A Perspective on Secondary Seed Dormancy in Arabidopsis thaliana
Abstract
1. Introduction
2. Primary Dormancy
3. Secondary Dormancy
4. Assessing Primary Dormancy
5. Assessing Secondary Dormancy
6. Different Ecological Functions of Primary and Secondary Dormancy
7. Overlap and Differences between Primary and Secondary Dormancy
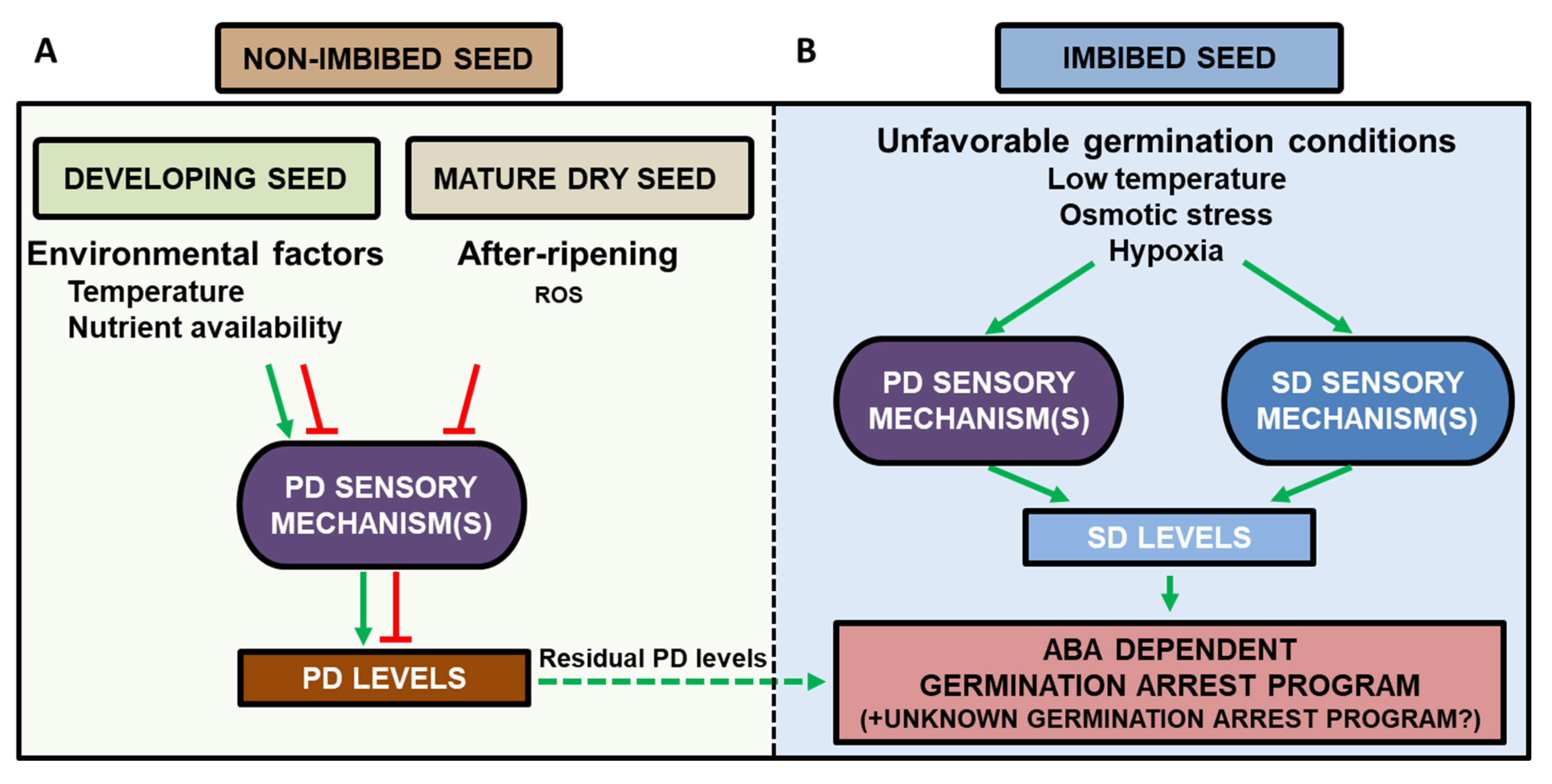
8. A Model for the Regulation of Secondary Dormancy
9. Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Benech-Arnold, R.L.; Sánchez, R.A.; Forcella, F.; Kruk, B.C.; Ghersa, C.M. Environmental control of dormancy in weed seed banks in soil. Field Crop. Res. 2000, 67, 105–122. [Google Scholar] [CrossRef]
- Postma, F.M.; Lundemo, S.; Ågren, J. Seed dormancy cycling and mortality differ between two locally adapted populations of Arabidopsis thaliana. Ann. Bot. 2016, 117, 249–256. [Google Scholar]
- Nikolaeva, M.G. Factors controlling the seed dormancy pattern. In The Physiology and Biochemistry of Seed Dormancy; Khan, A.A., Ed.; North Holland: Amsterdam, The Netherlands, 1977; pp. 51–74. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Piskurewicz, U.; Turečková, V.; Strnad, M.; Lopez-Molina, L. A seed coat bedding assay shows that RGL2-dependent release of abscisic acid by the endosperm controls embryo growth in Arabidopsis dormant seeds. Proc. Natl. Acad. Sci. USA 2010, 107, 19108–19113. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Oracz, K.; El-Maarouf Bouteau, H.; Farrant, J.M.; Cooper, K.; Belghazi, M.; Job, C.; Job, D.; Corbineau, F.; Bailly, C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 2007, 50, 452–465. [Google Scholar] [CrossRef]
- Leymarie, J.; Vitkauskaité, G.; Hoang, H.H.; Gendreau, E.; Chazoule, V.; Meimoun, P.; Corbineau, F.; El-Maarouf-Bouteau, H.; Bailly, C. Role of reactive oxygen species in the regulation of arabidopsis seed dormancy. Plant Cell Physiol. 2012, 53, 96–106. [Google Scholar] [CrossRef]
- Alonso-Blanco, C.; Hanhart, C.J.; Blankestijn-de Vries, H.; Koornneef, M. Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics 2003, 164, 711–729. [Google Scholar]
- Clerkx, E.J.M.; El-Lithy, M.E.; Vierling, E.; Ruys, G.J.; Blankestijn-De Vries, H.; Groot, S.P.C.; Vreugdenhil, D.; Koornneef, M. Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol. 2004, 135, 432–443. [Google Scholar] [CrossRef]
- Bentsink, L.; Soppe, W.; Koornneef, M. Genetic aspects of seed dormancy. Annu. Plant Rev. 2007, 27, 113–132. [Google Scholar]
- Atwell, S.; Huang, Y.S.; Vilhjálmsson, B.J.; Willems, G.; Horton, M.; Li, Y.; Meng, D.; Platt, A.; Tarone, A.M.; Hu, T.T.; et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 2010, 465, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Vidigal, D.S.; Marques, A.C.S.S.; Willems, L.A.J.; Buijs, G.; Méndez-Vigo, B.; Hilhorst, H.W.M.; Bentsink, L.; Picó, F.X.; Alonso-Blanco, C. Altitudinal and climatic associations of seed dormancy and flowering traits evidence adaptation of annual life cycle timing in Arabidopsis thaliana. Plant Cell Environ. 2016, 39, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- He, H.; De Souza Vidigal, D.; Basten Snoek, L.; Schnabel, S.; Nijveen, H.; Hilhorst, H.; Bentsink, L. Interaction between parental environment and genotype affects plant and seed performance in Arabidopsis. J. Exp. Bot. 2014, 65, 6603–6615. [Google Scholar] [CrossRef]
- Springthorpe, V.; Penfield, S. Flowering time and seed dormancy control use external coincidence to generate life history strategy. eLife 2015, 4, e05557. [Google Scholar] [CrossRef]
- Footitt, S.; Walley, P.G.; Lynn, J.R.; Hambidge, A.J.; Penfield, S.; Finch-Savage, W.E. Trait analysis reveals DOG1 determines initial depth of seed dormancy, but not changes during dormancy cycling that result in seedling emergence timing. New Phytol. 2019, 225, 2035–2047. [Google Scholar] [CrossRef]
- Buijs, G.; Kodde, J.; Groot, S.P.C.; Bentsink, L. Seed dormancy release accelerated by elevated partial pressure of oxygen is associated with DOG loci. J. Exp. Bot. 2018, 69, 3601–3608. [Google Scholar] [CrossRef]
- Chahtane, H.; Kim, W.; Lopez-Molina, L. Primary seed dormancy: A temporally multilayered riddle waiting to be unlocked. J. Exp. Bot. 2017, 68, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Ali-Rachedi, S.; Bouinot, D.; Wagner, M.H.; Bonnet, M.; Sotta, B.; Grappin, P.; Jullien, M. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: Studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 2004, 219, 479–488. [Google Scholar] [CrossRef]
- Millar, A.A.; Jacobsen, J.V.; Ross, J.J.; Helliwell, C.A.; Poole, A.T.; Scofield, G.; Reid, J.B.; Gubler, F. Seed dormancy and ABA metabolism in Arabidopsis and barley: The role of ABA 8’-hydroxylase. Plant J. 2006, 45, 942–954. [Google Scholar] [CrossRef]
- Nonogaki, H. ABA responses during seed development and germination. Adv. Bot. Res. 2019, 92, 171–217. [Google Scholar]
- Née, G.; Xiang, Y.; Soppe, W.J. The release of dormancy, a wake-up call for seeds to germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H. Seed biology updates—Highlights and new discoveries in seed dormancy and germination research. Front. Plant Sci. 2017, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Footitt, S. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. J. Exp. Bot. 2017, 68, 843–856. [Google Scholar] [CrossRef]
- Footitt, S.; Douterelo-Soler, I.; Clay, H.; Finch-Savage, W.E. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. PNAS 2011, 108, 20236–20241. [Google Scholar] [CrossRef]
- Buijs, G.; Vogelzang, A.; Nijveen, H.; Bentsink, L. Dormancy cycling: Translation related transcripts are the main difference between dormant and non-dormant seeds in the field. Plant J. 2019. [Google Scholar] [CrossRef]
- Footitt, S.; Müller, K.; Kermode, A.R.; Finch-Savage, W.E. Seed dormancy cycling in Arabidopsis: Chromatin remodelling and regulation of DOG1 in response to seasonal environmental signals. Plant J. 2015, 81, 413–425. [Google Scholar] [CrossRef]
- Penfield, S.; Springthorpe, V. Understanding chilling responses in Arabidopsis seeds and their contribution to life history. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 291–297. [Google Scholar] [CrossRef]
- Auge, G.A.; Blair, L.K.; Burghardt, L.T.; Coughlan, J.; Edwards, B.; Leverett, L.; Donohue, K. Secondary dormancy dynamics depends on primary dormancy status in Arabidopsis thaliana. Seed Sci. Res. 2015, 25, 230–246. [Google Scholar] [CrossRef]
- De Giorgi, J.; Piskurewicz, U.; Loubery, S.; Utz-Pugin, A.; Bailly, C.; Mène-Saffrané, L.; Lopez-Molina, L. An endosperm-associated cuticle is required for Arabidopsis seed viability, dormancy and early control of germination. PLoS Genet. 2015, 11. [Google Scholar] [CrossRef]
- Cadman, C.S.C.; Toorop, P.E.; Hilhorst, H.W.M.; Finch-Savage, W.E. Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. Plant J. 2006, 46, 805–822. [Google Scholar] [CrossRef] [PubMed]
- Footitt, S.; Ölçer-Footitt, H.; Hambidge, A.J.; Finch-Savage, W.E. A laboratory simulation of Arabidopsis seed dormancy cycling provides new insight into its regulation by clock genes and the dormancy-related genes DOG1, MFT, CIPK23 and PHYA. Plant Cell Environ. 2017, 40, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, J.M.; Saha, A.; Donohue, K. Effects of pre- and post-dispersal temperature on primary and secondary dormancy dynamics in contrasting genotypes of Arabidopsis thaliana (Brassicaceae). Plant Species Biol. 2017, 32, 210–222. [Google Scholar] [CrossRef]
- Geshnizjani, N.; Ghaderi-Far, F.; Willems, L.A.J.; Hilhorst, H.W.M.; Ligterink, W. Characterization of and genetic variation for tomato seed thermo-inhibition and thermo-dormancy. BMC Plant Biol. 2018, 18, 229. [Google Scholar] [CrossRef]
- Nagel, M.; Alqudah, A.M.; Bailly, M.; Rajjou, L.; Pistrick, S.; Matzig, G.; Börner, A.; Kranner, I. Novel loci and a role for nitric oxide for seed dormancy and preharvest sprouting in barley. Plant Cell Environ. 2019, 42, 1318–1327. [Google Scholar] [CrossRef]
- Footitt, S.; Clewes, R.; Feeney, M.; Finch-Savage, W.E.; Frigerio, L. Aquaporins influence seed dormancy and germination in response to stress. Plant Cell Environ. 2019, 42, 2325–2339. [Google Scholar] [CrossRef]
- Burghardt, L.T.; Metcalf, C.J.E.; Wilczek, A.M.; Schmitt, J.; Donohue, K. Modeling the influence of genetic and environmental variation on the expression of plant life cycles across landscapes. Am. Nat. 2015, 185, 212–227. [Google Scholar] [CrossRef]
- Bentsink, L.; Jowett, J.; Hanhart, C.J.; Koornneef, M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 17042–17047. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Bartsch, M.; Xiang, Y.; Miatton, E.; Pellengahr, S.; Yano, R.; Seo, M.; Soppe, W.J.J. The time required for dormancy release in Arabidopsis is determined by DELAY OF GERMINATION1 protein levels in freshly harvested seeds. Plant Cell 2012, 24, 2826–2838. [Google Scholar] [CrossRef]
- Seo, M.; Nambara, E.; Choi, G.; Yamaguchi, S. Interaction of light and hormone signals in germinating seeds. Plant Mol. Biol. 2009, 69, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H. The long-standing paradox of seed dormancy unfolded? Trends Plant Sci. 2019, 24, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef] [PubMed]
- Soppe, W.J.J.; Bentsink, L. Seed dormancy back on track; its definition and regulation by DOG1. New Phytol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H. Seed dormancy and germination-emerging mechanisms and new hypotheses. Front. Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Hyvarinen, L.; Piskurewicz, U.; Lopez-Molina, L. Non-canonical RNA-directed DNA methylation participates in maternal and environmental control of seed dormancy. eLife 2019, 8. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buijs, G. A Perspective on Secondary Seed Dormancy in Arabidopsis thaliana. Plants 2020, 9, 749. https://doi.org/10.3390/plants9060749
Buijs G. A Perspective on Secondary Seed Dormancy in Arabidopsis thaliana. Plants. 2020; 9(6):749. https://doi.org/10.3390/plants9060749
Chicago/Turabian StyleBuijs, Gonda. 2020. "A Perspective on Secondary Seed Dormancy in Arabidopsis thaliana" Plants 9, no. 6: 749. https://doi.org/10.3390/plants9060749
APA StyleBuijs, G. (2020). A Perspective on Secondary Seed Dormancy in Arabidopsis thaliana. Plants, 9(6), 749. https://doi.org/10.3390/plants9060749



