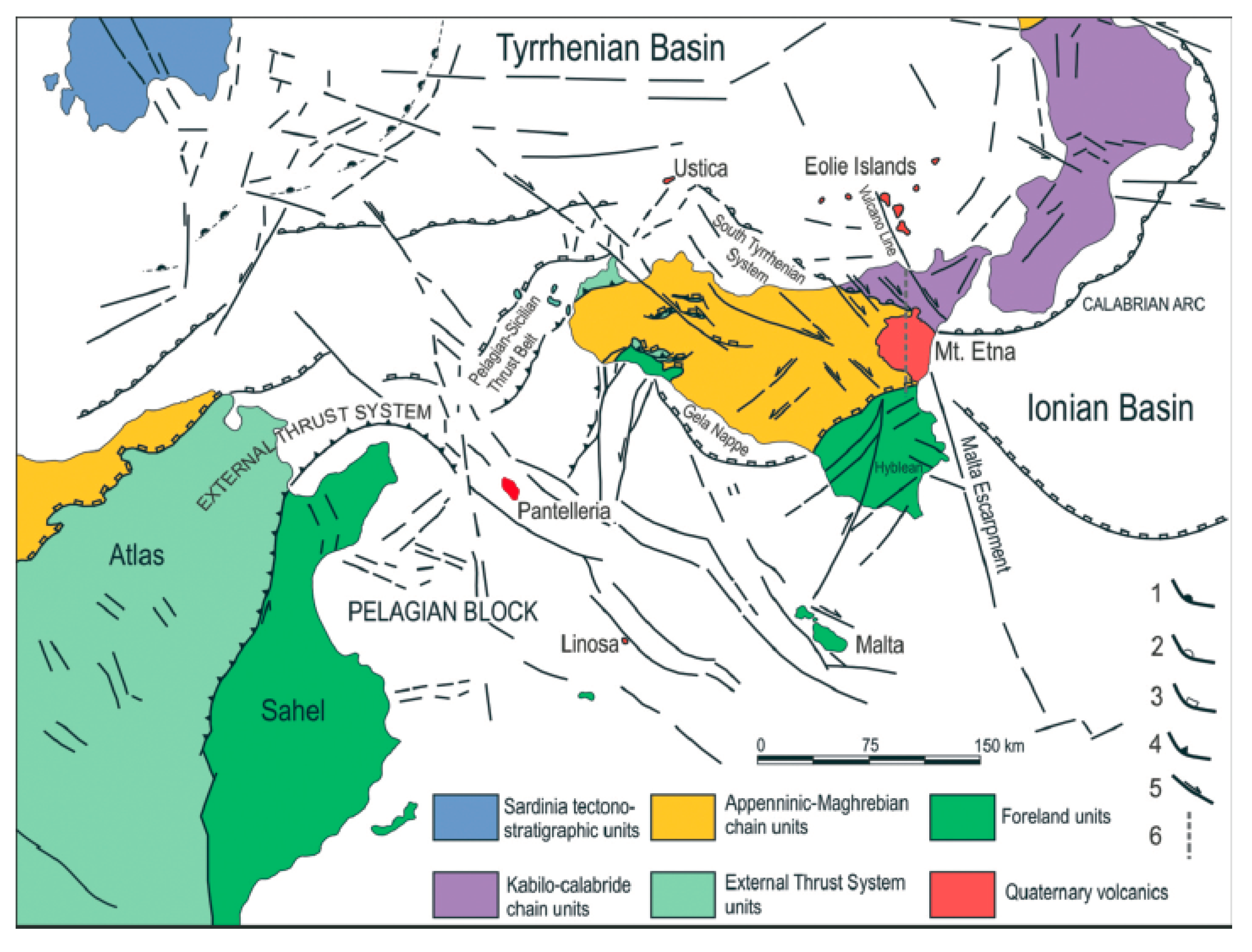
- Branca, S.; Coltelli, M.; Groppelli, G.; Lentini, F. Geological map of Etna volcano, 1:50,000 scale. Ital. J. Geosci. 2011, 130, 265–291. [Google Scholar] [CrossRef]
- Ferranti, L.; Pepe, F.; Barreca, G.; Meccariello, M.; Monaco, C. Multi-temporal tectonic evolution of Capo Granitola and Sciacca foreland transcurrent faults (Sicily channel). Tectonophysics 2019, 765, 187–204. [Google Scholar] [CrossRef]
- Nigro, F.; Renda, P. Un modello di evoluzione tettono-sedimentaria dell’avanfossa neogenica siciliana. Boll. Soc. Geosci. Ital. 2000, 119, 667–686. [Google Scholar]
- Gasparo Morticelli, M.; Valenti, V.; Catalano, R.; Sulli, A.; Agate, M.; Avellone, G.; Albanese, C.; Basilone, L.; Gugliotta, C. Deep controls on foreland basin system evolution along the Sicilian fold and thrust belt. Bull. Soc. Géol. Fr. 2015, 186, 273–290. [Google Scholar] [CrossRef]
- Sulli, A.; (Università degli Studi di Palermo, Palermo, Italy). Personal communication, 2020.
- Geraci, A.; Raimondo, F.M.; Troia, A. Genetic diversity and local population structure in Ambrosina bassii (Araceae, Ambrosineae), a Mediterranean relict species. Biochem. Syst. Ecol. 2009, 37, 737–746. [Google Scholar] [CrossRef]
- Troia, A.; Raimondo, F.M.; Geraci, A. Does genetic population structure of Ambrosina bassii L. (Araceae, Ambrosineae) attest a post-Messinian land-bridge between Sicily and Africa? Flora 2012, 207, 646–653. [Google Scholar] [CrossRef]
- Sciandrello, S.; Guarino, R.; Minissale, P.; Spampinato, G. The endemic vascular flora of Peloritani Mountains (NE Sicily): Plant functional traits and phytogeographical relationships in the most isolated and fragmentary micro-plate of the Alpine orogeny. Plant Biosyst. 2015, 149, 838–854. [Google Scholar] [CrossRef]
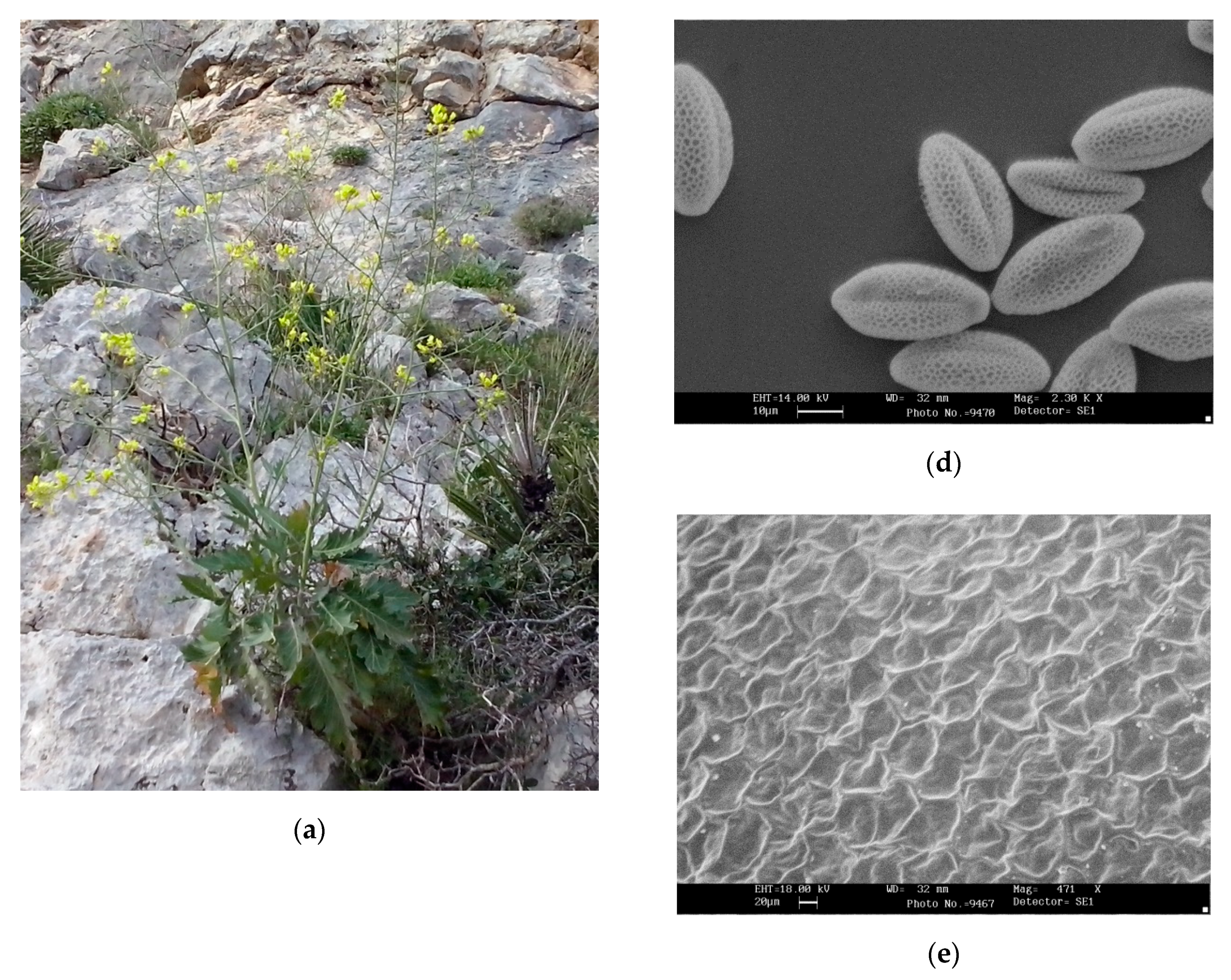
| Tardara Gorges population | Brassica rupestris subsp. rupestris | Brassica rupestris subsp. hispida | Brassica villosa subsp. brevisiliqua | |
|---|---|---|---|---|
| Stem indumentum | Glabrous | Glabrous | Glabrous | Glabrous |
| Seedling leaves | Hispid with bulbose hairs | Glabrous with rare bulbose hairs | Hispid with bulbose hairs | Glabrous with rare bulbose hairs |
| Adult leaves | Glabrous, sometimes with rare bulbose hairs in the sterile and in the young shoots, especially on the upper page | Glabrous or with rare bulbose hairs | Hispid with bulbose hairs mainly on the upper epidermis | Glabrous (shiny and thick) |
| Basal leaves: ovate–elliptic, sublyrate; petiole in upper part with 1−2 pairs of small lobes; the apical lobe is acute with deep teeth/incisions in the margin | Basal leaves ovate, lyrate, with margin more or less deeply toothed | Basal leaves ovate–elliptic, sublyrate, irregularly toothed | Basal leaves lobate–sublyrate; apical lobe is roundish with margin irregularly crenate | |
| Petal (mm) | 15.7 ± 1.57 × 7.15 ± 0.41 | 21.02 ± 1.99 × 9.84 ± 1.35 | 20.54 ± 2.05 × 9.71 ± 1.32 | 16,71± 1.02 × 6.68 ± 0.32 |
| Siliqua | Short, almost isodiametric, slightly compressed | Long, almost isodiametric, slightly compressed | Long, almost isodiametric, slightly compressed | Intermediate, tetragonous, laterally compressed |
| Ripening | Mid–end of May | Late May–early June | Mid-June | Early May |
| Pedicel of siliqua (mm) | 19.92 ± 2.46 | 13.52 ± 0.57 | 13.2 ± 2.1 | 14.02 ± 2.51 |
| Siliqua length (mm) | 30.19 ± 4.56 | 61.87 ± 3.34 | 54.1 ± 4.02 | 40.96 ± 5.04 |
| Siliqua width (mm) | 5.52 ± 0,42 | 3.7 ± 0.43 | 3.3 ± 0.6 | 6. 89 ± 0.73 |
| Siliqua beak (mm) | 4.71 ± 0.41 | 7.5 ± 2.16 | 3.8 ± 0.8 | 5.38 ± 0.65 |
| Seed in the beak | Absent | Absent | 1 (Rarely) | Absent |
| Ratio siliqua/pedicel | 1.53 ± 0.23 | 4.58 ± 0.26 | 4.09 ± 0.15 | 2.99 ± 0.57 |
| Valve, dorsal rib | Present | Slight | Slight | Present |
| Inflorescence | Very elongated inflorescence and infructescence (overall plant up to 1.5–1.8 m tall) | Very elongated inflorescence and infructescence (overall plant up to 1.5 m tall) | Very elongated inflorescence and infructescence (overall plant up to 1.5 m tall) | Contracted (not elongated) inflorescence and infructescence (overall plant up to 1.0–1.2 m tall) |
| Taste/flavor | Bitterish | Pungent/acrid | Bitterish | Pungent/acrid |
| Seed diameter (mm) | 2.51 ± 0.11 | 2.68 ± 0.32 | 2.5 ± 0.08 | 3.01 ± 0.10 |
| Seed color | Brown–blackish with small light streaks at the hilum | Brown–blackish | Brown–reddish | Brown–blackish |
| Seed surface | Alveolate–trabeculate (in relief in some parts of the seed) | Generally alveolate–trabeculate | Alveolate–trabeculate (in relief in some parts of the seed) | Alveolate regular |
| Hilum | A little prominent | Prominent | Prominent | Not prominent |
| Pollen length× width (µm) | 34.94 ± 1.66 × 18.13 ± 0.72 | 29.58 ± 0.82 × 16.36 ± 0.37 | 29.44 ± 1.91 × 16.82 ± 0.75 | 30.18 ± 0.71 × 16.82 ± 0.74 |
| Pollen ratio (polar/equatorial) | 1.93 ± 0.15 | 1.81 ± 0.07 | 1.75 ± 0.14 | 1.80 ± 0.08 |
| Pollen feature | Tricolpate, reticulate, elliptical, tapered at the poles (sometimes with prominence) | Tricolpate, reticulate, elliptical | Tricolpate, reticulate, elliptical, slightly tapered at the poles | Tricolpate, reticulate, elliptical |
| Stomata on upper blade (n/mm2) | 297.7 ± 20.6 | 295.5 ± 10.9 | 296.4 ± 9.95 | 242.7 ± 25.8 |
| Stomata on lower blade (n/mm2) | 693.96 ± 53.05 | 307.0 ± 18.3 | 325 ± 16.3 | 553.6 ± 21.48 |
| Stomata length (µm) | 8.3 ± 1.12 | 8.2 ± 1.14 | 8.5 ± 0.98 | 12.3 ± 1.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilardi, V.; Troia, A.; Geraci, A. Brassica tardarae (Brassicaceae), a New Species from a Noteworthy Biotope of South-Western Sicily (Italy). Plants 2020, 9, 947. https://doi.org/10.3390/plants9080947
Ilardi V, Troia A, Geraci A. Brassica tardarae (Brassicaceae), a New Species from a Noteworthy Biotope of South-Western Sicily (Italy). Plants. 2020; 9(8):947. https://doi.org/10.3390/plants9080947
Chicago/Turabian StyleIlardi, Vincenzo, Angelo Troia, and Anna Geraci. 2020. "Brassica tardarae (Brassicaceae), a New Species from a Noteworthy Biotope of South-Western Sicily (Italy)" Plants 9, no. 8: 947. https://doi.org/10.3390/plants9080947
APA StyleIlardi, V., Troia, A., & Geraci, A. (2020). Brassica tardarae (Brassicaceae), a New Species from a Noteworthy Biotope of South-Western Sicily (Italy). Plants, 9(8), 947. https://doi.org/10.3390/plants9080947






