The Use of Essential Oil and Hydrosol Extracted from Satureja hellenica for the Control of Meloidogyne incognita and M. javanica
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Essential Oil and Hydrosol
2.2. Nematicidal Effect of Essential Oil and Hydrosol
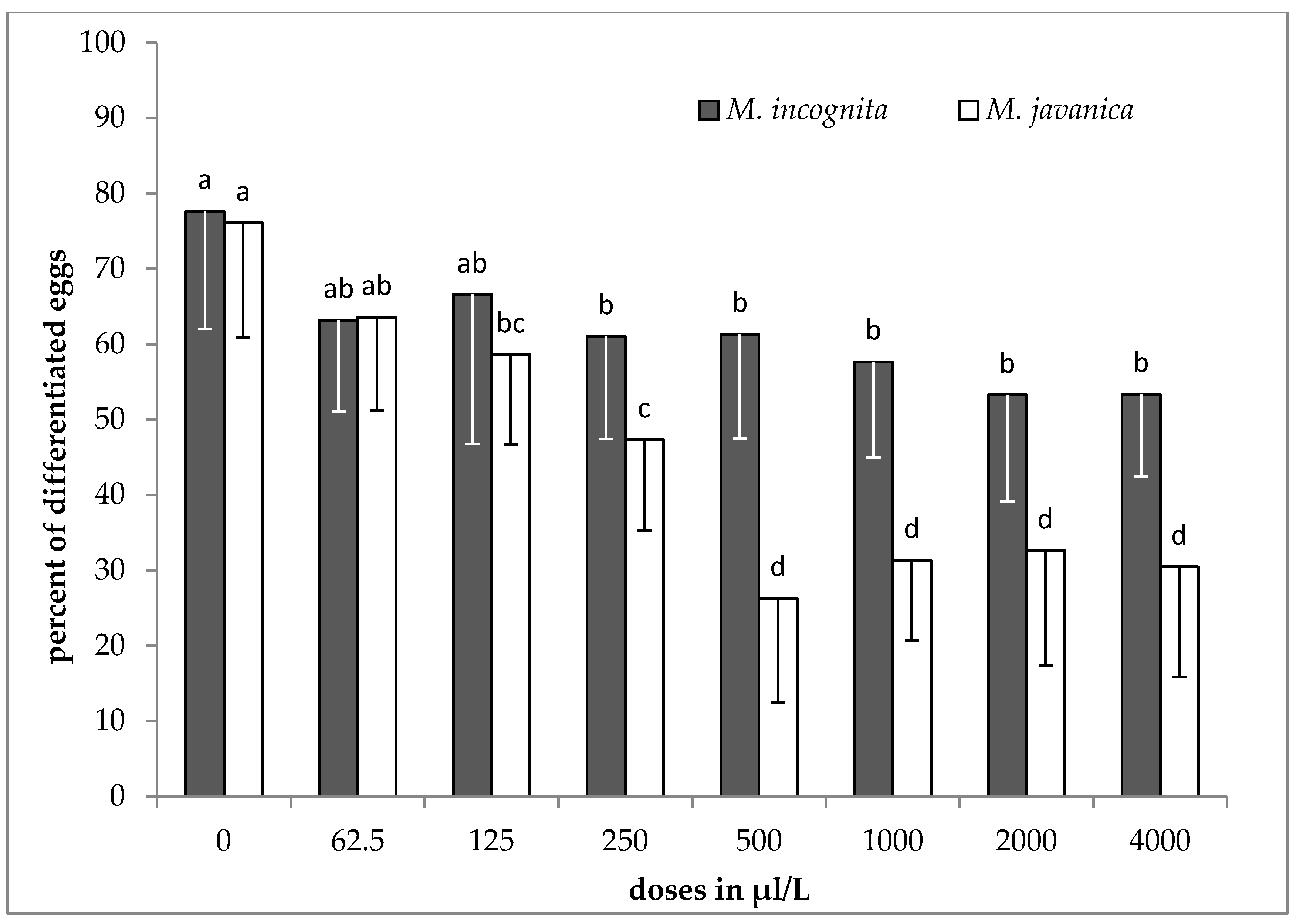
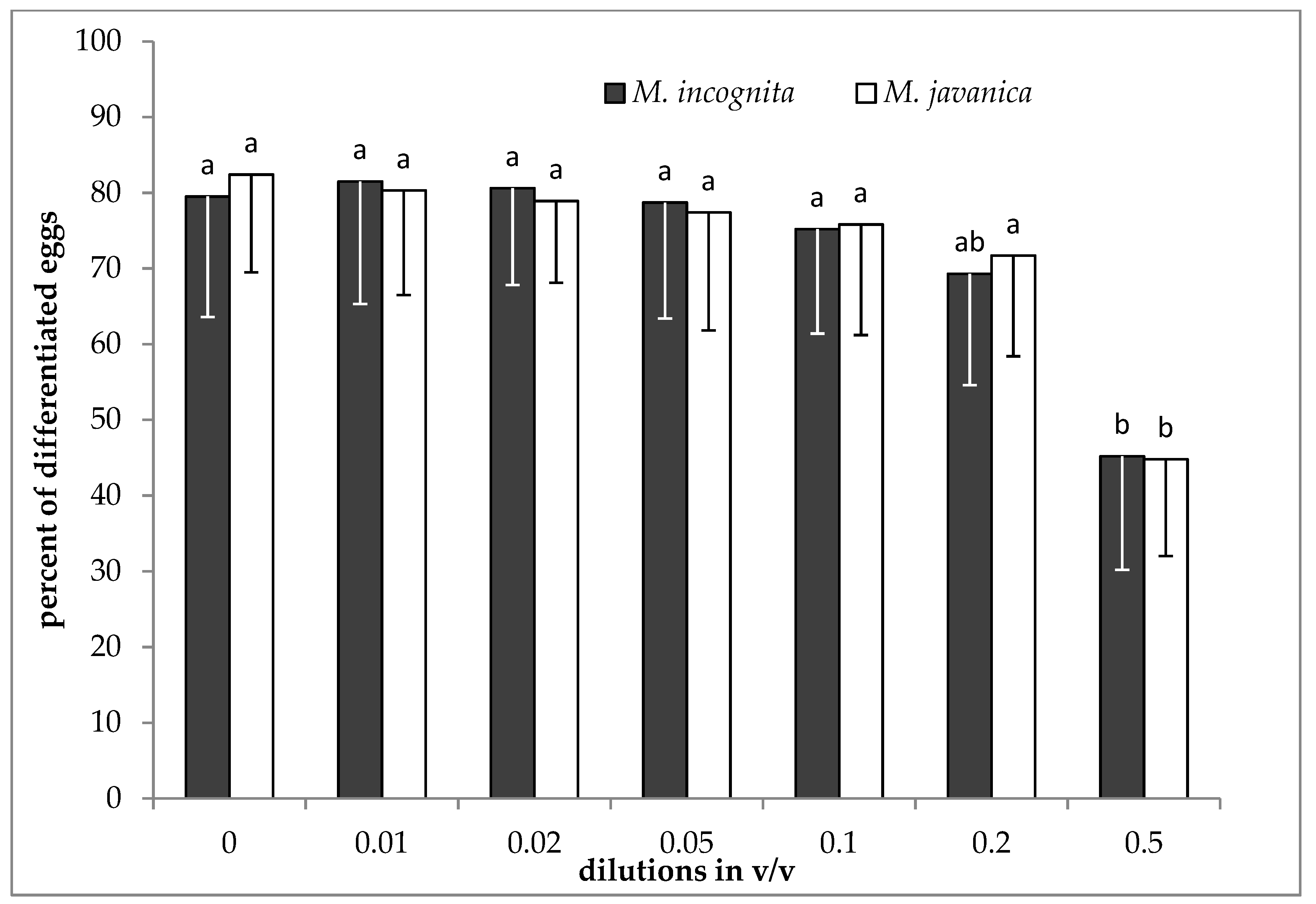
2.3. Effect of EO and HL on Egg Differentiation
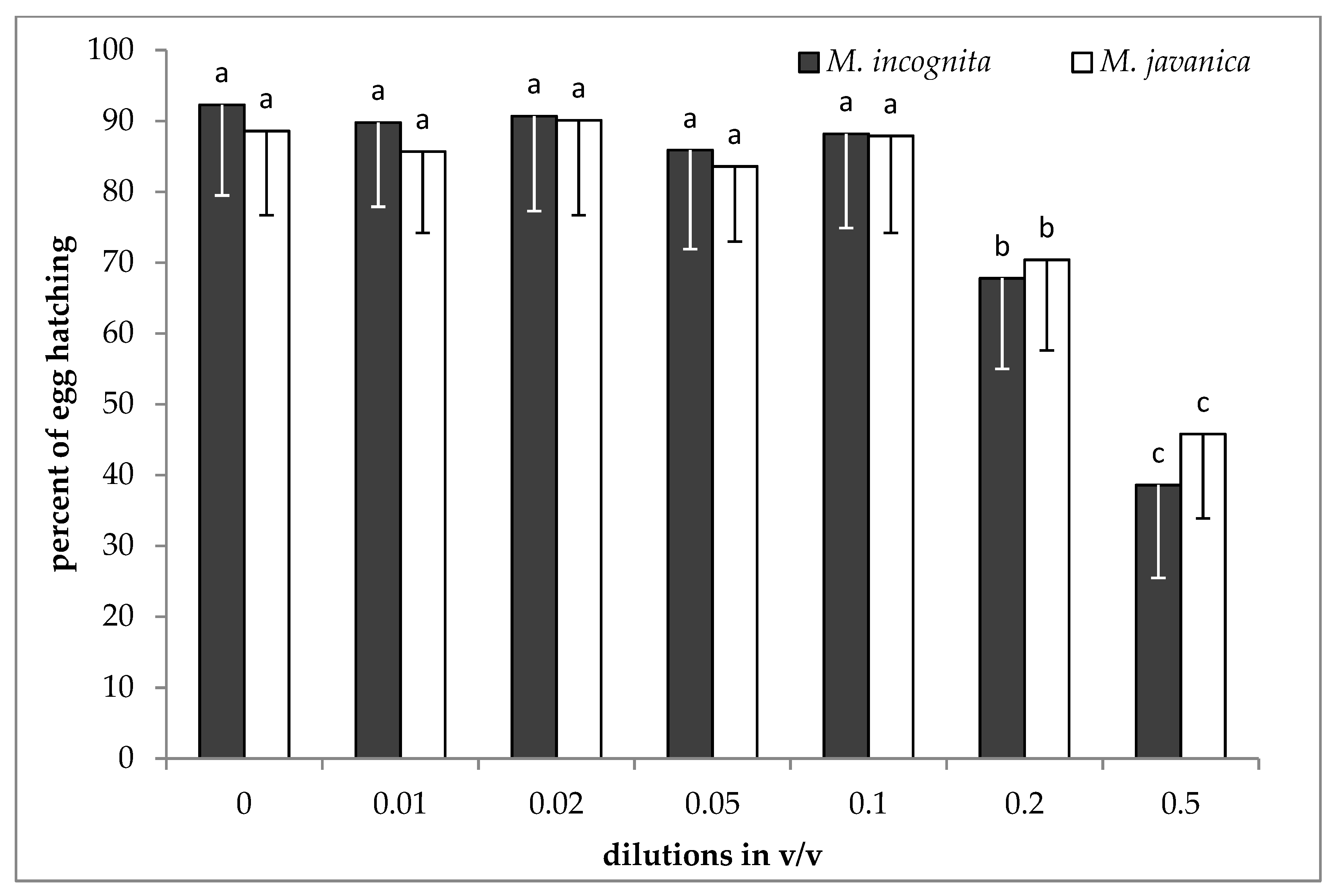
2.4. Hatching Inhibition as Affected by the Presence of EO and HL
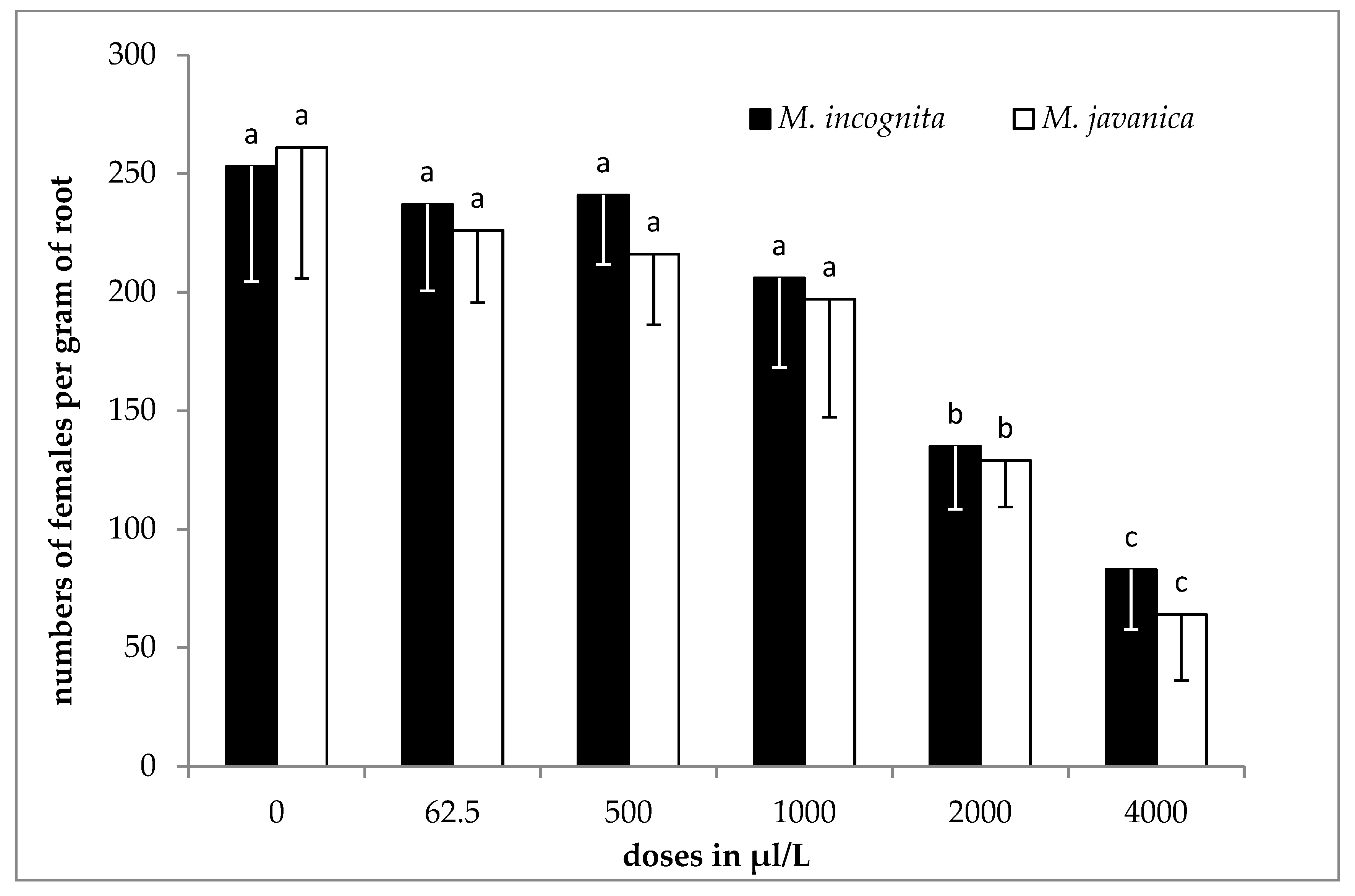
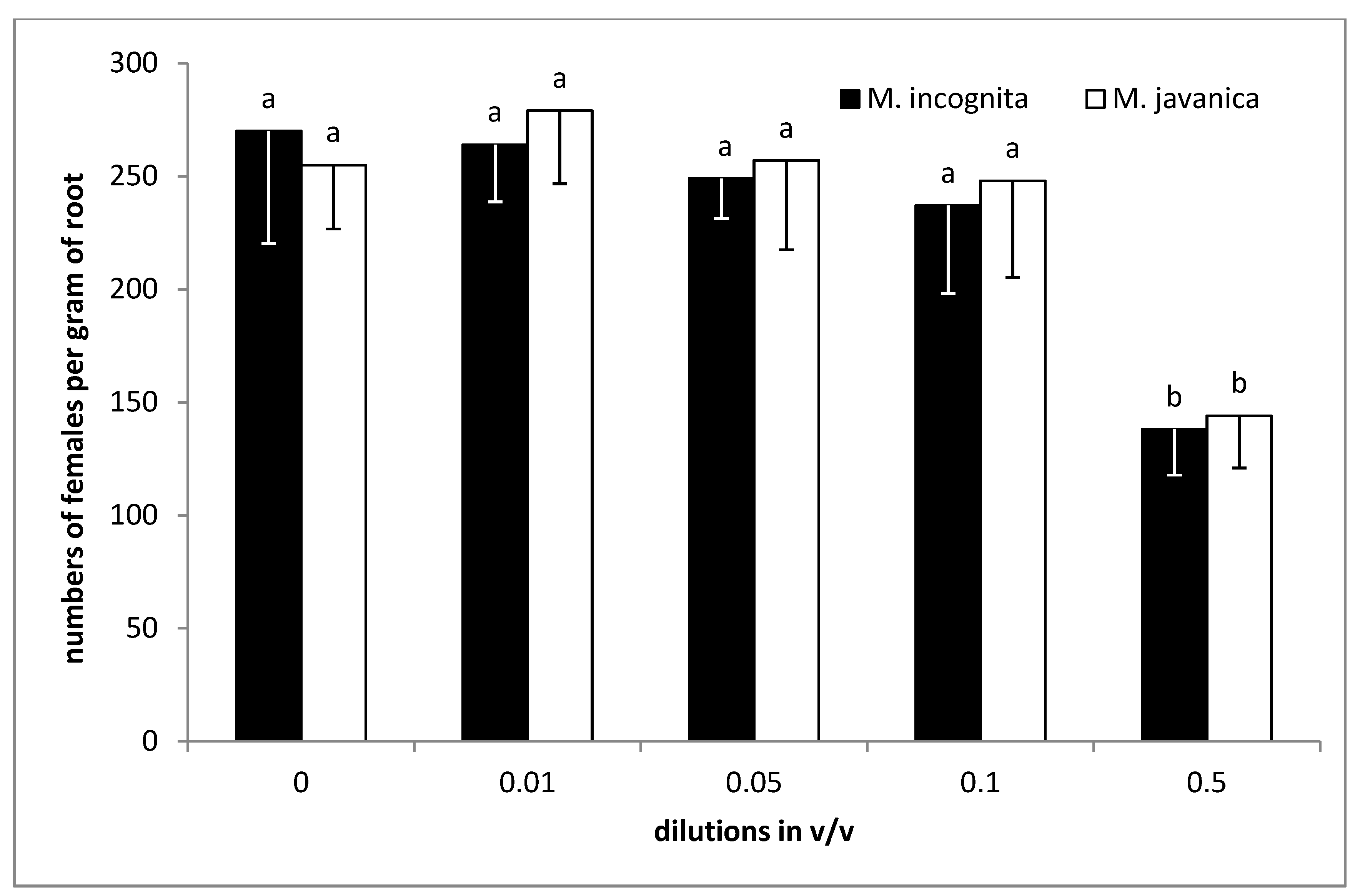
2.5. Effect of EO and HL on Juveniles in Soil
3. Discussion
4. Materials and Methods
4.1. Nematode Populations
4.2. Plant Material
4.3. Isolation of Essential Oil and Hydrosol and Determination of Their Chemical Composition
4.4. Effect of EO and Hydrosol on J2s Motility
4.5. Effect of EO and Hydrosol on Egg Differentiation
4.6. Hatching Inhibition as Affected by the Presence of EO and Hydrosol
4.7. Effect of EO and Hydrosol on Juveniles in Soil
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bird, D.M.K.; Williamson, V.M.; Abad, P.; McCarter, J.; Danchin, E.G.J.; Castagnone-Sereno, P.; Opperman, C.H. The Genomes of Root-Knot Nematodes. Annu. Rev. Phytopathol. 2009, 47, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Bleve-Zacheo, T.; Melillo, M.T.; Castagnone-Sereno, P. The contribution of biotechnology to root-knot nematode control in tomato plants. Pest Technol. 2007, 1, 1–16. [Google Scholar]
- Karpouzas, D.G.; Hatziapostolou, P.; Papadopoulou-Mourkidou, E.; Giannakou, I.O.; Georgiadou, A. The enhanced biodegradation of fenamiphos in soils from previously treated sites and the effect of soil fumigants. Environ. Toxicol. Chem. 2004, 23, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.S.; Lagos, S.; Spentza, F.; Vidiadakis, E.; Karas, P.A.; Klitsinaris, T.; Karpouzas, D.G. The dissipation of fipronil, chlorpyrifos, fosthiazate and ethoprophos in soils from potato monoculture areas: First evidence for the enhanced biodegradation of fosthiazate. Pest Manag. Sci. 2016, 72, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Mahmood, I. Potentiality of phytochemicals in nematode control: A review. Bioresour. Technol. 1994, 48, 189–201. [Google Scholar] [CrossRef]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef]
- Mendoza, A.R.; Kiewnick, S.; Sikora, R.A. In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis, the root-knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci. Biocontrol Sci. Technol. 2008, 18, 377–389. [Google Scholar] [CrossRef]
- Turatto, M.F.; Dourado, F.D.S.; Zilli, J.E.; Botelho, G.R. Control potential of Meloidogyne javanica and Ditylenchus spp. using fluorescent Pseudomonas and Bacillus spp. Braz. J. Microbiol. 2018, 49, 54–58. [Google Scholar] [CrossRef]
- Nyaku, S.T.; Affokpon, A.; Danquah, A.; Brentu, F.C. Harnesing Useful Rhizosphere Microorganisms for Nematode Control, Nematology-Concepts, Diagnosis and Control. Nematol.–Concepts Diagn. Control 2017, 8, 153–182. [Google Scholar]
- Andrés, M.F.; González-Coloma, A.; Sanz, J.; Burillo, J.; Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant Secondary Metabolites: Biosynthesis, Classification, Function and Pharmacological Properties. J. Pharm. Pharmacol. 2004, 2, 377–392. [Google Scholar]
- Petrakis, E.A.; Kimbaris, A.C.; Lykouressis, D.P.; Polisiou, M.G.; Perdikis, D.C.H. Hydrosols evaluation in pest control: Insecticidal and settling inhibition potential against Myzus persicae (Sulzer). J. Appl. Entomol. 2014, 139, 260–267. [Google Scholar] [CrossRef]
- Traka, C.K.; Petrakis, E.A.; Kimbaris, A.C.; Polisiou, M.G.; Perdikis, D.C. Effects of Ocinum basilicum and Ruta chalepensis hydrosols on Aphis gossypii and Tetranychus urticae. J. Appl. Entomol. 2017, 142, 413–420. [Google Scholar] [CrossRef]
- Sadiki, M.; Barkai, H.; Koraichi, S.I.; Elabed, S. The effect of the Thymusvulgaris extracts on the physicochemical characteristics of cedar wood using angle contact measurement. J. Adhes. Sci. Technol. 2014, 28, 1925–1934. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationship evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Karamanoli, K.I.; Stamopoulos, D.C.; Menkissoglu-Spiroudi, U. The relationship between the chemical composition of three essential oils and their insecticidal activity against Acanthoscelides obtectus (Say). Pest Manag. Sci. 2014, 60, 514–520. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.H.; Alaniz, N.M.; Saleh, M.A. Nematicidal activity of terpenoids. J. Environ. Sci. Health. 2013, 48, 16–22. [Google Scholar] [CrossRef]
- Park, I.K.; Kim, J.; Lee, S.G.; Sheen, S.C. Nematicidal activity of plant essential oils and components from ajowan (Trachysperum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against wood nematode (Bursaphelenchus xylophilus). J. Nematol. 2007, 39, 275–279. [Google Scholar]
- Oka, Y.; Nacar, S.; Putievsky, E.; Ravid, U.; Yaniv, Z.; Spiegel, Y. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 2000, 90, 710–715. [Google Scholar] [CrossRef]
- Moens, M.; Perry, N.P.; Starr, L.J. Root-Knot Nematodes; CAB International: Wallingford, UK, 2009. [Google Scholar]
- Barros, A.F.; Campos, V.P.; De Paula, L.L.; Oliveira, D.F.; De Silva, F.J.; Terra, W.C.; Silva, G.H.; Salimena, J.P. Nematicidal screening of essential oils and potent toxicity of Dysphania ambrosioides essential oil against Meloidogyne incognita in vitro and in vivo. J. Phytopathol. 2019, 167, 380–389. [Google Scholar] [CrossRef]
- Jeon, J.H.; Ko, H.R.; Kim, S.J.; Lee, J.K. Chemical composistion and nematicidal activities of essential oils on Meloidogyne hapla (Nematode: Tylenchida) Under Laboratory Conditions. Korean J. Pest. Sci. 2016, 20, 30–34. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Sena, I.; Ribeiro, B.; Rodrigues, A.M.; Figueiredo, A.C.S. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J. Pest Sci. 2016, 89, 207–217. [Google Scholar] [CrossRef]
- Nasiou, E.; Giannakou, I.O. The potential use of carvacrol for the control of Meloidogyne javanica. Eur. J. Plant Pathol. 2017, 149, 415–424. [Google Scholar] [CrossRef]
- Nasiou, E.; Giannakou, I.O. Effect of geraniol, a plant-based alcohol monoterpene oil, against Meloidogyne javanica. Eur. J. Plant Pathol. 2018, 152, 701–710. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Phytochemistry and nematicidal activity of the essential oils from 8 greek lamiaceae aromatic plants and 13 terpene components. J. Agric. Food Chem. 2010, 58, 7856–7863. [Google Scholar] [CrossRef]
- Laquale, S.; Avato, P.; Argentini, M.P.; Bellardi, M.G.; D’Addabbo, T. Nematotoxic activity of essential oils from Monarda species. J. Pest Sci. 2018, 91, 1115–1125. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Dardioti, A. Biosistimatiki Meleti tis Omadas Satureja Montana L. stin Ellada [Biosystematic study of Satureja Montana L. Group in Greece]. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 4 July 2005. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ. Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Byrd, D.W.; Krickpatrick, T.; Barker, K.R. An improved technique for cleaning and staining plant tissue for detection of nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar]

| No | RRI * | Compound | S. hellenica e.o. | S. hellenica Hydrosol |
|---|---|---|---|---|
| 1 | 940 | α-Pinene | 1.5 | - # |
| 2 | 948 | Camphene | 1.3 | - |
| 3 | 979 | 1-Octen-3-ol | - | 1.6 |
| 4 | 981 | β-Myrcene | 1.0 | - |
| 5 | 999 | 3-Octanol | - | 0.3 |
| 6 | 1014 | α-Terpinene | 1.1 | - |
| 7 | 1024 | p-Cymene | 27.5 | - |
| 8 | 1057 | γ-Terpinene | 4.6 | - |
| 9 | 1075 | cis-Sabinene hydrate | 2.4 | 4.6 |
| 10 | 1083 | Terpinolene | 0.4 | - |
| 11 | 1082 | cis-Linalool oxide | - | 0.7 |
| 12 | 1100 | Linalool | 0.6 | 0.7 |
| 13 | 1102 | trans-Sabinene hydrate | 0.4 | 2.5 |
| 14 | 1136 | 1-Terpineol | 0.3 | - |
| 15 | 1143 | cis-2-Pinanol | 0.6 | 1.3 |
| 16 | 1152 | Camphor | 0.5 | 1.2 |
| 17 | 1179 | Borneol | 6.8 | 20.4 |
| 18 | 1183 | 4-Terpineol | 3.6 | 6.7 |
| 19 | 1188 | p-Cymen-8-ol | 0.3 | 2.8 |
| 20 | 1195 | α-Terpineol | 0.6 | 1.9 |
| 21 | 1204 | cis-Dihydrocarvone | 0.2 | - |
| 22 | 1223 | Coahuilensol methyl ether | - | 0.2 |
| 23 | 1232 | Thymol methylether | 1.4 | - |
| 24 | 1243 | Carvacrol methylether | 6.8 | 0.5 |
| 25 | 1270 | Geranial | 0.1 | - |
| 26 | 1292 | Thymol | 0.3 | - |
| 27 | 1299 | Carvacrol | 23.3 | 50.1 |
| 28 | 1348 | Thymol acetate | - | 0.3 |
| 29 | 1354 | Eugenol | - | 0.2 |
| 30 | 1418 | (Ε)-Caryophyllene | 3.5 | 0.2 |
| 31 | 1438 | Aromadendrene | 0.4 | - |
| 32 | 1457 | α-Humulene | 0.2 | - |
| 33 | 1491 | Viridiflorene | 0.2 | |
| 34 | 1495 | Bicyclogermacrene | 0.3 | |
| 35 | 1507 | β-Bisabolene | 3.5 | - |
| 36 | 1575 | Spathulenol | 1.4 | 0.4 |
| 37 | 1580 | Caryophyllene oxide | 2.7 | 0.3 |
| 38 | 1652 | α-Eudesmol | 0.2 | 0.1 |
| Total (%) | 98.0 | 97.0 | ||
| Monoterpene Hydrocarbons (MH) | 37.4 | - | ||
| Oxygenated Monoterpenes (OM) | 48.2 | 93.7 | ||
| Sesquiterpene Hydrocarbons (SH) | 8.1 | 0.2 | ||
| Oxygenated Sesquiterpenes (OS) | 4.3 | 0.8 | ||
| Others | - | 2.3 | ||
| Dose (μL/L) | Exposure Time (h) | ||
|---|---|---|---|
| 24 | 48 | 96 | |
| Dead J2s (%) | Dead J2s (%) | Dead J2s (%) | |
| 0 | 0.0 d | 2.6 d | 10.4 e |
| 62.5 | 0.0 d | 2.0 d | 25.3 d |
| 125 | 0.0 d | 4.3 d | 25.7 d |
| 250 | 0.5 d | 7.5 d | 21.4 d |
| 500 | 1.1 d | 30.6 c | 56.6 c |
| 1000 | 7.7 c | 38.5 c | 85.6 b |
| 2000 | 31.1 b | 81.2 b | 100 a |
| 4000 | 99.1 a | 100 a | 100 a |
| Dose (μL/L) | Exposure Time (h) | ||
|---|---|---|---|
| 24 | 48 | 96 | |
| Dead J2s (%) | Dead J2s (%) | Dead J2s (%) | |
| 0 | 0.3 d | 1.1 d | 12.4 d |
| 62.5 | 3.7 d | 6.9 cd | 23.2 cd |
| 125 | 3.6 d | 5.2 cd | 31.5c |
| 250 | 2.4 d | 7.0 c | 22.7 cd |
| 500 | 4.6 d | 13.1 c | 46.2 c |
| 1000 | 12.7 c | 35.4 b | 82.5 b |
| 2000 | 48.5 b | 86.2 a | 100 a |
| 4000 | 90.1 a | 98.9 a | 100 a |
| Dilution (v/v) | Exposure Time (h) | ||
|---|---|---|---|
| 24 | 48 | 96 | |
| Dead J2s (%) | Dead J2s (%) | Dead J2s (%) | |
| 0 | 0.0 b | 0.5 b | 1.9 c |
| 0.01 | 0.6 b | 1.7 b | 5.2 bc |
| 0.02 | 0.5 b | 2.6 b | 7.7 b |
| 0.05 | 1.0 b | 1.9 b | 11.6 b |
| 0.1 | 0.8 b | 1.2 b | 8.3 b |
| 0.2 | 0.2 b | 1.0 b | 8.3 b |
| 0.5 | 13.1 a | 75.9 a | 93.3 a |
| Dilution (v/v) | Exposure Time (h) | ||
|---|---|---|---|
| 24 | 48 | 96 | |
| Dead J2s (%) | Dead J2s (%) | Dead J2s (%) | |
| 0 | 0.3 b | 0.8 b | 6.5 c |
| 0.01 | 0.9 b | 2.0 b | 14.0 bc |
| 0.02 | 0.8 b | 3.0 b | 18.4 b |
| 0.05 | 1.0 b | 1.6 b | 13.7 bc |
| 0.1 | 1.4 b | 2.4 b | 22.8 b |
| 0.2 | 2.5 b | 6.8 b | 28.8 b |
| 0.5 | 46.1 a | 79.7 a | 96.4 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardavella, I.; Nasiou, E.; Daferera, D.; Trigas, P.; Giannakou, I. The Use of Essential Oil and Hydrosol Extracted from Satureja hellenica for the Control of Meloidogyne incognita and M. javanica. Plants 2020, 9, 856. https://doi.org/10.3390/plants9070856
Pardavella I, Nasiou E, Daferera D, Trigas P, Giannakou I. The Use of Essential Oil and Hydrosol Extracted from Satureja hellenica for the Control of Meloidogyne incognita and M. javanica. Plants. 2020; 9(7):856. https://doi.org/10.3390/plants9070856
Chicago/Turabian StylePardavella, Iro, Eleni Nasiou, Dimitra Daferera, Panayiotis Trigas, and Ioannis Giannakou. 2020. "The Use of Essential Oil and Hydrosol Extracted from Satureja hellenica for the Control of Meloidogyne incognita and M. javanica" Plants 9, no. 7: 856. https://doi.org/10.3390/plants9070856
APA StylePardavella, I., Nasiou, E., Daferera, D., Trigas, P., & Giannakou, I. (2020). The Use of Essential Oil and Hydrosol Extracted from Satureja hellenica for the Control of Meloidogyne incognita and M. javanica. Plants, 9(7), 856. https://doi.org/10.3390/plants9070856







