Impacts of Verticillium Wilt on Photosynthesis Rate, Lint Production, and Fiber Quality of Greenhouse-Grown Cotton (Gossypium hirsutum)
Abstract
1. Introduction
2. Results
2.1. Disease Ratings
2.2. Photosynthesis
2.3. Yield and Fiber Quality
3. Discussion
4. Materials and Methods
4.1. Germplasm
4.2. Fungal Preparation and Soil Inoculation
4.3. Experiment Layout
4.4. Disease Ratings
4.5. Physiological Measurements
4.6. Yield and Fiber Quality
4.7. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bell, A.A. Verticillium wilt. In Cotton Diseases; Hillocks, R.J., Ed.; CAB International: Wallingford, UK, 1992; pp. 87–126. [Google Scholar]
- Beckman, C.H. The Nature of Wilt Disease of Plants; The American Phytopathological Society: St. Paul, MN, USA, 1987. [Google Scholar]
- Bowden, R.L.; Rouse, D.I. Effects of Verticillium dahliae on gas exchange of potato. Phytopathology 1991, 81, 293–301. [Google Scholar] [CrossRef]
- Bowden, R.L.; Rouse, D.I. Chronology of gas exchange effects and growth effects of infection by Verticillium dahliae in potato. Phytopathology 1991, 81, 301–310. [Google Scholar] [CrossRef]
- Bowden, R.L.; Rouse, D.I.; Sharkey, T.D. Mechanism of photosynthesis decrease by Verticillium dahliae in potato. Plant Physiol. 1990, 94, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, N.; Aguirreolea, J.; Cenoz, S.; Garcı’a-Mina, M.J.M. Gas exchange and flowering in Verticillium-wilted pepper plants. J. Phytopathl. 2001, 149, 281–286. [Google Scholar] [CrossRef]
- Pascual, I.; Azcona, I.; Morales, F.; Aguirreolea, J.; Sánchez-Díaz, M. Photosynthetic response of pepper plants to wilt induced by Verticillium dahliae and soil water deficit. J. Plant Physiol. 2010, 1678, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Pennypacker, B.W.; Knievel, D.P.; Leath, K.T.; Pell, E.J.; Hill, R.R., Jr. Analysis of photosynthesis in resistant and susceptible alfalfa clones infected with Verticillium albo-atrum. Phytopathology 1990, 80, 1300–1306. [Google Scholar] [CrossRef]
- Hampton, R.E.; Wullschleger, S.D.; Oosterhuis, D.M. Impact of Verticillium wilt on net photosynthesis, respiration, and photorespiration in field-grown cotton (Gossypium hirsutum L.). Physiol. Mol. Plant Pathol. 1990, 37, 271–280. [Google Scholar] [CrossRef]
- Ashworth, L.J.; McCutcheon, O.D.; George, A.G. Verticillium albo-atrum: The quantitative relationship between inoculum density and infection of cotton. Phytopathology 1972, 62, 901–903. [Google Scholar] [CrossRef]
- Erdogan, O.; Sezener, V.; Ozbek, V.N.; Bozbek, T.; Yavas, I.; Unay, A. The effects of Verticillium wilt (Verticillium dahliae Kleb.) on cotton yield and fiber quality. Asian J. Plant Sci. 2006, 5, 867–870. [Google Scholar] [CrossRef]
- Kabir, A.; Fennimore, S.A.; Duniway, J.M.; Martin, F.N.; Browne, G.T.; Winterbottom, C.Q.; Ajwa, H.A.; Westerdahl, B.B.; Goodhue, R.E.; Haar, M.J. Alternatives to methyl bromide for strawberry runner plant production. HortScience 2005, 40, 1709–1715. [Google Scholar] [CrossRef]
- Huisman, O.C.; Ashworth, L.J. Influence of crop rotation on the survival of Verticillium albo-atrum in soils. Phytopathology 1976, 66, 978–981. [Google Scholar] [CrossRef]
- Wheeler, T.A.; Bordovsky, J.P.; Keeling, J.W. The effectiveness of crop rotation on management of Verticillium wilt over time. Crop Prot. 2019, 121, 157–162. [Google Scholar] [CrossRef]
- Wilhelm, S. Longevity of the Verticillium wilt fungus in the laboratory and field. Phytopathology 1955, 45, 180–181. [Google Scholar]
- Shetty, K.G.; Subbarao, K.V.; Huisman, O.C.; Hubbard, J.C. Mechanism of broccoli-mediated Verticillium wilt reduction in cauliflower. Phytopathology 2000, 90, 305–310. [Google Scholar] [CrossRef]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef]
- Land, C.J.; Lawrence, K.S.; Burmester, C.H.; Meyer, B. Cultivar, irrigation, and soil contribution to the enhancement of Verticillium wilt disease in cotton. Crop Prot. 2017, 96, 1–6. [Google Scholar] [CrossRef]
- Chawla, S.; Woodward, J.E.; Wheeler, T.A.; Dever, J.K. Effect of cultivar selection on soil population of Verticillium dahliae and wilt development in cotton. Plant Health Prog. 2012. [Google Scholar] [CrossRef]
- Dever, J.; Wheeler, T.; Kelly, C. Registration of CA 4002, Cotton germplasm line partially resistant to Verticillium wilt. J. Plant Regist. 2013, 7, 1–7. [Google Scholar] [CrossRef]
- Khaskheli, M.I.; Sun, J.L.; He, S.P.; Du, X. Screening of cotton germplasm for resistance to Verticillium dahliae Kleb. under greenhouse and field conditions. Eur. J. Plant Pathol. 2013, 137, 259–272. [Google Scholar] [CrossRef]
- Wheeler, T.A.; Woodward, J.E. Field assessment of commercial cotton cultivars for Verticillium wilt resistance and yield. Crop Prot. 2016, 88, 1–6. [Google Scholar] [CrossRef]
- Leyendecker, P.J. Effects of certain practices on Verticillium wilt of cotton in New Mexico. New Mex. Agric. Exp. Stn. Bull. 1950, 356, 1–29. [Google Scholar]
- Song, R.; Li, J.; Xie, C.; Jian, W.; Yang, X. An overview of the molecular genetics of plant resistance to the Verticillium wilt pathogen Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 1120. [Google Scholar] [CrossRef]
- Misaghi, J.; DeVay, J.E.; Duniway, J.M. Relationship between occlusion of xylem elements and disease symptoms in leaves of cotton plants infected with Verticillium dahliae. Can. J. Bot. 1978, 56, 339–342. [Google Scholar] [CrossRef]
- Cabrales, L.; Abidi, N. Kinetics of cellulose deposition in developing cotton fibers studied by thermogravimetric analysis. Fibers 2019, 7, 78. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, W.; Liu, B.; Zhang, Y.; Song, X.; Cheng, Y.; Zhang, L.; Zhang, T. Molecular mechanisms of fiber differential development between Gossypium barbadense and G. hirsutum revealed by genetical genomics. PLoS ONE 2012, 7, e30056. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Cabrales, L.; Gannaway, J.; Wilkins, T.; Wells, L.W. Evaluating cell wall structure and composition of developing cotton fibers using Fourier transform infrared spectroscopy and thermogravimetric analysis. J. Appl. Polym. Sci. 2008, 107, 476–486. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E. Thermogravimetric analysis of cotton fibers and relationships with their physical properties. J. Appl. Polym. Sci. 2006, 103, 3476–3482. [Google Scholar] [CrossRef]
- Ayele, A.; Hequet, E.; Kelly, B. The impact of fiber maturity on estimating the number of cotton (Gossypium hirsutum L.) fibers per seed surface area. Ind. Crops Prod. 2017, 102, 16–22. [Google Scholar] [CrossRef]
- Ayele, A.; Hequet, E.; Kelly, B. Evaluating within-plant variability of cotton fiber length and maturity. Agron. J. 2018, 110, 47–55. [Google Scholar] [CrossRef]
- Hequet, E.F.; Wyatt, B.; Abidi, N.; Thibodeaux, D.P. Creation of a set of reference material for cotton fiber maturity measurements. Text. Res. J. 2006, 76, 576–586. [Google Scholar] [CrossRef]
- Francl, L.J.; Rowe, R.C.; Riedel, R.M.; Madden, L.V. Effects of three soil-types on potato early dying disease and associated yield reduction. Phytopathology 1988, 78, 159–166. [Google Scholar] [CrossRef]
- Kabir, Z.; Bhat, R.G.; Subbarao, K.V. Comparison of media for recovery of Verticillium dahliae from soil. Plant Dis. 2004, 88, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Azaddisfani, F.; Zangi, M.R. Verticillium wilt tolerance in some cotton genotypes. Plant Pathol. J. 2007, 6, 206–209. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, H.; Sanogo, S.; Hughs, S.E.; Jones, D.C.; Zhang, J. Evaluation of Verticillium wilt resistance in commercial cultivars and advanced breeding lines of cotton. Euphytica 2014, 196, 437–448. [Google Scholar] [CrossRef]
- Colella, C.; Miacola, C.; Amenduni, M.; D’Amico, M.; Bubici, G.; Cirulli, M. Sources of Verticillium wilt resistance in wild olive germplasm from the Mediterranean region. Phytopathology 2008, 57, 533–539. [Google Scholar] [CrossRef]
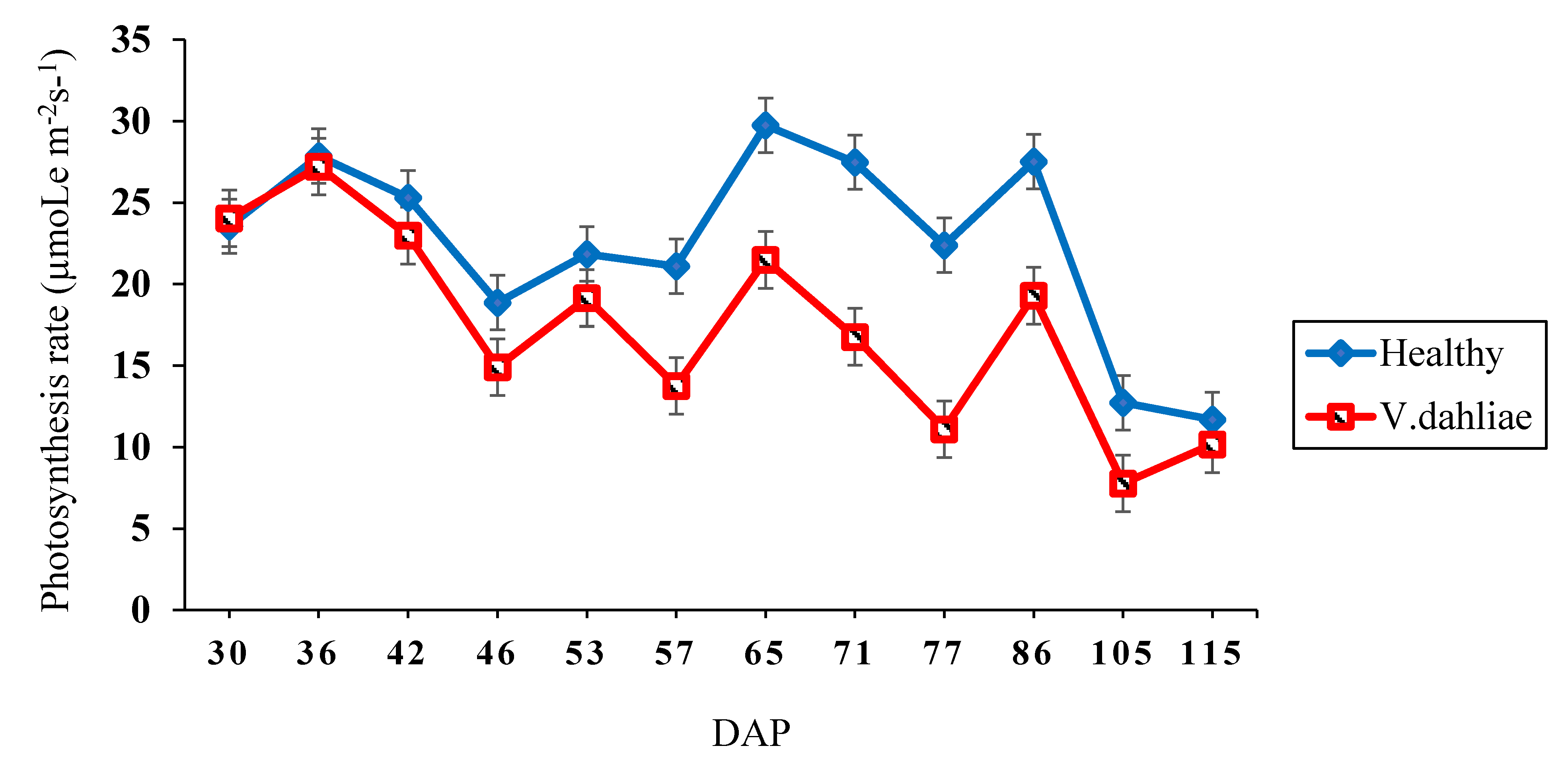
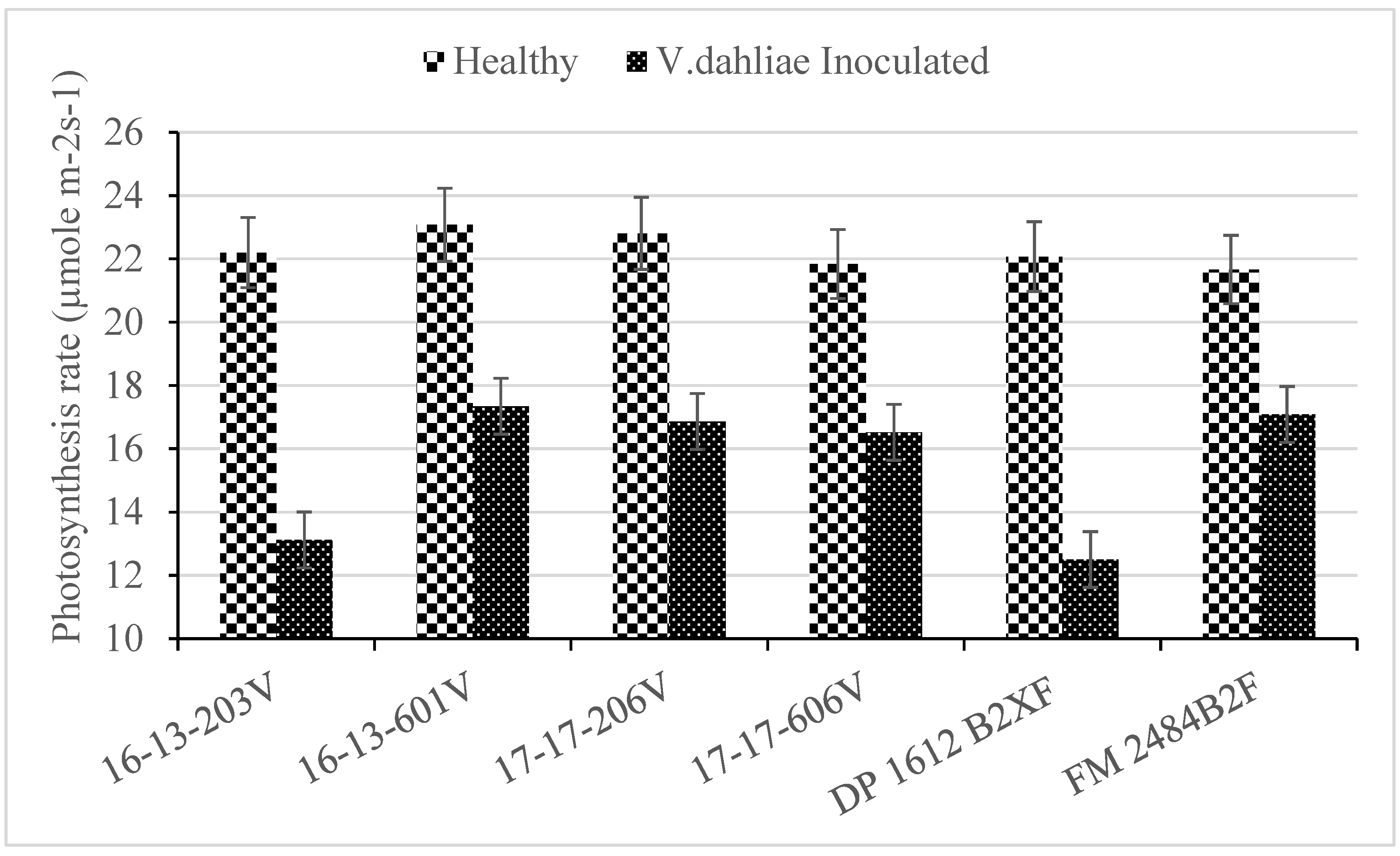
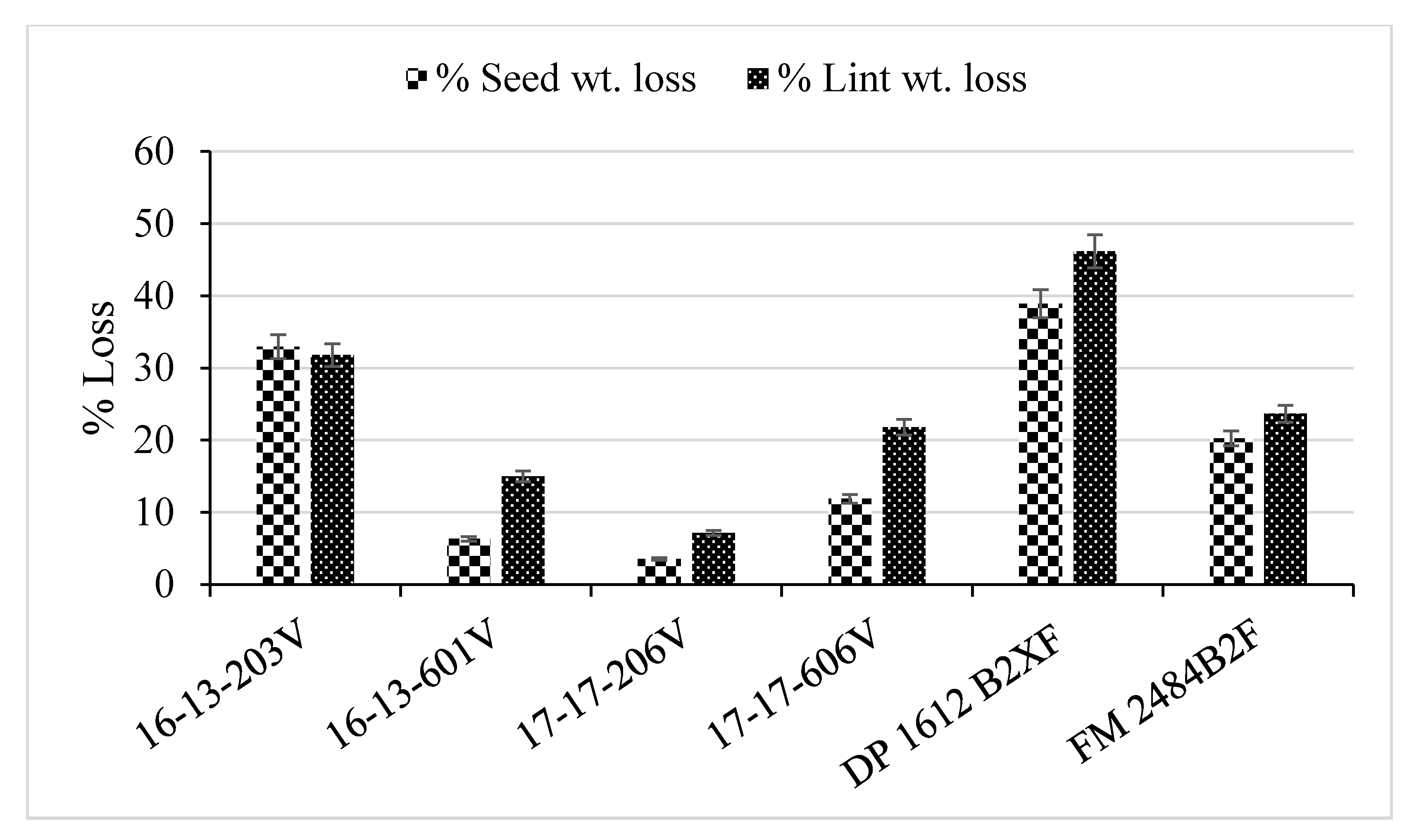


| Genotype | WILTi b (DAP) | DS c | VCDS d | Photosynthesis (µmoL m−2 s−1) | Lint (g) | Seed (g) |
|---|---|---|---|---|---|---|
| 16-13-203V | 44 b a | 3.7 a | 2.2 ab | 19.6 b | 37 bc | 53 bc |
| 16-13-601V | 67 a | 2.0 b | 1.0 c | 22.0 a | 42 ab | 57 ab |
| 17-17-206V | 56 ab | 2.6 b | 1.4 c | 22.2 a | 45 a | 62 a |
| 17-17-606V | 53 ab | 2.0 b | 1.5 bc | 21.7 a | 41 abc | 54 bc |
| DP 1612 B2XF | 55 ab | 3.5 a | 2.4 a | 19.2 b | 36 c | 48 c |
| FM 2484B2F | 47 b | 2.3 b | 1.0 c | 21.8 a | 45 a | 60 ab |
| Source | Mean Squares | |||
|---|---|---|---|---|
| DF | Photosynthesis Rate (µmoL m−2 s−1) | Ci (µmoL CO2 moL−1) | Transpiration Rate (mmoL H2O m−2 s−1) | |
| Inoculum b (+/−) | 1 | 209.89 ***a | 2.70 * | 96.62 *** |
| Genotype | 5 | 5.97 *** | 2.80 | 2.61 ** |
| Genotype × Inoculum | 5 | 4.97 ** | 3.76 | 3.39 ** |
| DAP | 13 | 52.12 ** | 19.75 ** | 242.46 ** |
| Inoculum × DAP | 13 | 8.51 ** | 1.62 | 17.73 ** |
| Genotype × DAP | 65 | 0.98 | 1.88 ** | 1.17 |
| Inoculum × Genotype × DAP | 65 | 0.91 | 1.10 | 1.01 |
| Error | 854 | 41 | 3548 | 7.5 |
| No Verticillium Wilt | ||||||
|---|---|---|---|---|---|---|
| Genotypes | Mic | Neps | SFC (n) | Fine (mTex) | IFC | MR |
| 16-13-203V | 4.5 b a | 117 a | 19.1 a | 167 c | 6.2 b | 0.92 a |
| 16-13-601V | 4.1 c | 156 a | 15.7 c | 152 d | 7.0 a | 0.92 a |
| 17-17-206V | 4.6 b | 118 a | 19.3 a | 174 ab | 6.0 b | 0.91 ab |
| 17-17-606V | 3.9 c | 166 a | 18.6 a | 153 d | 7.0 a | 0.90 ab |
| DP 1612 B2XF | 5.0 a | 145 a | 16.1 bc | 178 a | 5.9 b | 0.90 bc |
| FM 2484B2F | 4.4 b | 136 a | 17.7 ab | 168 bc | 5.9 b | 0.90 bc |
| With Verticillium Wilt | ||||||
| 16-13-203V | 3.4 bc | 331 ab | 25.3 ab | 146 bc | 7.8 ab | 0.85 bc |
| 16-13-601V | 3.9 ab | 210 b | 18.9 c | 153 abc | 7.3 abc | 0.88 ab |
| 17-17-206V | 4.0 a | 243 b | 22.3 bc | 164 a | 6.9 bc | 0.88 ab |
| 17-17-606V | 3.4 c | 258 ab | 23.1 bc | 143 c | 7.9 ab | 0.85 bc |
| DP 1612 B2XF | 3.6 bc | 565 a | 29.3 a | 144 c | 8.3 a | 0.83 c |
| FM 2484B2F | 4.1 a | 191 b | 20.4 bc | 159 ab | 6.5 c | 0.90 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayele, A.G.; Wheeler, T.A.; Dever, J.K. Impacts of Verticillium Wilt on Photosynthesis Rate, Lint Production, and Fiber Quality of Greenhouse-Grown Cotton (Gossypium hirsutum). Plants 2020, 9, 857. https://doi.org/10.3390/plants9070857
Ayele AG, Wheeler TA, Dever JK. Impacts of Verticillium Wilt on Photosynthesis Rate, Lint Production, and Fiber Quality of Greenhouse-Grown Cotton (Gossypium hirsutum). Plants. 2020; 9(7):857. https://doi.org/10.3390/plants9070857
Chicago/Turabian StyleAyele, Addissu G., Terry A. Wheeler, and Jane K. Dever. 2020. "Impacts of Verticillium Wilt on Photosynthesis Rate, Lint Production, and Fiber Quality of Greenhouse-Grown Cotton (Gossypium hirsutum)" Plants 9, no. 7: 857. https://doi.org/10.3390/plants9070857
APA StyleAyele, A. G., Wheeler, T. A., & Dever, J. K. (2020). Impacts of Verticillium Wilt on Photosynthesis Rate, Lint Production, and Fiber Quality of Greenhouse-Grown Cotton (Gossypium hirsutum). Plants, 9(7), 857. https://doi.org/10.3390/plants9070857






