Abstract
A cold-related protein, cold shock protein 3 (BcCSP3), was isolated from non-heading Chinese cabbage in this study. BcCSP3 can encode 205 amino acids (aa) with an open reading frame (ORF) of 618 base pairs (bp). Multiple sequence alignment and phylogenetic tree analyses showed that BcCSP3 contains an N-terminal cold shock domain and is highly similar to AtCSP2, their kinship is recent. Real-time quantitative polymerase chain reaction (RT-qPCR) showed that the expression level of BcCSP3 in stems and leaves is higher than that in roots. Compared with other stress treatments, the change in BcCSP3 expression level was most pronounced under cold stress. In addition, a BcCSP3–GFP fusion protein was localized to the nucleus and cytoplasm. These results indicated that BcCSP3 may play an important role in response to cold stress in non-heading Chinese cabbage. This work may provide a reference for the identification and expression analysis of other CSP genes in non-heading Chinese cabbage.
1. Introduction
Non-heading Chinese cabbage (Brassica rapa ssp. chinensis), a widely grown vegetable crop, is also an important Brassica crop with exceptional cold resistance [1,2]. As the global climate continues to change, plants (including Pak-choi) must be equipped with appropriate regulatory mechanisms to be able to respond to changes in the surrounding environment [3]. As one of the important environmental factors (abiotic stress), cold stress can affect crop yield and quality by restricting plant growth and development [4]. After a period of low temperature (no freezing), some plants can obtain higher resistance to freezing. This process is called cold acclimation and has been proven by previous studies [5]. Cold acclimation is a complex process with a series of changes in gene expression and protein metabolism [6].
Cold-induced protein, cold shock domain (CSD) protein (CSP), is an evolutionarily conserved nucleic-acid binding domain. Studies have shown these proteins are widely found in animals and plants [7]. Karlson and Imai found that in plants, CSP usually contains an N-terminal CSD region and a glycine-rich region, which is randomly scattered with multiple CCHC zinc fingers (ZnF) [8]. In a prior study, they identified and isolated WCSP1 from wheat (Triticum aestivum L.), which was the first CSP isolated from plants [9]. However, previous experiments have shown that WCSP1 mRNA can only be induced by cold and without being regulated by other environmental abiotic stresses (such as salt or ABA stress). These results indicate that WCSP1 induction may be cold-specific [9]. In addition, WCSP1 can bind to DNA and unlock the double-stranded structure of its nucleotides [9,10] and partially supplements the cold sensitivity of the E. coli CSP mutant. These findings indicate that WCSP1 has similar functions as E. coli CSPs during cold adaptation [11]. The above predecessors’ research on CSP from wheat is more in-depth, but there is relatively little research on CSP from Pak-choi. Based on the above mentioned studies, further research on the cold stress tolerance of Pak-choi could be valuable in improving the growth and development and by extension, the yield and quality of this economically important crop.
To date, four CSD proteins (AtCSP1, AtCSP2, AtCSP3 and AtCSP4) from Arabidopsis have been identified and functionally analyzed [12]. Further analysis showed that AtCSP3 enhanced both the crop cold resistance pathway and C-repeat/dehydration responsive element-binding factor (CBF) pathway, which were two unrelated pathways [13]. AtCSP2 plays a role as the negative regulator of freezing tolerance and is considered functionally redundant to AtCSP4 and AtCSP2, as it can regulate flowering time and frost resistance of plants compared to AtCSP3 [14]. It was further found that AtCSP2 had obvious activity in meristems and developmental tissues of plants, while AtCSP1 overexpression did not increase the tolerance to low temperature stress [15,16].
Although CSP has been identified in many other plants and functional studies of CSP have been conducted, information about CSPs in Pak-choi (Brassica rapa ssp. chinensis) is still limited. Moreover, their response mechanism to abiotic stresses is not yet clear. Through sequence alignment and evolutionary tree analysis, we found that BcCSP3 and AtCSP2 proteins are highly similar and have a closer relationship. Therefore, we speculate whether they have similar cold stress emergency response. In this study, we analyzed the protein structure, protein interaction prediction, phylogenetic relationships, conserved motifs and subcellular localization of BcCSP3. In addition, the expression level changes of BcCSP3 in different vegetative organs (roots, stems and leaves) and under multiple abiotic stresses were examined through RT-qPCR test analysis. In all, this work may help to reveal the biologic function of BcCSP3 and lay the foundation for a deeper understanding of BcCSP3 in Pak-choi.
2. Materials and Methods
2.1. Experiment Material and Stresses
Seeds of Pak-choi (Brassica rapa ssp. chinensis cv. Suzhouqing) were obtained from the Cabbage System Biology Laboratory of Nanjing Agricultural University. The seeds were geminated and planted in a mixture of soil and sand (3:1, volume ratio). The seeds were then placed in an artificial climate room (16 h/8 h; 22/18 °C) and allowed to grow. After four weeks, the seedlings were exposed to various abiotic stress treatments including ABA, cold, salt and dehydration. For ABA, salt and dehydration treatments, 100 uM ABA, 200-mM NaCl and 15% (w/v) polyethylene glycol (PEG) was used, respectively [17]. For cold treatment, the seedlings were placed in a 4 °C incubator. Leaf tissues were collected at 0, 12, 24 and 48 h after initial exposure and then immediately placed in liquid nitrogen and promptly stored in a −80 °C freezer for further testing. To measure the expression level of BcCSP3 throughout the plant, samples were taken from the roots, stems and leaves.
2.2. Identification and Isolation of BcCSP3
According to the sequence of Arabidopsis CSP protein, blasting in the non-heading Chinese cabbage database. In addition, in the non-heading Chinese cabbage database by blasting with the Arabidopsis genome, we found 6 genes containing CSP domains in the B. rapa genome, named BcCSP1, BcCSP2, BcCSP3, BcCSP4, BcCSP5 and BcCSP6 based on their sequence length [18]. Here, the focus of this work was BcCSP3. BcCSP3 gene was identified and analyzed, then the primer design was performed using the method of comparing nucleotide sequences with the software Primer 5.0 (Supplementary Table S1). Total RNA was extracted from roots, stems and leaves with the RNAeasy mini kit (Tiangen, Beijing, China) following the manufacturer’s instructions. The cDNA of BcCSP3 was amplified from a cDNA library of Pak-choi treated with diverse abiotic stresses by RT-qPCR. The first-strand cDNA was synthesized with 1 ug of mixed total RNA to construct a stress-induced cDNA library of Pak-choi by using a Superscript II kit (Takara, Dalian, China). Conserved regions were identified based on sequence information from the Arabidopsis CSP gene family (http://arabidopsis.org/index.jsp). The amplification program: 94 °C for 5 min, 30 cycles of 94 °C for 30 s, 60 °C for 30 s, 1 min at 72 °C, and for 10 min at 72 °C. The PCR product was cloned into pMD18-T (Takara, Dalian, China). The ORF size of BcCSP3 was confirmed by sequencing. The molecular weight, isoelectric point (pI) and length of the putative protein were predicted using the online ExPasy program (http://www.expasy.org/tools/).
2.3. Bioinformatics Analysis of BcCSP3 Protein
Secondary structure and tertiary structure analysis were performed by online tool SOPMA [19] (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html) and SWISS-MODEL (https://www.swissmodel.expasy.org/), respectively; signal peptide prediction through the online tool SignalP-5.0 Server [20] (http://www.cbs.dtu.dk/services/SignalP); transmembrane regions prediction through the online tool TMpred [21] (http://www.ch.embnet.org/software/TMPRED_form.html); hydrophilic prediction through the online tool ProtScale [22] (https://web.expasy.org/protscale/).
2.4. Sequence Alignment and Phylogenetic Tree
Some CSP amino acid sequences from Arabidopsis were also obtained (Supplementary Table S2). The deduced amino acid sequence was analyzed using DNAMAN (Lynnon Biosoft, San Ramon, CA, USA). Multiple sequence alignment was performed by ClustalW [23] and MEGA 6.0 software [24] with the default parameters and manual correction. Phylogenetic trees were generated with MEGA 6.0 using the maximum likelihood method. Bootstrap values were estimated with 1000 replicates. The conserved motifs were analyzed using MEME suite (http://meme.nbcr.net/meme/) with the default settings except the maximum width was set to 200, and the maximum and minimum numbers of motifs were defined as 10 and 2, respectively.
2.5. Analysis of BcCSP3 under Stress Treatments
The roots, stems and leaves of non-heading Chinese cabbage were sampled separately, to study organ-specific expression analysis. To investigate the changes of BcCSP3 expression under diverse stress treatments, we performed RT-qPCR. Real-time quantitative PCR is a commonly used high-throughput technique to measure mRNA transcription levels [25]. Total RNA was extracted from the previously frozen plant tissues with an RNA extraction kit (RNAsimply total RNA Kit, Tiangen, Beijing, China). Genomic DNA contamination was removed by using DNase I (Takara, Dalian, China). The first-strand cDNA was synthesized by using the PrimeScript™ RT reagent Kit (Takara, Dalian, China). The RT-qPCR assay was carried out with the 7500 fast real-time qPCR System (Applied Biosystems, Foster City, CA, USA). The PCR program: 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s. The gene-specific and internal control primers are listed in Supplementary Table S1. The relative expression level of BcCSP3 was calculated by the 2−ΔΔCT method [26]. All of the above RT-qPCR experiments were performed with three biologic replicates and three technical replicates.
2.6. Subcellular Localization of BcCSP3 Protein
The WoLF PSORT (https://wolfpsort.hgc.jp/) online tool was used to predict the subcellular localization of the BcCSP3 protein. The gateway-specific primers (Supplementary Table S1) were used to amplify the full-length coding region of BcCSP3 then construct the cloned product into the corresponding vector. The vector was then introduced into pEarleyGate103 using the gateway system (Invitrogen, Carlsbad, CA, USA) to get the BcCSP3–GFP fusion construct protein. In order to achieve transient expression of BcCSP3, BcCSP3–GFP was bombarded into tobacco leaves cells. The infected tobacco was grown in the dark at 22 °C for approximately 48 h and then placed under a confocal laser scanning microscope to find the corresponding fluorescence (Zeiss, Jena, Germany).
2.7. Protein–Protein Interaction Analysis
As a protein interaction prediction method, protein–protein interaction (PPI) refers to two (or more) protein molecules forming a protein complex through non-covalent bonds [27]. In biologic processes, protein–protein interactions and recognition play a certain role [28]. In order to predict the interaction between proteins and build a protein interaction network based on the prediction results, the STRING online tool (https://string-db.org/) was used.
3. Results
3.1. Identification and Cloning of BcCSP3
The full-length of BcCSP3 gene was isolated. BcCSP3 has a 618 bp ORF, encoding 205 deduced aa (Figure 1), with molecular weight of 19.39 kDa, pI of 5.92, the grand average of hydropathicity (GRAVY) of −0.764 and an instability index of 59.39 (Table 1). The ORF was confirmed by three repeated sequencing events.

Figure 1.
Nucleotide acid and corresponding amino acid sequence of BcCSP3 in non-heading Chinese cabbage.

Table 1.
General information on cold shock proteins (CSPs).
3.2. Analysis of Proteins
SOPMA online tool was often used to predict the secondary structure of BcCSP3 protein, with result shows that the secondary structure consists of 0.0293 alpha helix, 0.2439 extended strand, 0.2341 beta turn and 0.4927 random coil. Random coil, extended strand and beta turn are scattered throughout the protein (Figure 2). In addition, the predicted tertiary structure of the protein was consistent with the predicted results of its secondary structure (Figure 3). SignalP-5.0 was used to predict the signal peptide, and the results showed that: Signal Peptide (Sec/SPI), 0.0017%; Other (the probability that the sequence does not have any kind of signal peptide), 0.9983%, which means that BcCSP3 has no signal peptide (Figure S1). Using TMHMM to predict the transmembrane domain of BcCSP3 the result shows no transmembrane domain (Figure S2). ProtScale was used to analyze the hydrophilicity of BcCSP3 protein, it is a hydrophilic protein (Figure S3).
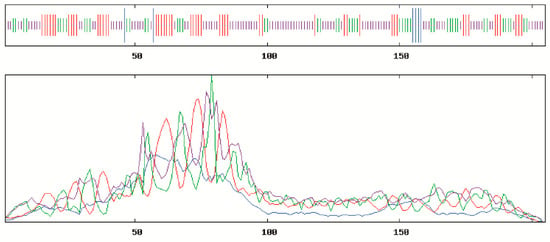
Figure 2.
Secondary structural prediction of the BcCSP3 protein. The successively shorter straight lines represent helix, turn, strand and coil, respectively; the blue, red, green and purple curves represent helix, turn, strand and coil, respectively.
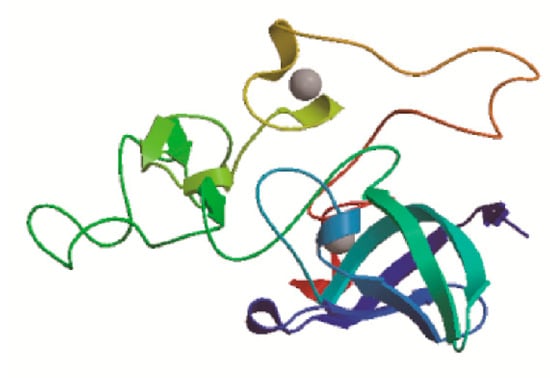
Figure 3.
Tertiary structure prediction of the BcCSP3 protein. Random coil, extended strand and beta turn make up most of the main body.
3.3. Sequence Alignment and Phylogenetic Analysis
Using DNAMAN (Lynnon Biosoft, San Ramon, CA, USA) software, the deduced amino acid sequence of BcCSP3 was compared with the CSP protein sequence of Arabidopsis. It was found that the protein sequence of BcCSP3 had a certain similarity with AtCSP, especially a higher sequence similarity was detected at the N-terminus. Sequence alignment revealed that the BcCSP3 and AtCSP2 proteins had approximately 75.11% amino acid sequence homology. It is interesting that five CSPs all contain a well-preserved CSD field (Figure 4).
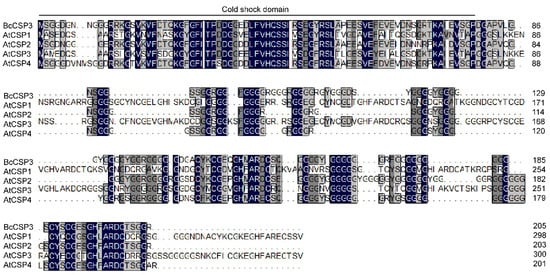
Figure 4.
Amino acid sequence alignment of the putative BcCSP3 protein with Arabidopsis CSP proteins. Perfectly matched residues, highly conserved residues, and less conserved residues are represented by a black, dark gray and gray box, respectively.
In order to reveal the evolutionary genetic relationship between BcCSP3 and AtCSPs, amino acid sequence alignment was used to establish a phylogenetic tree by setting 1000 bootstrap repeats with maximum likelihood method (Figure 5). The results showed that BcCSP3 had the closest genetic relationship with AtCSP2 in Arabidopsis. At the same time, after analyzing the motifs, it is interesting to find that the motifs of BcCSP3 and AtCSP2 are also very similar (both have 4 motif sites) (Figure 5).
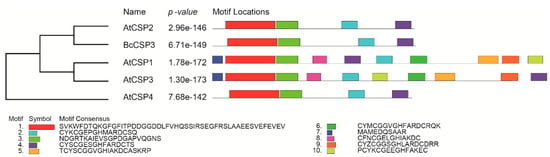
Figure 5.
Phylogenetic and motif analysis of the putative BcCSP3 protein and Arabidopsis CSPs. The unrooted tree is based on an alignment of the full-length protein sequences and was constructed by the neighbor joining method.
3.4. BcCSP3 Expression Pattern under Various Abiotic Stresses
Through RT-qPCR analysis, we found that the expression level of BcCSP3 in stems and leaves was much higher than that in roots (Figure 6). In addition, the relative expression of BcCSP3 under abiotic stress was tested by RT-qPCR. As shown in Figure 7A, under the ABA treatment, the expression level of BcCSP3 exhibited a tendency to increase first, then decrease before increasing again within 48 h. In the cold treatment, the expression level of BcCSP3 kept increasing over time within 48 h (Figure 7B). Under the salt stress treatment, the expression level of BcCSP3 increased first and then decreased and reached the maximum expression level at 24 h (Figure 7C). Under the dehydration stress, the expression trend of BcCSP3 was basically similar to that expressed during the ABA treatment (Figure 7D). After cold treatment, the expression level of BcCSP3 increased with time, indicating that cold treatment could affect expression level of BcCSP3.
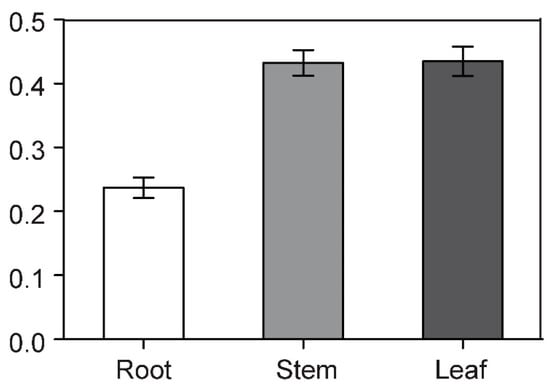
Figure 6.
RT-qPCR analysis of the expression patterns of BcCSP3 in roots, stems and leaves of Pak-choi. Each column represents the average and standard deviation of three replicates. Each bar value represents the mean ± SD (p < 0.05).
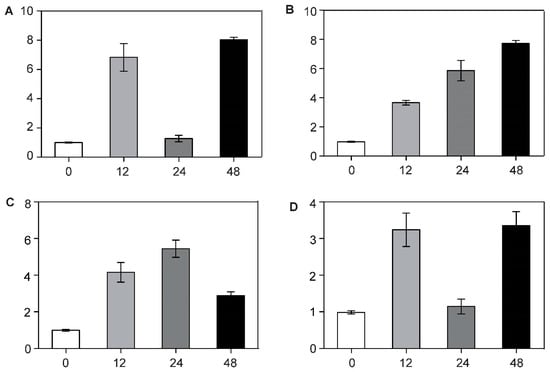
Figure 7.
RT-qPCR analysis of the expression patterns of BcCSP3 in Pak-choi (leaves) under abiotic stresses. (A) BcCSP3 expression under ABA treatment; (B) BcCSP3 expression under cold treatment; (C) BcCSP3 expression under salt treatment; (D) BcCSP3 expression under dehydration treatment. Each column represents the average and standard deviation of three replicates. Each bar value represents the mean ± SD (p < 0.05).
3.5. Subcellular Localization Analysis
Through WoLF PSORT, BcCSP3 was predicted to be located in the nucleus, and its nuclear localization score was 14.0 (KNN = 14). The subcellular localization of BcCSP3 was tested in tobacco leaf cells by expressing transiently the BcCSP3–GFP. Results indicate the BcCSP3–GFP localized in the nucleus and cytoplasm (Figure 8).
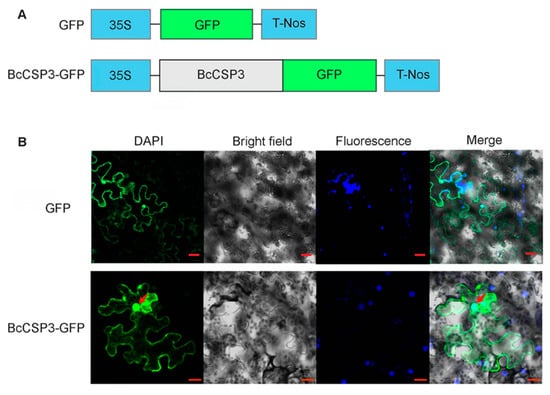
Figure 8.
Subcellular localization of BcCSP3. (A) Constructs used in the experiments; (B) BcCSP3–GFP was transferred to tobacco leaf cells to test and verify the subcellular localization of BcCSP3 (bar = 20 μm).
3.6. Protein–Protein Interaction Network
By using the online tool STRING, we constructed a protein interaction network (Figure 9). From the selected database, it was found that ten proteins may have some relationship with BcCSP3 (Supplementary Table S3). Seven proteins (except Bra040811.1-P, Bra032735.1-P and Bra013111.1-P) were identified to interact with BcCSP3 through previous experimentally determined; further research found that Bra040811.1-P and Bra032735.1-P proteins were identified as having gene co-occurrence relationship; while nine proteins (except Bra013111.1-P) had a co-expression relationship with BcCSP3.
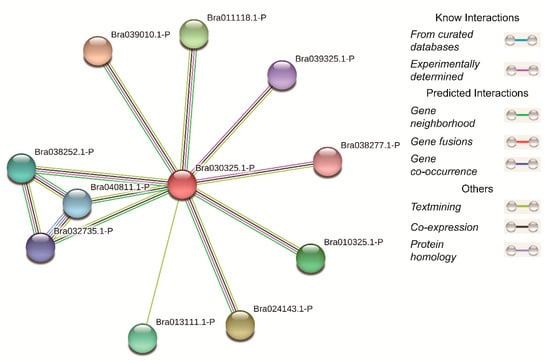
Figure 9.
Online prediction of protein interactions.
4. Discussion
CSP genes have been reported in many plants, including Arabidopsis, rice and wheat [8]. It is well documented that cold stress often affects the normal growth and development of plants. Studies have revealed that CSP is a protein associated with cold stress [29]. In this study, BcCSP3 gene was isolated from Pak-choi by homologous sequence alignment analysis. Further research shows that the BcCSP3 protein has an N-terminal CSD domain. The sequence alignment of BcCSP3 and the Arabidopsis CSP family proteins indicates that they have a highly conserved N-terminus (CSD domain). Based on the above results, we speculate that BcCSP3 has similar functions as AtCSP (Figure 4). The phylogenetic analysis shows that BcCSP3 and AtCSP2 are orthologous genes, so we predict that these two genes may have been conserved through evolution and possibly come from the same ancestor (Figure 5).
A large number of studies have shown that plant CSP genes can actively respond to abiotic stresses (especially cold stress) from the surrounding environment during growth. For example, AtCSP3 [13] and AtCSP1 [12] can exhibit a response to cold stress. Although overexpressed AtCSP1 did not show a significant increase in frost resistance, AtCSP1 was able to protect against cold damage by saving cold-sensitive grp7 mutants [16]. AtCSP3 knock out plants showed more sensitivity to cold stress [12], while plants that overexpressed AtCSP3 showed higher tolerance to cold stress [13]. Because BcCSP3 and AtCSPs have similarities in amino acid sequence structure, we speculate that BcCSP3 has similar functions and may also participate in the cold stress response.
Previous studies have found that drought, salt and ABA stress treatments show up-regulated expression level of AtCSP3 [30]. Here, RT-qPCR analysis showed that the expression pattern of BcCSP3 is different from AtCSPs in some respects. BcCSP3 showed a positive response to cold, ABA and salt stress treatment while previous studies have shown that AtCSP1 exhibits a down-regulated response under the drought and salt stresses [16]. Further research found that under the dehydration and ABA stress treatment, BcCSP3 will be induced to show similar expression patterns and response trends (Figure 7). These results indicate that BcCSP3 may not behave exactly the same under various stresses, compared with AtCSPs.
Previous research findings indicate that the subcellular localization of AtCSP1 [31] and AtCSP3 [13] are both located in the nucleus. AtCSP1 shows selective binding to the corresponding mRNA and can continue to maintain translation in response to various stresses [31]. A further study found that AtCSP3 also plays an important role in RNA processing and metabolism [30]. Here, we used the transient expression of BcCSP3–GFP fusion protein in tobacco leaf cells to show that BcCSP3 is located in the nucleus and cytoplasm (Figure 8). Based on the similarity of the amino acid sequences of AtCSP1, AtCSP3 and BcCSP3, their conservative structure under the cold stress and similar subcellular localization, we speculate that it is very likely that the BcCSP3 protein plays the role of RNA chaperone during cold adaptation.
5. Conclusions
In conclusion, a protein related to cold stress was identified and isolated from non-heading Chinese cabbage, BcCSP3, which is a new member of the Pak-choi CSP family and has the closest evolutionary relationship with AtCSP2 in Arabidopsis. BcCSP3 is a hydrophilicity cold-related protein with no signal peptide and no transmembrane domain. The expression level of BcCSP3 in stems and leaves are higher than in roots of Pak-choi. At the same time, BcCSP3 also shows response to other abiotic stresses and participates in active regulation. This study may help to further provide ideas for the mining of genes related to cold stress in non-heading Chinese cabbage.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/7/890/s1, Figure S1: Signal peptide prediction of BcCSP3 protein, Figure S2: Transmembrane domain prediction of BcCSP3 protein, Figure S3: Hydrophilicity prediction of BcCSP3 protein, Table S1: Primer sequences used in this paper, Table S2: CSP protein sequences used in this paper, Table S3: Information of predicted functional partner proteins.
Author Contributions
F.H. and J.W. completed the relevant experiments and wrote the study, they contributed equally to this work. W.D. did proofreading and analysis. X.H. modified and approved the manuscript. All authors have read and finalized the draft, agreeing that the study will be submitted to this prestigious journal. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Projects of National Key Research and Development Plan of China (2017YFD0101803), the China Agriculture Research System (CARS-23-A-06), the National Key Research and Development Plan of China (2016YFD0101701), the Natural Science Youth Foundation of Jiangsu province (BK20190513) and the Natural Science Foundation of Jiangsu province (BK20170462). The funding is mainly used for the purchase of reagents and consumables used in this experiment and biologic sequencing, etc.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
aa—amino acids; bp—base pairs; CSD—cold shock domain; CSP3—cold shock protein 3; h—hour; min—minute; ORF—open-reading frame; PEG—polyethylene glycol; pI—isoelectric point; PPI—protein–protein interact; RT-qPCR—real-time quantitative polymerase chain reaction; s—second.
References
- Jansen, P.; Westphal, E.; Siemonsma, J.; Piluek, K. Plant Resources of South-East Asia 8; Vegetables. Pudoc: Wageningen, The Netherlands, 1993. [Google Scholar]
- Jiang, F.; Wang, F.; Wu, Z.; Li, Y.; Shi, G.; Hu, J.; Hou, X. Components of the Arabidopsis CBF cold-response pathway are conserved in non-heading Chinese cabbage. Plant Mol. Biol. Report 2011, 29, 525–532. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, Y.; Zhang, X.; Xin, X.; Qi, L.; Guo, H.; Li, J.; Yang, S. PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E6695–E6702. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Hodges, H.F.; McKinion, J.M. A comparison of scenarios for the effect of global climate change on cotton growth and yield. Funct. Plant Biol. 1997, 24, 707–713. [Google Scholar] [CrossRef]
- Graham, D.; Patterson, B.D. Responses of plants to low, nonfreezing temperatures: Proteins, metabolism, and acclimation. Annu. Rev. Plant Physiol. 1982, 33, 347–372. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Karlson, D.; Imai, R. Conservation of the cold shock domain protein family in plants. Plant Physiol. 2003, 131, 12–15. [Google Scholar] [CrossRef]
- Sasaki, K.; Imai, R. Pleiotropic roles of cold shock domain proteins in plants. Front. Plant Sci. 2012, 2, 116. [Google Scholar] [CrossRef]
- Karlson, D.; Nakaminami, K.; Toyomasu, T.; Imai, R. A cold-regulated nucleic acid-binding protein of winter wheat shares a domain with bacterial cold shock proteins. J. Biol. Chem. 2002, 277, 35248–35256. [Google Scholar] [CrossRef]
- Nakaminami, K.; Sasaki, K.; Kajita, S.; Takeda, H.; Karlson, D.; Ohgi, K.; Imai, R. Heat stable ssDNA/RNA-binding activity of a wheat cold shock domain protein. FEBS Lett. 2005, 579, 4887–4891. [Google Scholar] [CrossRef]
- Nakaminami, K.; Karlson, D.T.; Imai, R. Functional conservation of cold shock domains in bacteria and higher plants. Proc. Natl. Acad. Sci. USA 2006, 103, 10122–10127. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, S.J.; Kwak, K.J.; Kim, Y.O.; Kim, J.Y.; Song, J.; Jang, B.; Jung, C.H.; Kang, H. Cold shock domain proteins and glycine-rich RNA-binding proteins from Arabidopsis thaliana can promote the cold adaptation process in Escherichia coli. Nucleic Acids Res. 2006, 35, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Sasaki, K.; Imai, R. Cold shock domain protein 3 regulates freezing tolerance in Arabidopsis thaliana. J. Biol. Chem. 2009, 284, 23454–23460. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kim, M.H.; Imai, R. Arabidopsis cold shock domain protein 2 is a negative regulator of cold acclimation. New Phytol. 2013, 198, 95–102. [Google Scholar] [CrossRef]
- Fusaro, A.F.; Bocca, S.N.; Ramos, R.L.B.; Barrôco, R.M.; Magioli, C.; Jorge, V.C.; Coutinho, T.C.; Rangel-Lima, C.M.; De Rycke, R.; Inzé, D.; et al. AtGRP2, a cold-induced nucleo-cytoplasmic RNA-binding protein, has a role in flower and seed development. Planta 2007, 225, 1339–1351. [Google Scholar] [CrossRef]
- Park, S.J.; Kwak, K.J.; Oh, T.R.; Kim, Y.O.; Kang, H. Cold shock domain proteins affect seed germination and growth of Arabidopsis thaliana under abiotic stress conditions. Plant Cell Physiol. 2009, 50, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, F.; Wang, Z.; Huang, Z.; Xiong, A.; Hou, X. Characterization and co-expression analysis of WRKY orthologs involved in responses to multiple abiotic stresses in Pak-choi (Brassica campestris ssp. chinensis). BMC Plant Biol. 2013, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, J.; Tang, J.; Hou, X. Identification, evolution and functional inference on the cold-shock domain protein family in Pak-choi (Brassica rapa ssp. chinensis) and Chinese cabbage (Brassica rapa ssp. pekinensis). J. Plant Interact. 2019, 14, 232–241. [Google Scholar] [CrossRef]
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Hofmann, K. TMbase-A database of membrane spanning proteins segments. Biol. Chem. Hoppe Seyler 1993, 374, 166. [Google Scholar]
- Jones, D. ProtScale Tool: Amino acid scale: Refractivity. J. Theor. Biol 1975, 50, 167–184. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 1, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; Van Poucke, M.; Van Soom, A.; Vandesompele, J.; Van Zeveren, A.; Peelman, L.J. Selection of reference genes for quantitative real-time PCR in bovine preimplantation embryos. BMC Dev. Biol. 2005, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Luo, X.; Zhu, W.; Yu, K.; Chen, K.; Li, Y.; Jiang, H. Predicting protein-protein interactions based only on sequences information. Proc. Natl. Acad. Sci. USA 2007, 104, 4337–4341. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Z. Functional features, biological pathways, and protein interaction networks of addiction-related genes. Chem. Biodivers. 2010, 7, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Graumann, P.L.; Marahiel, M.A. A superfamily of proteins that contain the cold-shock domain. Trends Biochem. Sci. 1998, 23, 286–290. [Google Scholar] [CrossRef]
- Kim, M.-H.; Sato, S.; Sasaki, K.; Saburi, W.; Matsui, H.; Imai, R. Cold shock domain protein 3 is involved in salt and drought stress tolerance in Arabidopsis. FEBS Open Biol. 2013, 3, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Juntawong, P.; Sorenson, R.; Bailey-Serres, J. Cold shock protein 1 chaperones mRNA s during translation in Arabidopsis thaliana. Plant J. 2013, 74, 1016–1028. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).