Ecological Risk Assessment of Heavy Metals along Three Main Drains in Nile Delta and Potential Phytoremediation by Macrophyte Plants
Abstract
1. Introduction
2. Results and Discussion
2.1. Sediment Analysis
2.1.1. Physiochemical Parameters
2.1.2. Heavy Metal Distribution in Sediments
2.1.3. Correlation between Sediment Parameters and Heavy Metals
2.2. Contamination Assessment of Heavy Metals in Sediment
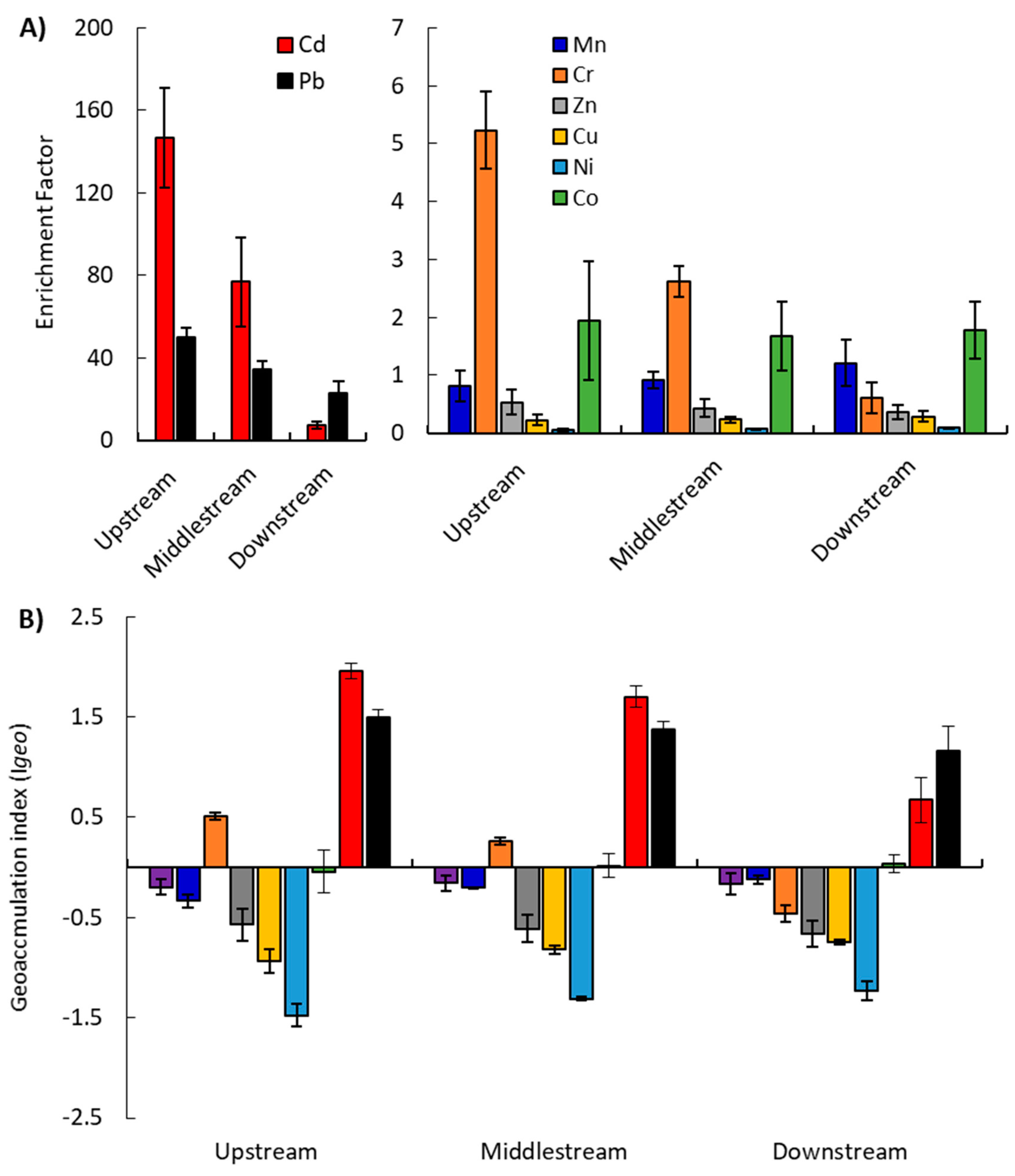
2.2.1. Enrichment Factor (EF)
2.2.2. Geo-accumulation Index (Igeo)
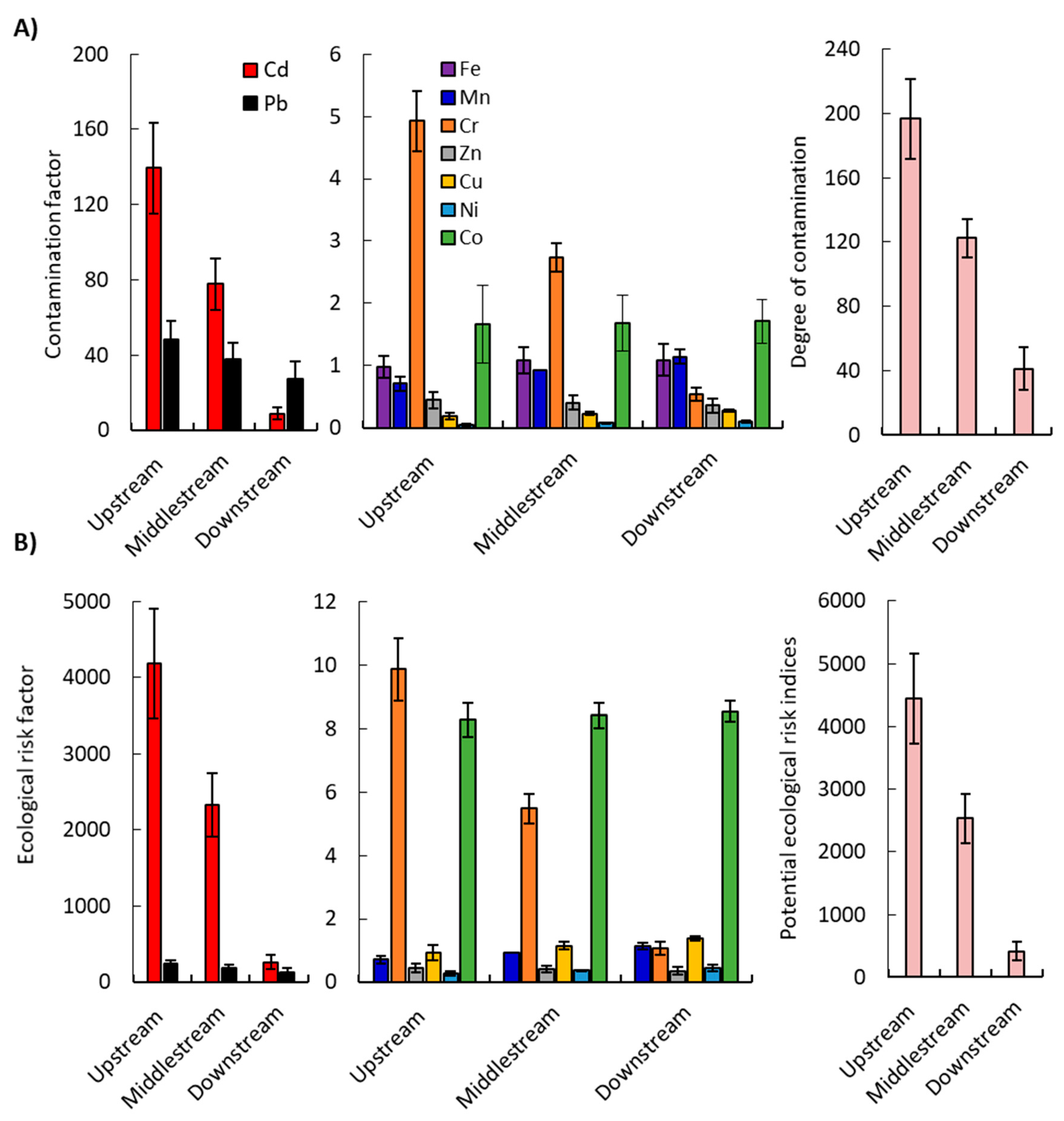
2.2.3. Contamination Factor (Cf) and Contamination Degree (CD)
2.2.4. Ecological Risk Assessment
2.3. Heavy Metal Phytoremediation
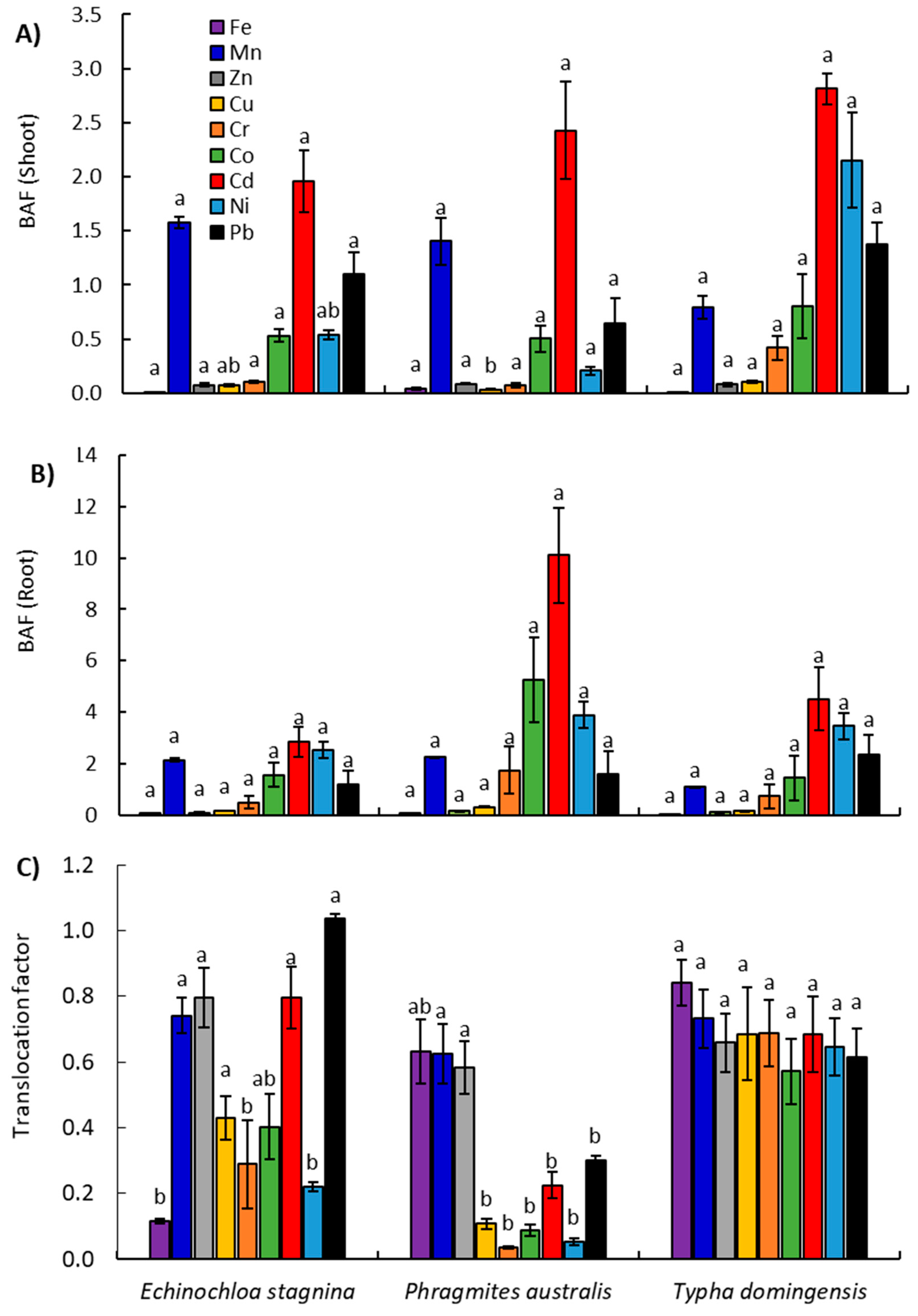
2.3.1. Heavy Metal Concentrations in Plants
2.3.2. Assessment of Plants Ability for Heavy Metal Bioaccumulation
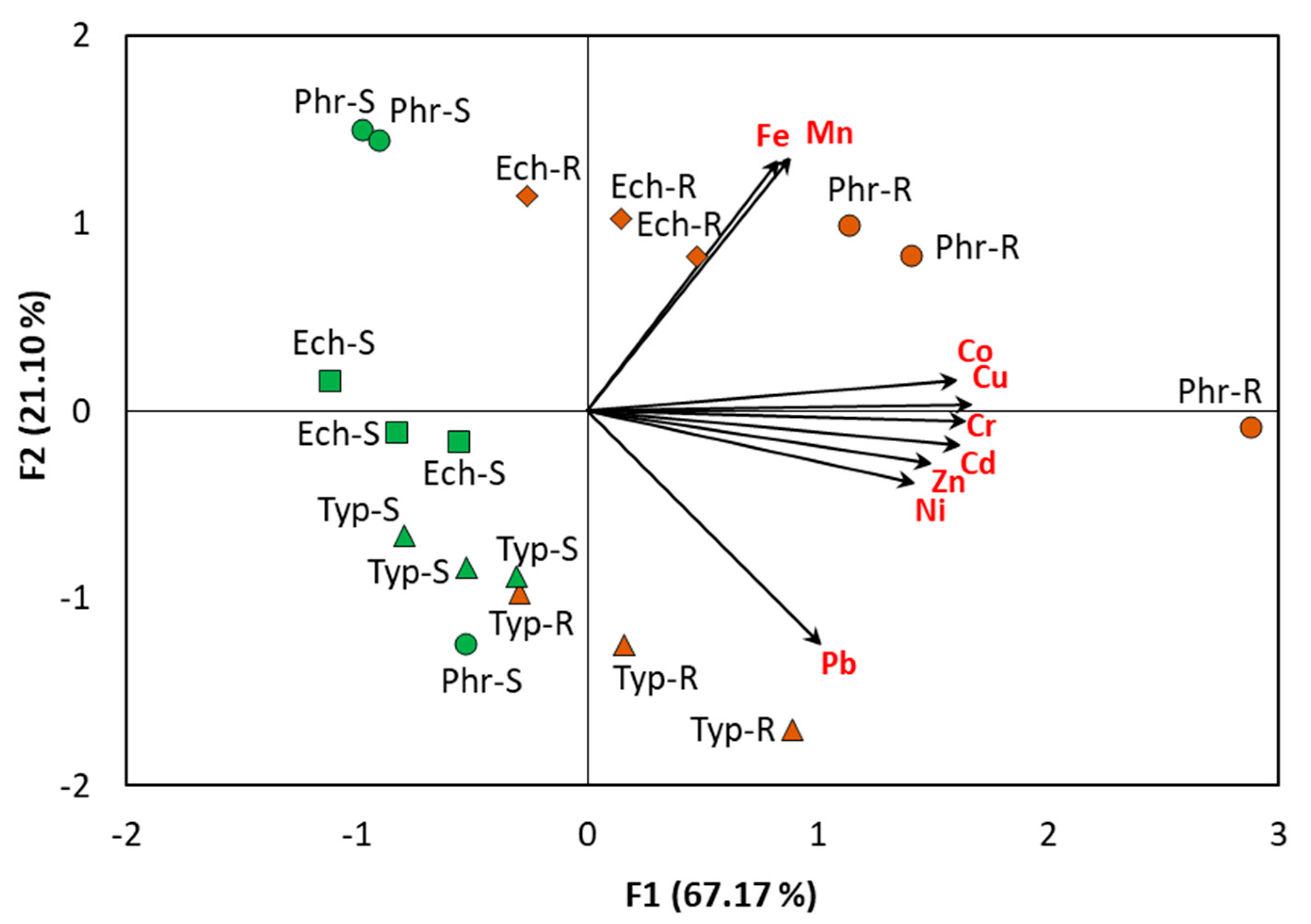
2.3.3. Principal Component Analysis of the Plant Species and Heavy Metals
3. Materials and Methods
3.1. Study Area
3.2. Sample Collection and Preparation
3.3. Samples Analyses
3.3.1. Physicochemical Analysis of Sediment Samples
3.3.2. Chemical Analysis of Sediments and Plants
3.3.3. Assessment of Sediment Heavy Metals Contamination
3.3.4. Assessment of Plants Ability for Heavy Metals Bioaccumulation
3.4. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Ghonemy, M.R. Land, Food and Rural Development in North Africa; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Alam, M.N.; Elahi, F.; Didar-UL-Alam, M.D. Risk and water quality assessment over view of River Sitalakhya in Bangladesh. Acad. Open Internet J. 2006, 19, 1311–4360. [Google Scholar]
- Rodrigues, G.C.; Pereira, L.S. Assessing economic impacts of deficit irrigation as related to water productivity and water costs. Biosyst. Eng. 2009, 103, 536–551. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Xiao, R.; Zhao, Q.; Jia, J.; Cui, B.; Liu, X. Heavy metal fractions and ecological risk assessment in sediments from urban, rural and reclamation-affected rivers of the Pearl River Estuary, China. Chemosphere 2017, 184, 278–288. [Google Scholar] [CrossRef]
- Förstner, U.; Wittmann, G.T.W. Metal Pollution in the Aquatic Environment; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.H.; Hasan, M.R.; Khan, M.; Aktar, S.; Fatema, K. Distribution of heavy metals in surface sediments of the Bay of Bengal Coast. J. Toxicol. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Negm, A.M.; Saavedra, O.; El-Adawy, A. Nile Delta biography: Challenges and opportunities. In The Nile Delta; Negm, A.M., Ed.; Springer: Cham, Switzerland, 2016; pp. 3–18. [Google Scholar]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef]
- Satpathy, D.; Reddy, M.V.; Dhal, S.P. Risk assessment of heavy metals contamination in paddy soil, plants, and grains (Oryza sativa L.) at the East Coast of India. Biomed Res. Int. 2014, 2014, 545473. [Google Scholar] [CrossRef]
- Zhao, K.; Fu, W.; Ye, Z.; Zhang, C. Contamination and spatial variation of heavy metals in the soil-rice system in Nanxun County, Southeastern China. Int. J. Environ. Res. Public Health 2015, 12, 1577–1594. [Google Scholar] [CrossRef]
- Esposito, F.; Nardone, A.; Fasano, E.; Scognamiglio, G.; Esposito, D.; Agrelli, D.; Ottaiano, L.; Fagnano, M.; Adamo, P.; Beccaloni, E. A systematic risk characterization related to the dietary exposure of the population to potentially toxic elements through the ingestion of fruit and vegetables from a potentially contaminated area. A case study: The issue of the” Land of Fires” area in Campania region, Italy. Environ. Pollut. 2018, 243, 1781–1790. [Google Scholar]
- Cai, Q.; Long, M.; Zhu, M.; Zhou, Q.; Zhang, L.; Liu, J. Food chain transfer of cadmium and lead to cattle in a lead–zinc smelter in Guizhou, China. Environ. Pollut. 2009, 157, 3078–3082. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M. Quality of river Nile sediments from Idfo to Cairo. Egypt. J. Aquat. Res. 2005, 31, 182–199. [Google Scholar]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. (Eds.) Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. In Reviews of Environmental Contamination and Toxicology; Springer Nature: Cham, Switzerland, 2019; Volume 249, pp. 71–131. [Google Scholar]
- Rezania, S.; Taib, S.M.; Din, M.F.M.; Dahalan, F.A.; Kamyab, H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.A.; El-Alfy, M.A.; Nofal, M.M. Macrophytes potential for removal of heavy metals from aquatic ecosystem, Egypt: Using metal accumulation index (MAI). Plant Arch. 2018, 18, 2131–2144. [Google Scholar]
- Newete, S.W.; Byrne, M.J. The capacity of aquatic macrophytes for phytoremediation and their disposal with specific reference to water hyacinth. Environ. Sci. Pollut. Res. 2016, 23, 10630–10643. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, B.; Korstad, J. Phytoremediation Potential of Bioenergy Plants; Springer: Singapore, 2017. [Google Scholar]
- Sood, A.; Uniyal, P.L.; Prasanna, R.; Ahluwalia, A.S. Phytoremediation potential of aquatic macrophyte, Azolla. Ambio 2012, 41, 122–137. [Google Scholar] [CrossRef]
- Bonanno, G. Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef]
- Bonanno, G.; Giudice, R.L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Cicero-Fernández, D.; Peña-Fernández, M.; Expósito-Camargo, J.A.; Antizar-Ladislao, B. Role of Phragmites australis (common reed) for heavy metals phytoremediation of estuarine sediments. Int. J. Phytoremediation 2016, 18, 575–582. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H. Monthly variations of trace elements accumulation and distribution in above-and below-ground biomass of Phragmites australis (Cav.) Trin. ex Steudel in Lake Burullus (Egypt): A biomonitoring application. Ecol. Eng. 2014, 73, 17–25. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedzielski, P.; Klimaszyk, P.; Poniedziałek, B. Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ. Monit. Assess. 2014, 186, 3199–3212. [Google Scholar] [CrossRef]
- Eid, E.M.; Galal, T.M.; Sewelam, N.A.; Talha, N.I.; Abdallah, S.M. Phytoremediation of heavy metals by four aquatic macrophytes and their potential use as contamination indicators: A comparative assessment. Environ. Sci. Pollut. Res. 2020, 27, 12138–12151. [Google Scholar] [CrossRef] [PubMed]
- Hadad, H.R.; Mufarrege, M.M.; Pinciroli, M.; Di Luca, G.A.; Maine, M.A. Morphological response of Typha domingensis to an industrial effluent containing heavy metals in a constructed wetland. Arch. Environ. Contam. Toxicol. 2010, 58, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Klink, A. A comparison of trace metal bioaccumulation and distribution in Typha latifolia and Phragmites australis: Implication for phytoremediation. Environ. Sci. Pollut. Res. 2017, 24, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Luo, C.; Lou, L.; Li, X.; Shen, Z. Bioaccumulation of heavy metals by the aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. and their potential use for contamination indicators and in wastewater treatment. Sci. Total Environ. 2008, 392, 22–29. [Google Scholar] [CrossRef]
- El-Zeiny, A.; El-Kafrawy, S. Assessment of water pollution induced by human activities in Burullus Lake using Landsat 8 operational land imager and GIS. Egypt. J. Remote Sens. Space Sci. 2017, 20, S49–S56. [Google Scholar] [CrossRef]
- Kelepertzis, E.; Stathopoulou, E. Availability of geogenic heavy metals in soils of Thiva town (central Greece). Environ. Monit. Assess. 2013, 185, 9603–9618. [Google Scholar] [CrossRef]
- Khaledian, Y.; Pereira, P.; Brevik, E.C.; Pundyte, N.; Paliulis, D. The influence of organic carbon and pH on heavy metals, potassium, and magnesium levels in Lithuanian Podzols. Land Degrad. Dev. 2017, 28, 345–354. [Google Scholar] [CrossRef]
- Shabankareh, M.G.; Amanipoor, H.; Battaleb-Looie, S.; Khatooni, J.D. Statistical modeling the effect of sediment physicochemical properties on the concentration of heavy metal (case study: Musa Creek, SW Iran). Environ. Earth Sci. 2018, 77, 101–113. [Google Scholar] [CrossRef]
- Bang, J.; Hesterberg, D. Dissolution of trace element contaminants from two coastal plain soils as affected by pH. J. Environ. Qual. 2004, 33, 891–901. [Google Scholar] [CrossRef]
- Du Laing, G.; De Vos, R.; Vandecasteele, B.; Lesage, E.; Tack, F.M.; Verloo, M.G. Effect of salinity on heavy metal mobility and availability in intertidal sediments of the Scheldt estuary. Estuar. Coast. Shelf Sci. 2008, 77, 589–602. [Google Scholar] [CrossRef]
- FAO. Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006; ISBN 92-5-105521-1. [Google Scholar]
- Kierczak, J.; Pędziwiatr, A.; Waroszewski, J.; Modelska, M. Mobility of Ni, Cr and Co in serpentine soils derived on various ultrabasic bedrocks under temperate climate. Geoderma 2016, 268, 78–91. [Google Scholar] [CrossRef]
- Dube, A.; Zbytniewski, R.; Kowalkowski, T.; Cukrowska, E.; Buszewski, B. Adsorption and migration of heavy metals in soil. Pol. J. Environ. Stud. 2001, 10, 1–10. [Google Scholar]
- Farhat, H.I. Impact of Drain Effluent on Surficial Sediments in the Mediterranean Coastal Wetland: Sedimentological Characteristics and Metal Pollution Status at Lake Manzala, Egypt. J. Ocean Univ. China 2019, 18, 834–848. [Google Scholar] [CrossRef]
- Massas, L.; Kalivas, D.; Ehaliotis, C.; Gasparatos, D. Total and available heavy metal concentrations in soils of the Thriassio plain (Greece) and assessment of soil pollution indexes. Environ. Monit. Assess. 2013, 185, 6751–6766. [Google Scholar] [CrossRef]
- Bradl, H.B. Sources and origins of heavy metals. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 6, pp. 1–27. [Google Scholar]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Abayazid, H.; Al-Shinnawy, I. Coastal lake sustainability: Threats and opportunities with climate change. Iosr J. Mech. Civ. Eng. 2012, 1, 33–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, D.; O’Connor, D.; Shen, Z.; Shi, P.; Ok, Y.S.; Tsang, D.C.W.; Wen, Y.; Luo, M. Lead contamination in Chinese surface soils: Source identification, spatial-temporal distribution and associated health risks. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1386–1423. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Theologou, E.; Bompoti, N.; Dermatas, D.; Panagiotakis, I. Occurrence, origin and transformation processes of geogenic chromium in soils and sediments. Curr. Pollut. Rep. 2016, 2, 224–235. [Google Scholar] [CrossRef]
- Mills, C.T.; Goldhaber, M.B. Laboratory investigations of the effects of nitrification-induced acidification on Cr cycling in vadose zone material partially derived from ultramafic rocks. Sci. Total Environ. 2012, 435, 363–373. [Google Scholar] [CrossRef]
- Segarra, M.J.; Szefer, P.; Wilson, M.J.; Bacon, J.; Bolalek, J. Chemical Forms and Distribution of Heavy Metals in Core Sediments from the Gdańsk Basin, Baltic Sea. Pol. J. Environ. Stud. 2007, 16, 505–515. [Google Scholar]
- Panagos, P.; Ballabio, C.; Lugato, E.; Jones, A.; Borrelli, P.; Scarpa, S.; Orgiazzi, A.; Montanarella, L. Potential sources of anthropogenic copper inputs to European agricultural soils. Sustainability 2018, 10, 2380. [Google Scholar] [CrossRef]
- EU. Heavy Metals in Wastes, European Commission on Environment. 2002. Available online: http://ec.europa.eu/environment/waste/studies/pdf/heavy_metalsreport.pdf (accessed on 1 February 2002).
- CSQGD. Canadian Soil Quality Guidelines for the Protection of Environment and Human Health; Canadian Council of Ministers of the Environment (CCME): Winnipeg, MB, Canada, 2007. [Google Scholar]
- USA EPA. Screening Level Ecological Risk Assessment Protocol for Hazardous Waste Combustion Facilities; Appendix E: Toxicity Reference Values; EPA530-D99-001C; USA EPA: Dallas, TX, USA, 1999; Volume 3.
- Spurgeon, D.J.; Rowland, P.; Ainsworth, G.; Rothery, P.; Long, S.; Black, H.I. Geographical and pedological drivers of distribution and risks to soil fauna of seven metals (Cd, Cu, Cr, Ni, Pb, V and Zn) in British soils. Environ. Pollut. 2008, 153, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Dragović, S.; Mihailović, N.; Gajić, B. Heavy metals in soils: Distribution, relationship with soil characteristics and radionuclides and multivariate assessment of contamination sources. Chemosphere 2008, 72, 491–495. [Google Scholar] [CrossRef]
- Caeiro, S.; Costa, M.H.; Ramos, T.B.; Fernandes, F.; Silveira, N.; Coimbra, A.; Medeiros, G.; Painho, M. Assessing heavy metal contamination in Sado Estuary sediment: An index analysis approach. Ecol. Indic. 2005, 5, 151–169. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Liu, J.; Yin, P.; Chen, B.; Gao, F.; Song, H.; Li, M. Distribution and contamination assessment of heavy metals in surface sediments of the Luanhe River Estuary, northwest of the Bohai Sea. Mar. Pollut. Bull. 2016, 109, 633–639. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, C.; Ahn, B. Sedimentation pattern and sediments bioavailability in a wastewater discharging area by sequential metal analysis. Microchem. J. 2001, 68, 135–141. [Google Scholar] [CrossRef]
- Kowalska, J.; Mazurek, R.; Gąsiorek, M.; Setlak, M.; Zaleski, T.; Waroszewski, J. Soil pollution indices conditioned by medieval metallurgical activity—A case study from Krakow (Poland). Environ. Pollut. 2016, 218, 1023–1036. [Google Scholar] [CrossRef]
- Müller, G. Die Schwermetallbelstung der sediment des Neckars und seiner Nebenflusse: Eine Bestandsaufnahme. Chem. Ztg. 1981, 105, 156–164. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Dickinson, N.M. Strategies for sustainable woodland on contaminated soils. Chemosphere 2000, 41, 259–263. [Google Scholar] [CrossRef]
- Sawidis, T.; Chettri, M.K.; Zachariadis, G.A.; Stratis, J.A. Heavy metals in aquatic plants and sediments from water systems in Macedonia, Greece. Ecotoxicol. Environ. Saf. 1995, 32, 73–80. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Jt. FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods, 5th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xing, Y.; Zhang, B.; Cai, R.; Zhang, D.; Sun, G. Effective phytoremediation of low-level heavy metals by native macrophytes in a vanadium mining area, China. Environ. Sci. Pollut. Res. 2018, 25, 31272–31282. [Google Scholar] [CrossRef]
- FAO. Land Resources Information Systems in the Near East. In Regional Workshop; FAO: Cairo, Egypt, 2002. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 2005; Volume IV. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Interscience Publishers, Inc.: New York, NY, USA, 1947. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- ISO. Soil Quality—Microwave Assisted Extraction of the Aqua Regia Soluble Fraction for the Determination of Elements; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- ISO. Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICPOES); ISO 11885:2007; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Franco-Uría, A.; López-Mateo, C.; Roca, E.; Fernández-Marcos, M.L. Source identification of heavy metals in pastureland by multivariate analysis in NW Spain. J. Hazard. Mater. 2009, 165, 1008–1015. [Google Scholar] [CrossRef]
- Lu, S.; Bai, S. Contamination and potential mobility assessment of heavy metals in urban soils of Hangzhou, China: Relationship with different land uses. Environ. Earth Sci. 2010, 60, 1481–1490. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Baker, A.J.M. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L.F.J. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 24. [Google Scholar]

| Parameter | Upstream | Middlestream | Downstream | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S4 | S7 | Mean ± SE | S2 | S5 | S8 | Mean ± SE | S3 | S6 | S9 | Mean ± SE | ||
| pH | 7.89 | 8.01 | 8.10 | 8.00 a ± 0.06 | 8.05 | 7.83 | 7.90 | 7.93 a ± 0.06 | 7.78 | 7.71 | 8.02 | 7.84 a ± 0.09 | 0.36 ns |
| EC (dS/m) | 2.14 | 1.89 | 1.91 | 1.98 c ± 0.08 | 2.88 | 2.46 | 3.15 | 2.83 b ± 0.20 | 3.55 | 3.22 | 3.72 | 3.50 a ± 0.15 | 0.001 ** |
| OM (%) | 1.32 | 1.37 | 1.51 | 1.40 b ± 0.06 | 1.65 | 2.17 | 2.08 | 1.97 ab ± 0.16 | 1.98 | 2.88 | 2.92 | 2.59 a ± 0.31 | 0.017 * |
| CaCO3 (%) | 2.95 | 2.80 | 1.79 | 2.51 a ± 0.36 | 2.43 | 2.28 | 2.42 | 2.38 a ± 0.05 | 2.63 | 2.68 | 2.88 | 2.73 a ± 0.08 | 0.54 ns |
| Sand (%) | 81.10 | 70.97 | 78.73 | 76.93 b ± 3.06 | 85.32 | 77.78 | 83.29 | 82.13 ab ± 2.25 | 89.75 | 86.89 | 90.12 | 88.92 a ± 1.02 | 0.027 * |
| Silt (%) | 13.24 | 17.58 | 12.52 | 14.45 a ± 1.58 | 11.57 | 12.58 | 9.67 | 11.27 ab ± 0.85 | 8.27 | 9.57 | 7.22 | 8.35 b ± 0.68 | 0.023 * |
| Clay (%) | 5.66 | 11.45 | 8.75 | 8.62 a ± 1.67 | 3.11 | 9.64 | 7.04 | 6.60 ab ± 1.90 | 1.98 | 3.54 | 2.66 | 2.73 a ± 0.45 | 0.076 ns |
| Locations | Site | Fe | Mn | Pb | Cr | Zn | Cu | Ni | Co | Cd |
|---|---|---|---|---|---|---|---|---|---|---|
| Upstream | S1 | 45,624.00 | 776.70 | 441.20 | 397.90 | 51.20 | 7.33 | 5.22 | 9.98 | 54.68 |
| S4 | 61,201.00 | 1350.30 | 599.60 | 531.20 | 18.40 | 5.16 | 2.08 | 33.37 | 40.95 | |
| S7 | 32,079.00 | 776.80 | 772.20 | 401.40 | 58.00 | 12.78 | 3.66 | 50.86 | 29.76 | |
| Mean | 46,301.33 a | 967.93 a | 604.33 b | 443.50 a | 42.53 a | 8.42 a | 3.65 a | 31.40 a | 41.80 a | |
| ± SE | 8413.62 | 191.18 | 95.58 | 43.86 | 12.23 | 2.27 | 0.91 | 11.84 | 7.21 | |
| Middlestream | S2 | 40,742.50 | 782.13 | 467.48 | 232.55 | 38.75 | 10.40 | 4.53 | 19.48 | 30.90 |
| S5 | 71,617.00 | 801.78 | 1073.20 | 287.39 | 19.50 | 8.47 | 5.21 | 28.14 | 22.47 | |
| S8 | 41,711.00 | 781.00 | 723.10 | 218.48 | 56.75 | 12.13 | 5.11 | 48.14 | 16.50 | |
| Mean | 51,356.83 a | 788.30 ab | 754.59 a | 246.14 b | 38.33 a | 10.33 a | 4.95 a | 31.92 a | 23.29 b | |
| ± SE | 10,133.94 | 6.75 | 175.56 | 21.02 | 10.76 | 1.06 | 0.21 | 8.49 | 4.18 | |
| Downstream | S3 | 30,861.00 | 1123.05 | 158.25 | 67.19 | 26.30 | 13.46 | 3.84 | 28.98 | 0.71 |
| S6 | 72,033.00 | 1003.95 | 796.10 | 43.58 | 20.60 | 11.78 | 8.33 | 22.90 | 3.99 | |
| S9 | 51,343.00 | 789.80 | 669.40 | 35.55 | 55.50 | 11.48 | 6.56 | 45.41 | 3.24 | |
| Mean | 51,412.33 a | 972.27 a | 541.25 a | 48.77 c | 34.13 a | 12.24 a | 6.24 a | 32.43 a | 2.65 c | |
| ± SE | 11,885.38 | 97.50 | 194.96 | 9.50 | 10.81 | 0.62 | 1.31 | 6.72 | 0.99 | |
| p−value | 0.92 ns | 0.0450 * | 0.34 ns | 0.0002 *** | 0.87 ns | 0.27 ns | 0.22 ns | 0.99 ns | 0.0037 ** | |
| Permissible limits worldwide | ||||||||||
| EU (2002) | - | - | 300 | 150 | 300 | 140 | 75 | 11.6 | 3 | |
| CSQGD (2007) | - | - | 70 | 64 | - | - | 50 | 40 | 1.4 | |
| US EPA (1999) | - | 550 | 19 | 54 | 60 | 25 | 19 | 9.1 | 0.01–41 | |
| Average Shale | 47,200 | 850 | 20 | 90 | 95 | 45 | 68 | 19 | 0.3 | |
| Toxic response factor | - | 1 | 5 | 2 | 1 | 5 | 5 | 5 | 30 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Amier, Y.A.; Bonanomi, G.; Al-Rowaily, S.L.; Abd-ElGawad, A.M. Ecological Risk Assessment of Heavy Metals along Three Main Drains in Nile Delta and Potential Phytoremediation by Macrophyte Plants. Plants 2020, 9, 910. https://doi.org/10.3390/plants9070910
El-Amier YA, Bonanomi G, Al-Rowaily SL, Abd-ElGawad AM. Ecological Risk Assessment of Heavy Metals along Three Main Drains in Nile Delta and Potential Phytoremediation by Macrophyte Plants. Plants. 2020; 9(7):910. https://doi.org/10.3390/plants9070910
Chicago/Turabian StyleEl-Amier, Yasser A., Giuliano Bonanomi, Saud L. Al-Rowaily, and Ahmed M. Abd-ElGawad. 2020. "Ecological Risk Assessment of Heavy Metals along Three Main Drains in Nile Delta and Potential Phytoremediation by Macrophyte Plants" Plants 9, no. 7: 910. https://doi.org/10.3390/plants9070910
APA StyleEl-Amier, Y. A., Bonanomi, G., Al-Rowaily, S. L., & Abd-ElGawad, A. M. (2020). Ecological Risk Assessment of Heavy Metals along Three Main Drains in Nile Delta and Potential Phytoremediation by Macrophyte Plants. Plants, 9(7), 910. https://doi.org/10.3390/plants9070910









