Effect of Seedling Nitrogen Condition on Subsequent Vegetative Growth Stages and Its Relationship to the Expression of Nitrogen Transporter Genes in Rice
Abstract
1. Introduction
2. Results
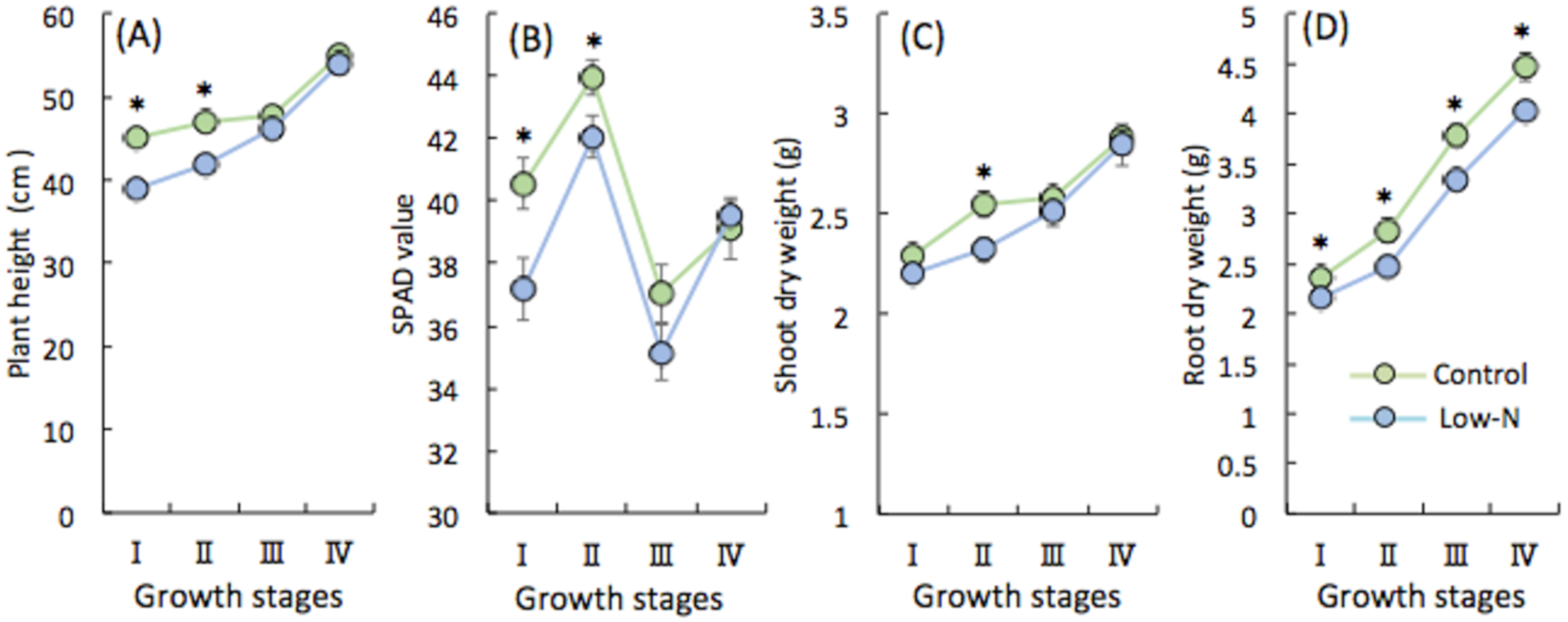
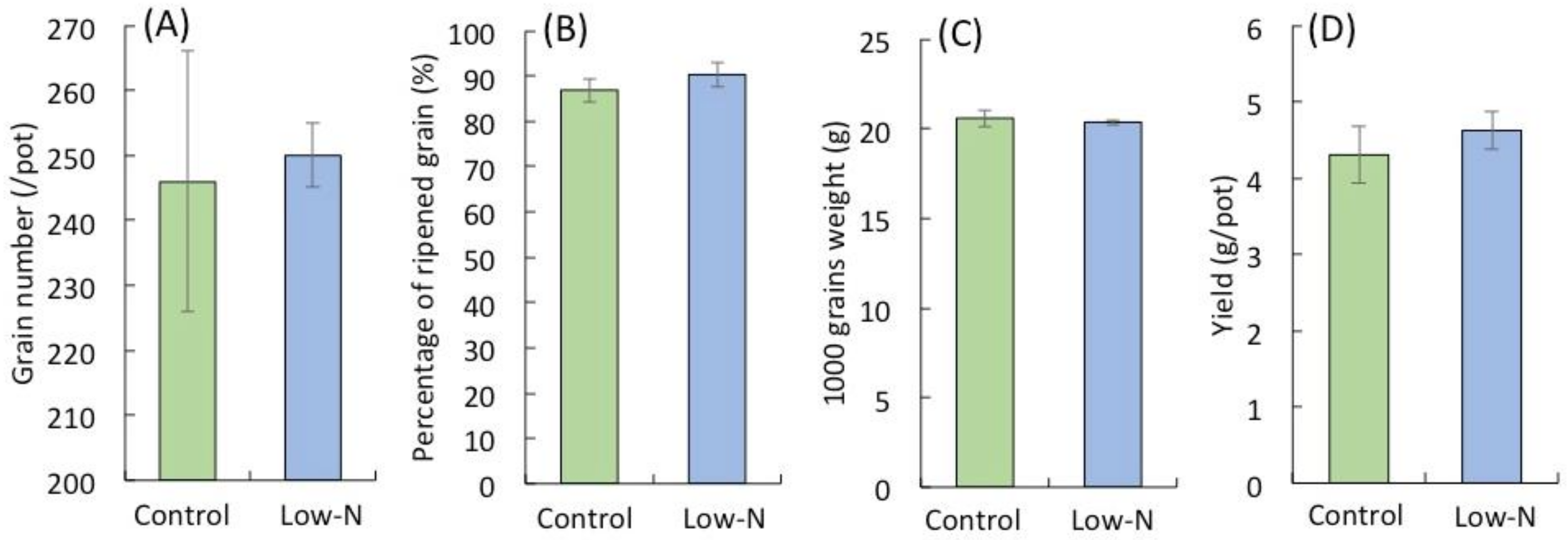
2.1. Analysis of Rice Growth at Different Growth Stages
2.2. Accumulation of N in Shoots and Roots at Various Growth Stages
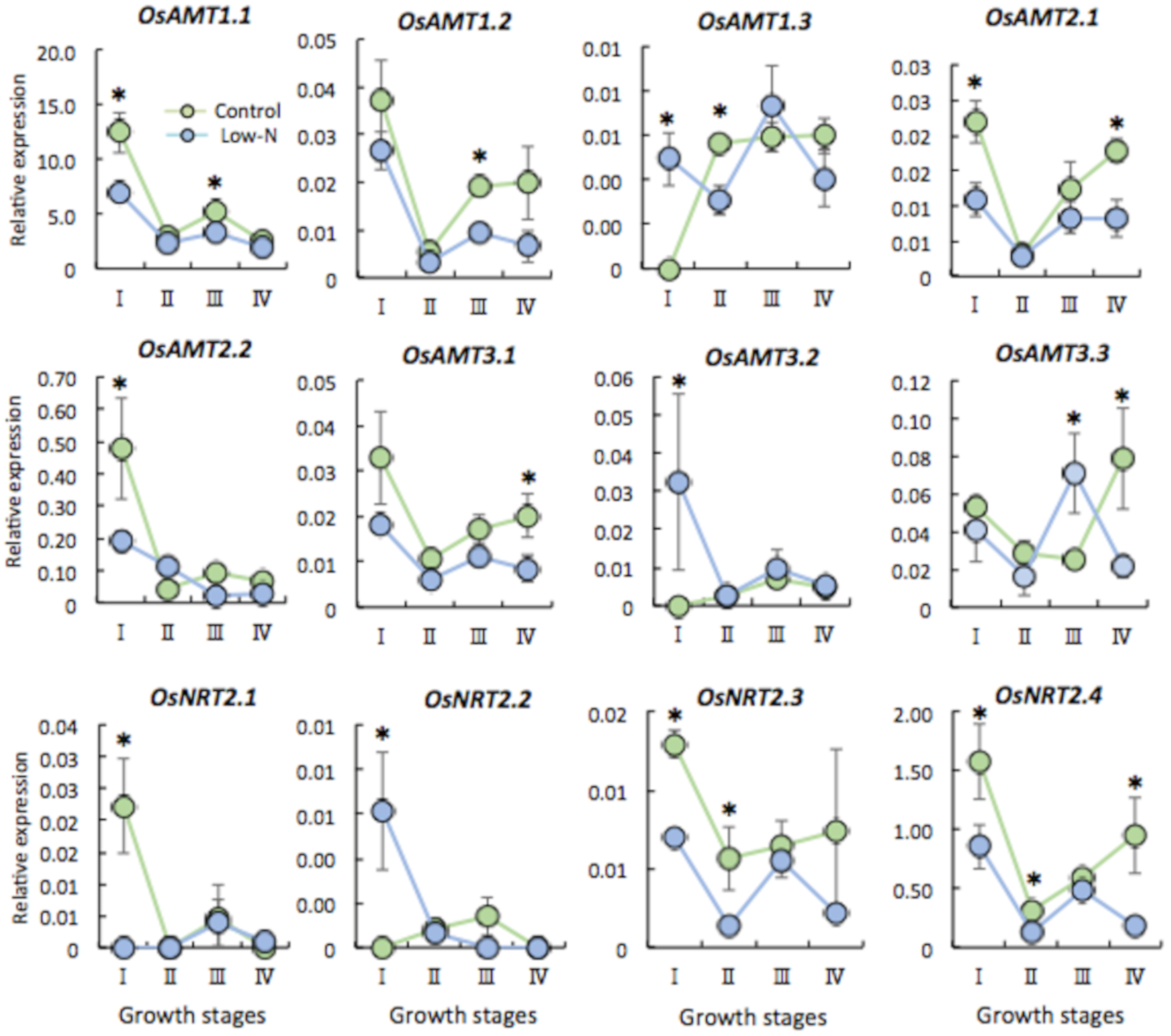
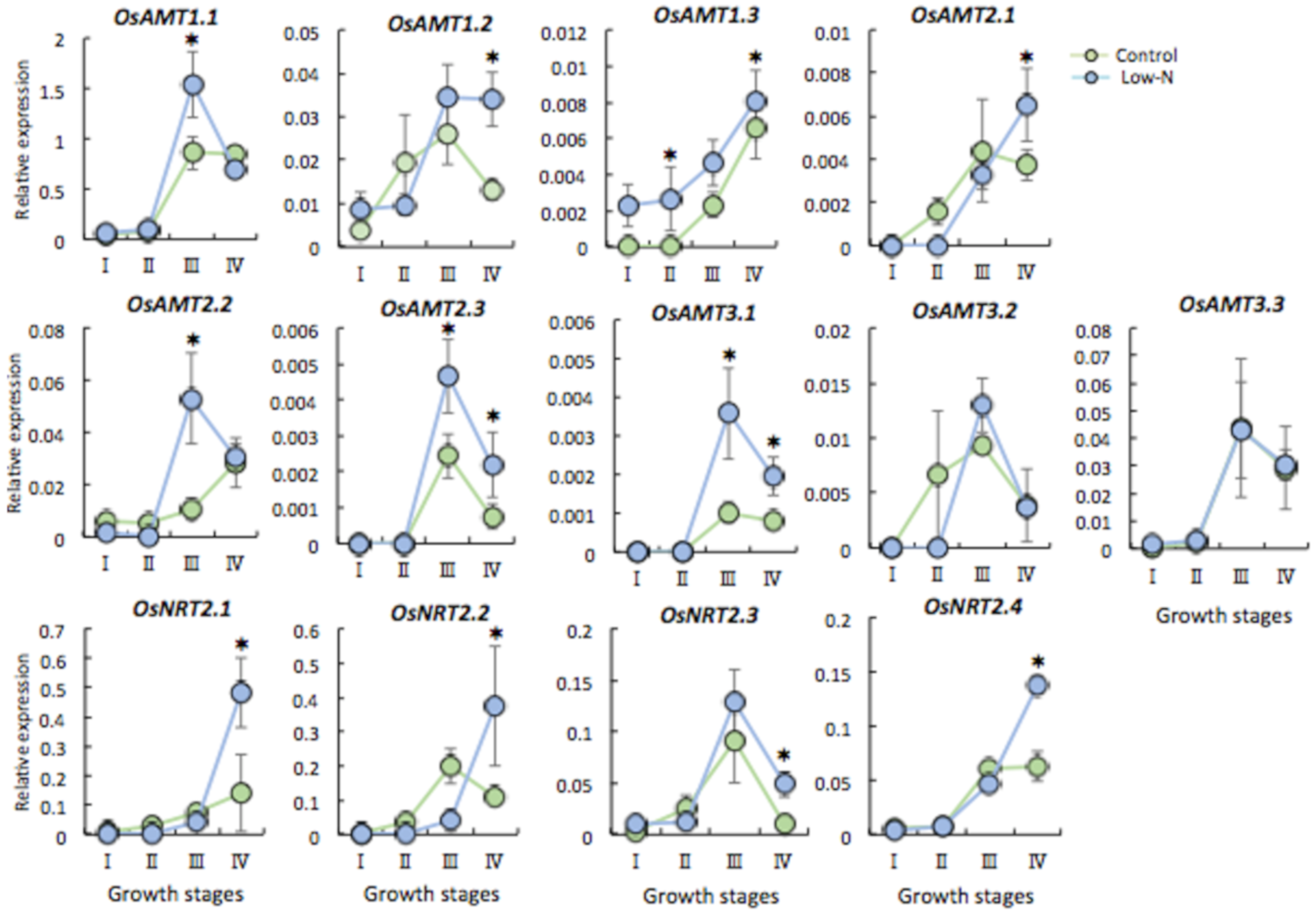
2.3. Gene Expression of N Transporter Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. N Treatment
4.3. SPAD Value, Length and Dry Weight of Shoots and Roots
4.4. N Content
4.5. RNA Extraction and Quantitative Real-Time PCR
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rütting, T.; Aronsson, H.; Delin, S. Efficient use of nitrogen in agriculture. Nutr. Cycl. Agroecosyst. 2018, 110, 1–5. [Google Scholar] [CrossRef]
- Russo, S. Rice yield as affected by the s plit method of “ N ” application and nitrification inhibitor DCD. Cah. Options Mediterr. 1996, 52, 43–52. [Google Scholar]
- Zhou, W.; Lv, T.; Yang, Z.; Wang, T.; Fu, Y.; Chen, Y.; Hu, B.; Ren, W. Morphophysiological mechanism of rice yield increase in response to optimized nitrogen management. Sci. Rep. 2017, 7, 17226. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Maruyama, S. Proteins and carbohydrates in developing rice panicles with different numbers of spikelets cultivar difference and the effect of nitrogen topdressing. Plant Prod. Sci. 2004, 7, 16–21. [Google Scholar] [CrossRef]
- Tac, T.H. Effect of top-dressing and planting density on the number of spikelets and yield of rice cultivated with nitrogen-free basal dressing. Plant Prod. Sci. 1998, 1, 191–198. [Google Scholar] [CrossRef]
- Panda, M.M.; Reddy, M.D.; Sharma, A.R. Yield performance of rainfed lowland rice as affected by nursery fertilization under conditions of intermediate deepwater (15–50 cm) and flash floods. Plant Soil 1991, 132, 65–71. [Google Scholar] [CrossRef]
- Tsukaguchi, T.; Taniguchi, Y.; Ito, R. The effects of nitrogen uptake before and after heading on grain protein content and the occurrence of basal- and back-white grains in rice (Oryza sativa L.). Plant Prod. Sci. 2016, 19, 508–517. [Google Scholar] [CrossRef]
- Xiong, Q.; Tang, G.; Zhong, L.; He, H.; Chen, X. Response to nitrogen deficiency and compensation on physiological characteristics, yield formation, and nitrogen utilization of rice. Front. Plant Sci. 2018, 9, 1075. [Google Scholar] [CrossRef]
- Huang, M.; Yang, C.; Ji, Q.; Jiang, L.; Tan, J.; Li, Y. Tillering responses of rice to plant density and nitrogen rate in a subtropical environment of southern China. Field Crop. Res. 2013, 149, 187–192. [Google Scholar] [CrossRef]
- Hasegawa, T.; Koroda, Y.; Seligman, N.G.; Horie, T. Response of spikelet number to plant nitrogen concentration and dry weight in paddy rice. Agron. J. 1994, 86, 673–676. [Google Scholar] [CrossRef]
- Kou, H.P.; Li, Y.; Song, X.X.; Ou, X.F.; Xing, S.C.; Ma, J.; Von Wettstein, D.; Liu, B. Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance by progenies to the stress in rice (Oryza sativa L.). J. Plant Physiol. 2011, 168, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Richard-Molard, C.; Krapp, A.; Brun, F.; Ney, B.; Daniel-Vedele, F.; Chaillou, S. Plant response to nitrate starvation is determined by N storage capacity matched by nitrate uptake capacity in two Arabidopsis genotypes. J. Exp. Bot. 2008, 59, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wu, P.; Ling, H.; Xu, G.; Xu, F.; Zhang, Q. Plant nutriomics in China: An overview. Ann. Bot. 2006, 98, 473–482. [Google Scholar] [CrossRef]
- Yoneyama, T. Absorption and assimilation of nitrogen by rice plants. JARQ 1986, 12, 2–7. [Google Scholar]
- Sasakawa, H.; Yamamoto, Y. Comparison of the uptake of nitrate and ammonium by rice seedlings: Influences of light, temperature, oxygen concentration, exogenous sucrose, and metabolic inhibitors. Plant Physiol. 1978, 62, 665–669. [Google Scholar] [CrossRef]
- Nour-Eldin, H.H.; Andersen, T.G.; Burow, M.; Madsen, S.R.; Jørgensen, M.E.; Olsen, C.E.; Dreyer, I.; Hedrich, R.; Geiger, D.; Halkier, B.A. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 2012, 488, 531–534. [Google Scholar] [CrossRef]
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A unified nomenclature of nitrate transporter 1/peptide transporter family members in plants. Trends Plant Sci. 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Araki, R.; Hasegawa, H. Expression of rice (Oryza sativa L.) genes involved in high-affinity nitrate transport during the period of nitrate induction. Breed. Sci. 2006, 56, 295–302. [Google Scholar] [CrossRef]
- Cai, C.; Wang, J.-Y.; Zhu, Y.-G.; Shen, Q.-R.; Li, B.; Tong, Y.-P.; Li, Z.-S. Gene structure and expression of the high-affinity nitrate transport system in rice roots. J. Integr. Plant Biol. 2008, 50, 443–451. [Google Scholar] [CrossRef]
- Feng, H.; Yan, M.; Fan, X.; Li, B.; Shen, Q.; Miller, A.J.; Xu, G. Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J. Exp. Bot. 2011, 62, 2319–2332. [Google Scholar] [CrossRef]
- Wei, J.; Zheng, Y.; Feng, H.; Qu, H.; Fan, X.; Yamaji, N.; Ma, J.F.; Xu, G. OsNRT2.4 encodes a dual-affinity nitrate transporter and functions in nitrate-regulated root growth and nitrate distribution in rice. J. Exp. Bot. 2018, 69, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y.; Ikeda, A.; Saiki, S.; Von Wirén, N.; Yamaya, T.; Yamaguchi, J. Distinct expression and function of three ammonium transporter genes (OsAMT1;1–1;3) in rice. Plant Cell Physiol. 2003, 44, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, A.; Moriya, K.; Sonoda, Y.; Ikeda, A.; Von Wirén, N.; Hayakawa, T.; Yamaguchi, J.; Yamaya, T. Constitutive expression of a novel-type ammonium transporter OsAMT2 in rice plants. Plant Cell Physiol. 2003, 44, 206–211. [Google Scholar] [CrossRef]
- Li, C.; Tang, Z.; Wei, J.; Qu, H.; Xie, Y.; Xu, G. The OsAMT1.1 gene functions in ammonium uptake and ammonium–potassium homeostasis over low and high ammonium concentration ranges. J. Genet. Genom. 2016, 43, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Lezhneva, L.; Boutet-Mercey, S.; Orsel, M.; Bréhaut, V.; Miller, A.; Daniel-Vedele, F.; Sakakibara, H.; et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 2012, 24, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought “train” transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities inwheat seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef]
- Wibowo, A.; Becker, C.; Marconi, G.; Durr, J.; Price, J.; Hagmann, J.; Papareddy, R.; Putra, H.; Kageyama, J.; Becker, J.; et al. Hyperosmotic stress memory in arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by dna glycosylase activity. Elife 2016, 5, e13546. [Google Scholar] [CrossRef]
- Takehisa, H.; Sato, Y.; Antonio, B.A.; Nagamur, Y. Global transcriptome profile of rice root in response to essential macronutrient deficiency. Plant Signal Behav. 2013, 8, e24409. [Google Scholar] [CrossRef]
- Dechorgnat, J.; Francis, K.L.; Dhugga, K.S.; Rafalski, J.A.; Tyerman, S.D.; Kaiser, B.N. Tissue and nitrogen-linked expression profiles of ammonium and nitrate transporters in maize. BMC Plant Biol. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Wen, Z.; Tyerman, S.D.; Dechorgnat, J.; Ovchinnikova, E.; Dhugga, K.S.; Kaiser, B.N. Maize NPF6 proteins are homologs of arabidopsis CHL1 that are selective for both nitrate and chloride. Plant Cell 2017, 29, 2581–2596. [Google Scholar] [CrossRef]
- Nadeem, F.; Ahmad, Z.; Wang, R.; Han, J.; Shen, Q.; Chang, F.; Diao, X.; Zhang, F.; Li, X. Foxtail millet [Setaria italica (L.) beauv.] grown under low nitrogen shows a smaller root system, enhanced biomass accumulation, and nitrate transporter expression. Front. Plant Sci. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Li, Z.; Zhao, Y.; Peng, C.; Zhao, C. An integrated analysis of the rice transcriptome and metabolome reveals differential regulation of carbon and nitrogen metabolism in response to nitrogen availability. Int. J. Mol. Sci. 2019, 20, 2349. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Kan, C.C.; Hung, T.H.; Hsieh, P.H.; Wang, S.Y.; Hsieh, W.Y.; Hsieh, M.H. Identification of early ammonium nitrate-responsive genes in rice roots. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Cai, H.; Lu, Y.; Xie, W.; Zhu, T.; Lian, X. Transcriptome response to nitrogen starvation in rice. J. Biosci. 2012, 37, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Ruffe, S.; Freixes, S.; Balzergue, S.; Tillard, P.; Jeudy, C.; Martin-Magniette, M.L.; Van Der Merwe, M.J.; Kakar, K.; Gouzy, J.; Fernie, A.R.; et al. Systemic signaling of the plant nitrogen status triggers specific transcriptome responses depending on the nitrogen source in Medicago truncatula. Plant Physiol. 2008, 146, 2020–2035. [Google Scholar] [CrossRef]
- Zhang, H.; Forde, B.G. Regulation of arabidopsis root development by nitrate availability. J. Exp. Bot. 2000, 51, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Bhalerao, R.; Casimiro, I.; Eklöf, J.; Casero, P.J.; Bennett, M.; Sandberg, G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 2002, 14, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Sun, H.; Li, J.; Gong, X.; Huang, S.; Zhu, X.; Zhang, Y.; Xu, G. Auxin distribution is differentially affected by nitrate in roots of two rice cultivars differing in responsiveness to nitrogen. Ann. Bot. 2013, 112, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, W.; Ou, S.; Tang, J.; Li, H.; Che, R.; Zhang, Z.; Chai, X.; Wang, H.; Wang, Y.; et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 2015, 47, 834–838. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Yuan, D.; Liu, Y.; Che, R.; Hu, Y.; Ou, S.; Liu, Y.; Zhang, Z.; Wang, H.; et al. Expression of the nitrate transporter gene OsNRT1.1A/ OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 2018, 30, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, T.; Zhang, Y.; Duan, F.; Neuhaüser, B.; Ludewig, U.; Schulze, W.X.; Yuan, L. Ammonium and nitrate regulate NH4+ uptake activity of Arabidopsis ammonium transporter AtAMT1;3 via phosphorylation at multiple C-terminal sites. J. Exp. Bot. 2019, 70, 4919–4929. [Google Scholar] [CrossRef]
- Widiez, T.; El Kafafi, E.S.; Girin, T.; Berr, A.; Ruffel, S.; Krouk, G.; Vayssières, A.; Shen, W.H.; Coruzzi, G.M.; Gojon, A.; et al. High nitrogen insensitive 9 (HNI9)-mediated systemic repression of root NO 3- uptake is associated with changes in histone methylation. Proc. Natl. Acad. Sci. USA 2011, 108, 13329–13334. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hao, D.; Cong, Y.; Jin, M.; Su, Y. The rice OsAMT1;1 is a proton-independent feedback regulated ammonium transporter. Plant Cell Rep. 2015, 34, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Yamauchi, M.; Biswas, J.K. Rice cultivar difference in seedling establishment in flooded soil. Plant Soil 1997, 189, 145–153. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Chen, S.; Ge, Q.; Chu, G.; Xu, C.; Yan, J.; Zhang, X.; Wang, D. Seasonal differences in the rice grain yield and nitrogen use efficiency response to seedling establishment methods in the middle and lower reaches of the Yangtze river in China. Field Crops Res. 2017, 205, 157–169. [Google Scholar] [CrossRef]
- Danying, W.; Chang, Y.; Chunmei, X.; Zaiman, W.; Song, C.; Guang, C.; Xiufu, Z. Soil nitrogen distribution and plant nitrogen utilization in direct-seeded rice in response to deep placement of basal fertilizer-nitrogen. Rice Sci. 2019, 26, 404–415. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, X.; Li, J.; Xin, C. Effects of seedling age and cultivation density on agronomic characteristics and grain yield of mechanically transplanted rice. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Fang, B.; Fang, Y.; Chen, K.; Zhang, Y.; Zhang, H. Potential for high yield with increased seedling density and decreased N fertilizer application under seedling-throwing rice cultivation. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-G.; Zhu, X.-G.; Raines, C. Source-sink interaction: A century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, D.; Watanabe, C.K.A.; Betsuyaku, E.; Terashima, I. Sink–source balance and down-regulation of photosynthesis in Raphanus sativus: Effects of grafting, N and CO2. Plant Cell Physiol. 2017, 58, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.P. Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Staswick, P.E. Novel regulation of vegetative storage protein genes. Plant Cell 1990, 2, 1–6. [Google Scholar] [CrossRef]
- Macduff, J.H.; Jarvis, S.C.; Mosquera, A. Nitrate nutrition of grasses from steady-state supplies in flowing solution culture following nitrate deprivation and /or defoliation. II: Assimilation of NO3− and short-term effects on NO3− uptake. J. Exp. Bot. 1989, 40, 977–984. [Google Scholar] [CrossRef]
- Wiley, E.; Huepenbecker, S.; Casper, B.B.; Helliker, B.R. The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013, 33, 1216–1228. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thi Nong, H.; Tateishi, R.; Suriyasak, C.; Kobayashi, T.; Oyama, Y.; Chen, W.J.; Matsumoto, R.; Hamaoka, N.; Iwaya-Inoue, M.; Ishibashi, Y. Effect of Seedling Nitrogen Condition on Subsequent Vegetative Growth Stages and Its Relationship to the Expression of Nitrogen Transporter Genes in Rice. Plants 2020, 9, 861. https://doi.org/10.3390/plants9070861
Thi Nong H, Tateishi R, Suriyasak C, Kobayashi T, Oyama Y, Chen WJ, Matsumoto R, Hamaoka N, Iwaya-Inoue M, Ishibashi Y. Effect of Seedling Nitrogen Condition on Subsequent Vegetative Growth Stages and Its Relationship to the Expression of Nitrogen Transporter Genes in Rice. Plants. 2020; 9(7):861. https://doi.org/10.3390/plants9070861
Chicago/Turabian StyleThi Nong, Hue, Ryota Tateishi, Chetphilin Suriyasak, Takuya Kobayashi, Yui Oyama, Wun Jin Chen, Ryo Matsumoto, Norimitsu Hamaoka, Mari Iwaya-Inoue, and Yushi Ishibashi. 2020. "Effect of Seedling Nitrogen Condition on Subsequent Vegetative Growth Stages and Its Relationship to the Expression of Nitrogen Transporter Genes in Rice" Plants 9, no. 7: 861. https://doi.org/10.3390/plants9070861
APA StyleThi Nong, H., Tateishi, R., Suriyasak, C., Kobayashi, T., Oyama, Y., Chen, W. J., Matsumoto, R., Hamaoka, N., Iwaya-Inoue, M., & Ishibashi, Y. (2020). Effect of Seedling Nitrogen Condition on Subsequent Vegetative Growth Stages and Its Relationship to the Expression of Nitrogen Transporter Genes in Rice. Plants, 9(7), 861. https://doi.org/10.3390/plants9070861



