Structural Adaptation and Physiological Mechanisms in the Leaves of Anthyllis vulneraria L. from Metallicolous and Non-Metallicolous Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants Collection
2.2. Determination of Trace Metal Content in the Substrate and Plant Tissues
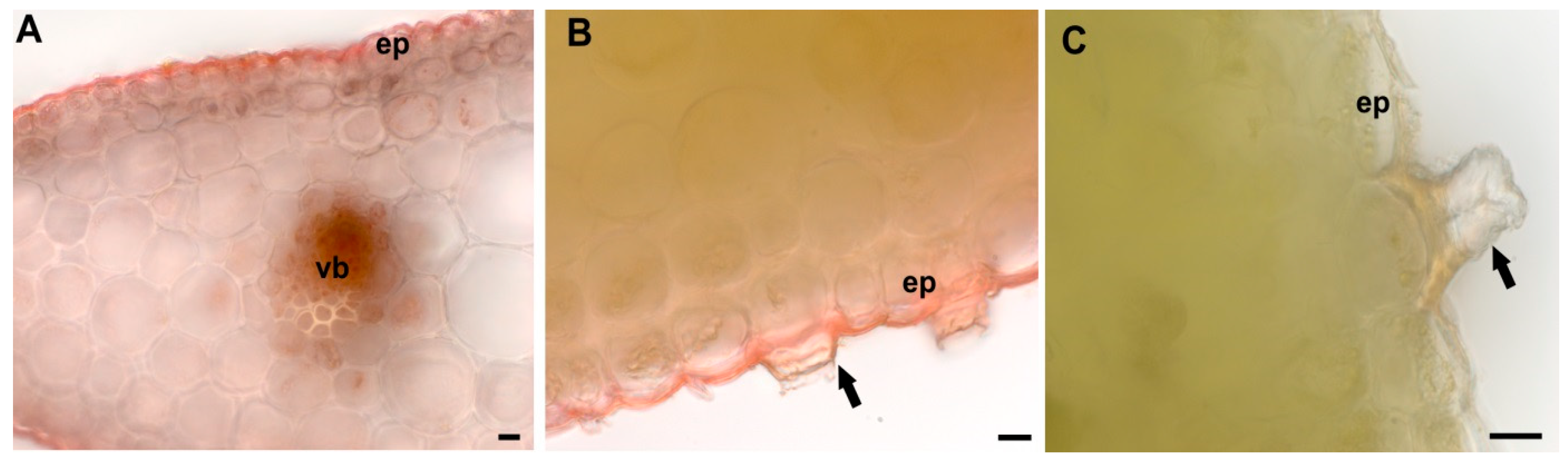
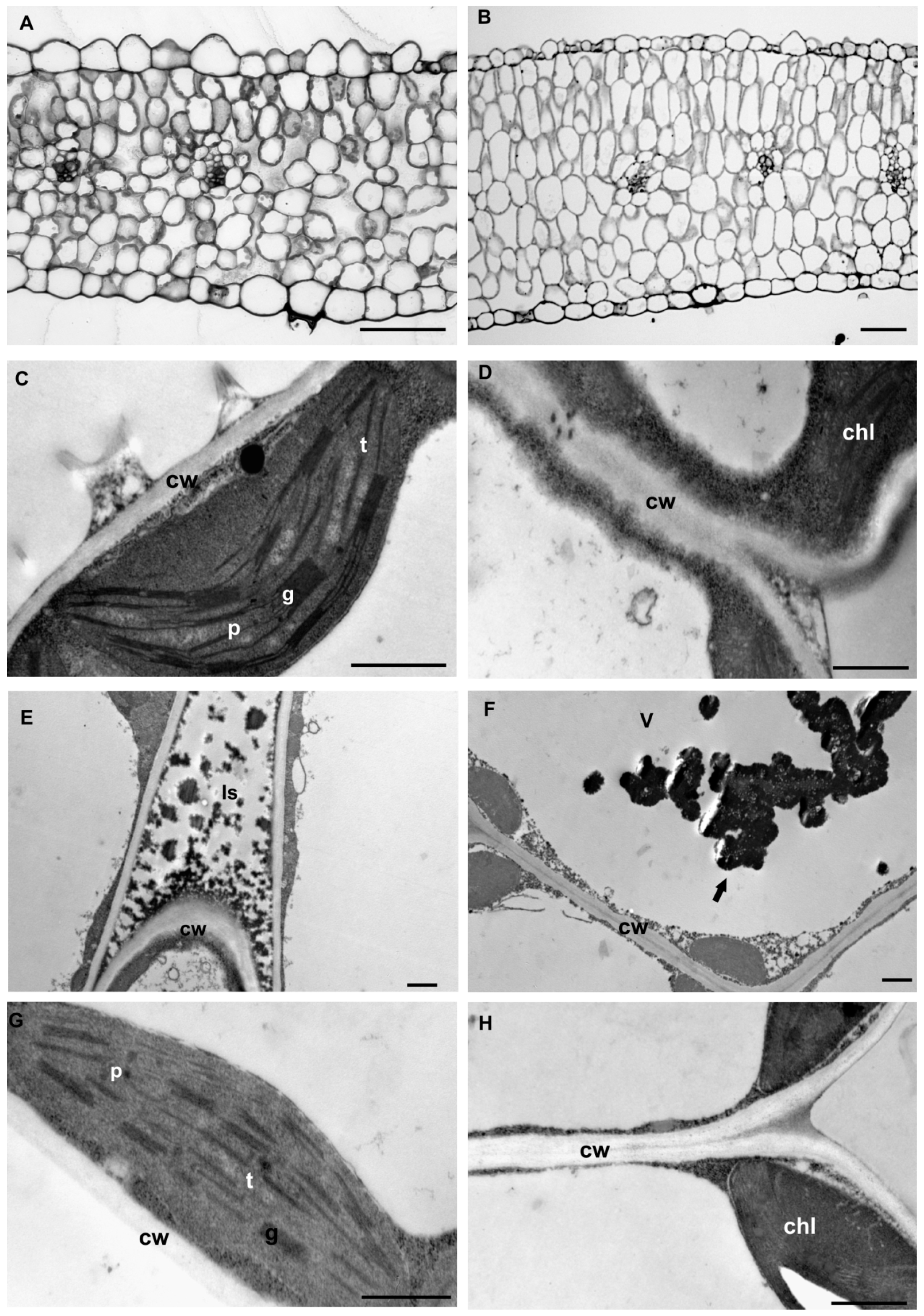
2.3. Leaf Structural Examination
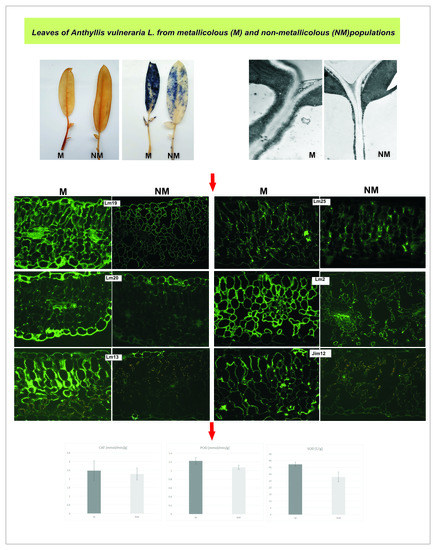
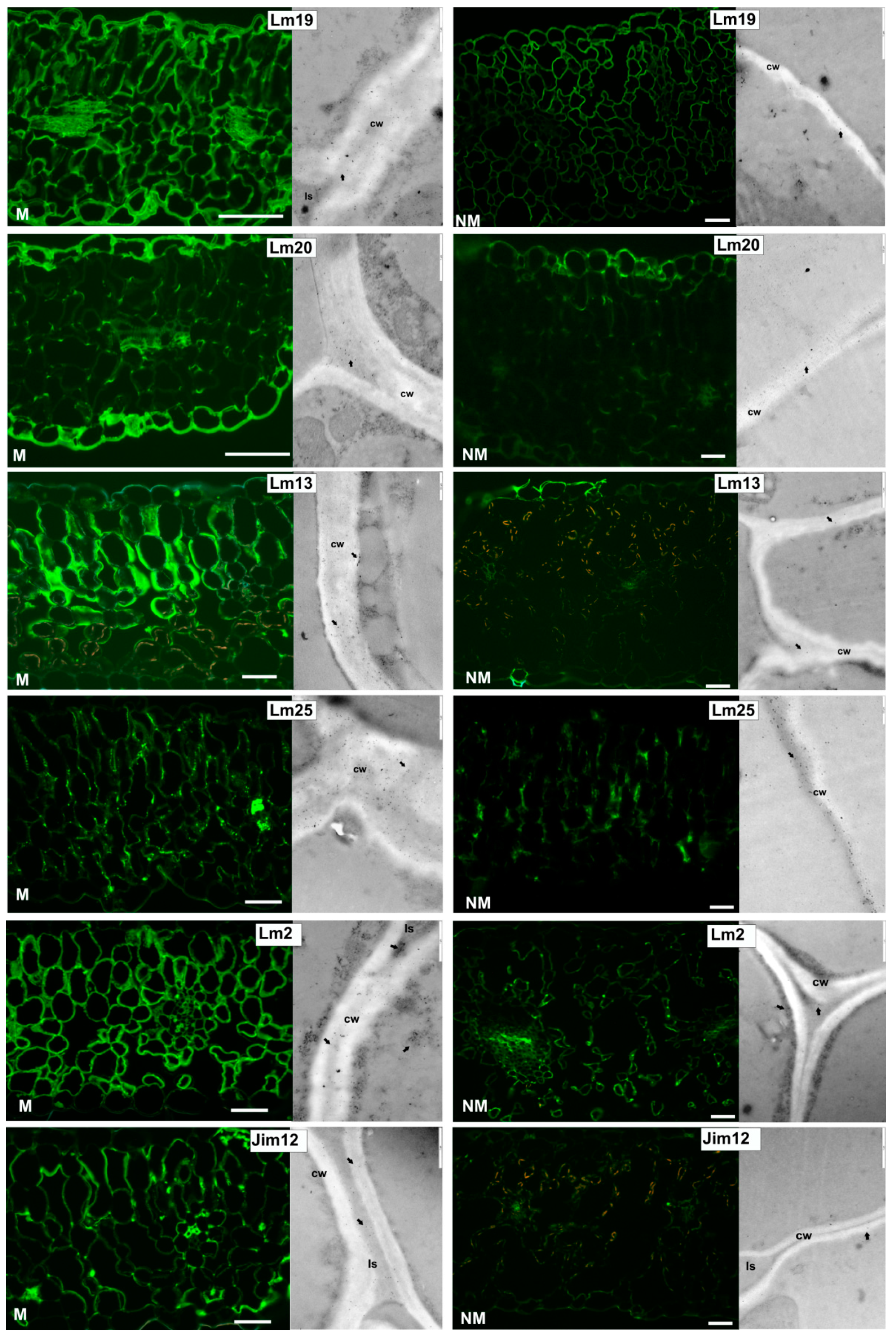
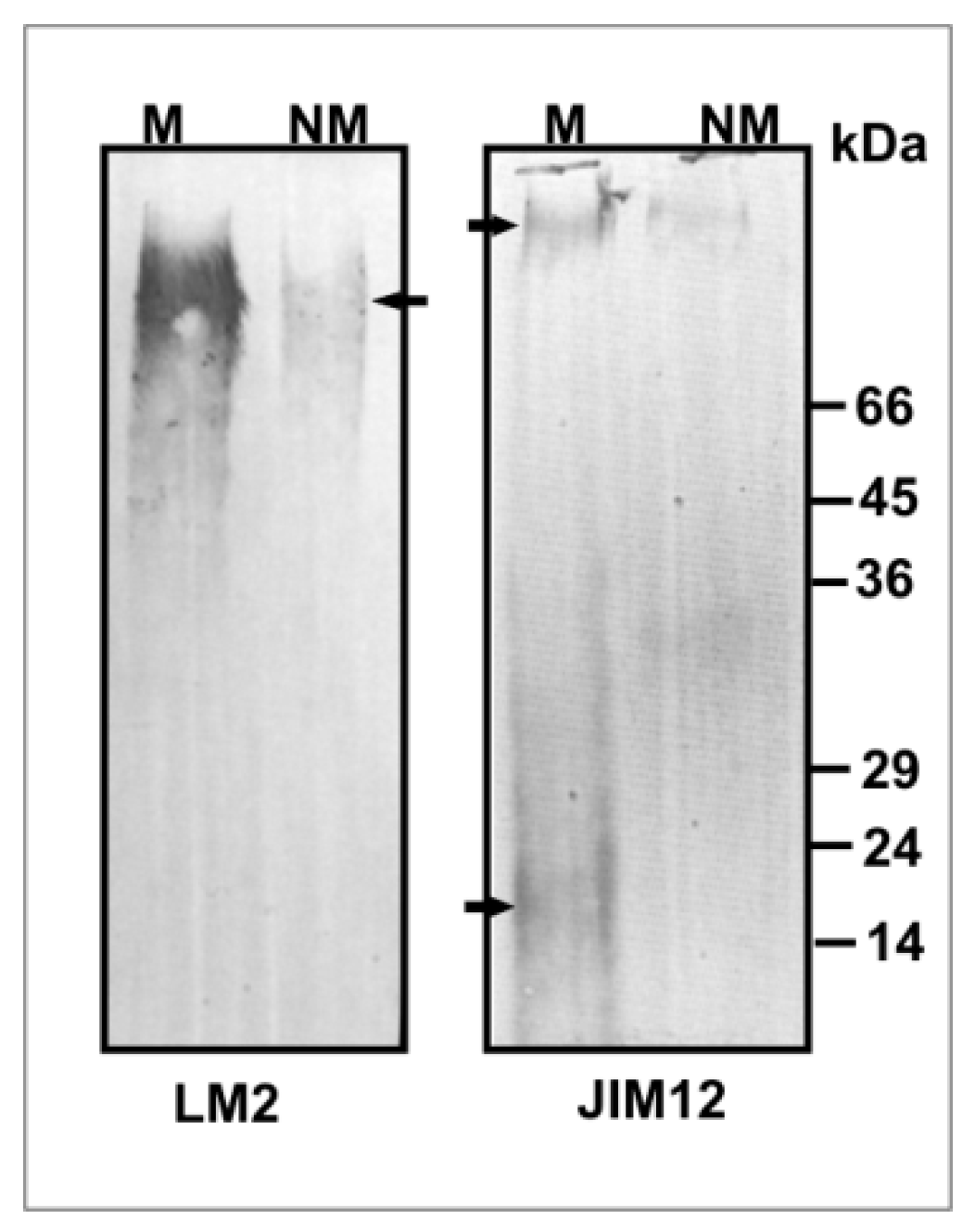
2.4. Cell Wall Component Immunolocalization
2.5. Electrophoresis and Western Blotting
2.6. Photosynthetic Pigment Content Estimation
2.7. Phenolic Compounds
2.8. Radical Scavenging Activity (RSA)
2.9. Thiobarbituric Acid-Reactive-Substances (TBARs)
2.10. ROS Accumulation
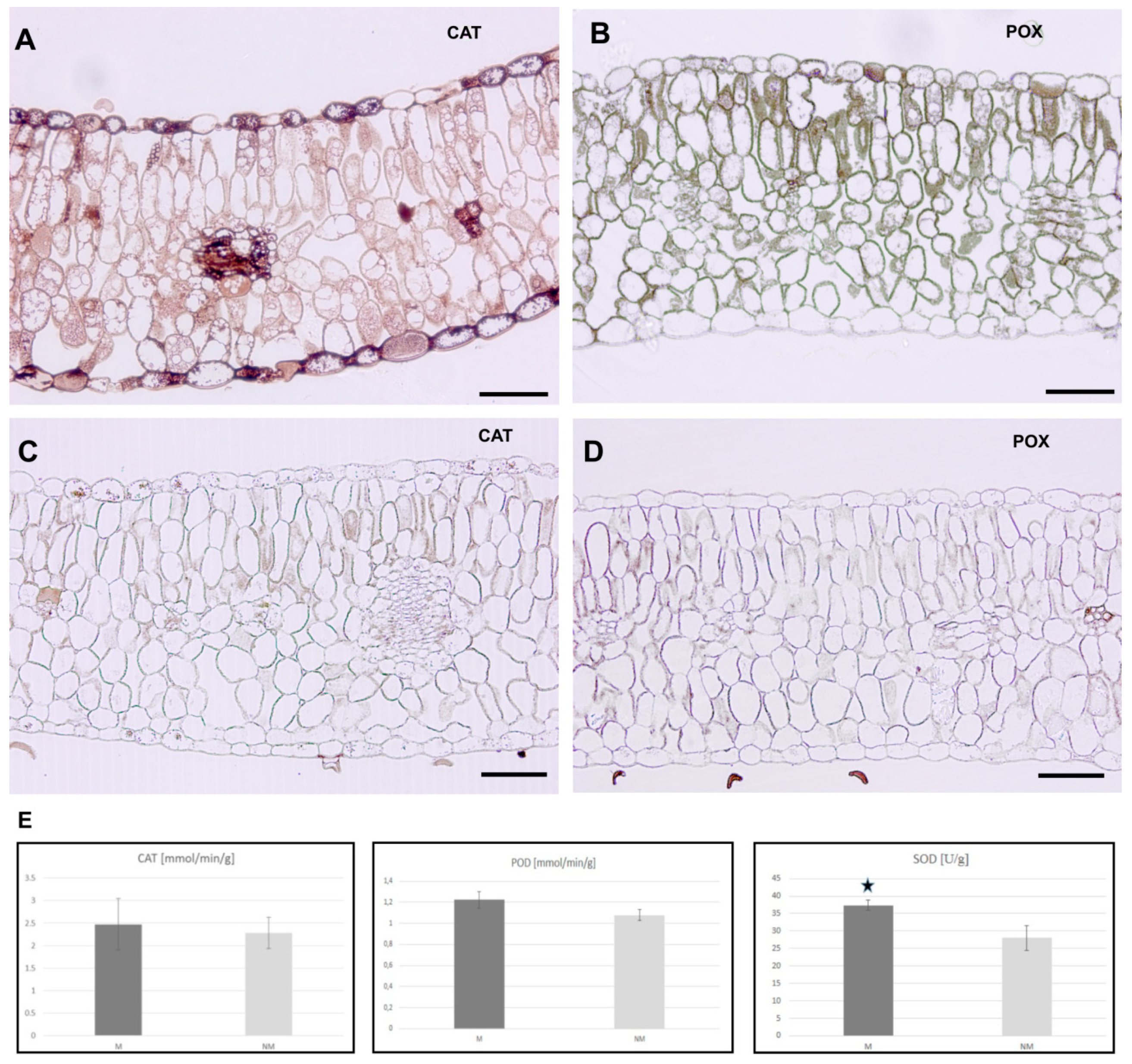
2.11. Antioxidant Enzymes
2.12. Statistical Analyses
3. Results
3.1. Trace Metal Content in the Soil and Plant Tissues
3.2. Leaf Anatomical and Ultrastructural Studies
3.3. Leaf Apoplast Alterations
3.4. ROS Accumulation
3.5. Activity of ROS Detoxifying Enzymes
3.6. Plant Vitality-Physiological Measurements
4. Discussion
4.1. Zn, Cd, and Pb Contents in the Soil and Their Accumulation in Plant Tissues
4.2. Leaf Anatomy
4.3. Leaf Apoplast Modifications
4.4. Plant Vitality
4.5. Activation of Antioxidant System under Metal Stress
4.6. Symbiotic Associations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGP | arabinogalactan protein |
| CAT | catalase |
| DW | dry weight |
| EXT | extensin |
| FW | fresh weight |
| TMs | trace metals |
| M | metallicolous ecotype |
| NM | non-metallicolous ecotype |
| POX | peroxidase |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TBARs | thiobarbituric acid-reactive-substances |
| TEM | transmission electron microscope |
| TF | translocation factor |
References
- Wójcik, M.; Sugier, P.; Siebielec, G. Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci. Total Environ. 2014, 487, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Grodzińska, K.; Szarek-Łukaszewska, G. Hałdy cynkowo-ołowiowe w okolicach Olkusza—Przeszłość, teraźniejszość i przyszłość. Kosmos 2002, 51, 127–138. [Google Scholar]
- Mahieu, S.; Frerot, H.; Vidal, C.; Galiana, A.; Heulin, K.; Maure, L.; Brunel, B.; Lefebvre, C.; Escarre, J.; Cleyet–Marel, J.-C. Anthyllis vulneraria/Mesorhizobium metallidurans, an efficient symbiotic nitrogen fixing association able to grow in mine tailings highly contaminated by Zn, Pb and Cd. Plant Soil. 2011, 342, 405–417. [Google Scholar] [CrossRef]
- Grison, C.M.; Jackson, S.; Merlot, S.; Dobson, A.; Grison, C. Rhizobium metallidurans sp. nov., a symbiotic heavy metal resistant bacterium isolated from the Anthyllis vulneraria Zn-hyperaccumulator. Int. J. Syst. Evol. Microbiol. 2015, 65, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Hanus-Fajerska, E.; Piwowarczyk, B.; Augustynowicz, J.; Ciarkowska, K.; Czech, T. From laboratory to field studies—The assessment of Biscutella laevigata suitability to biological reclamation of areas contaminated with lead and cadmium. Ecol. Environ. Saf. 2017, 142, 266–273. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Różańska, E.; Hanus-Fajerska, E.; Koszelnik-Leszek, A. Structural, physiological and genetic diversification of Silene vulgaris ecotypes from heavy metal-contaminated areas and their synchronous in vitro cultivation. Planta 2019, 249, 1761–1778. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Rostański, A. Microevolutionary changes in ecotypes of calamine waste heap vegetation near Olkusz, Poland: A review. Acta Biol. Crac. Ser. Bot. 2002, 44, 7–19. [Google Scholar]
- Sujkowska-Rybkowska, M.; Ważny, R. Metal resistant rhizobia and ultrastructure of Anthyllis vulneraria nodules from zinc and lead contaminated tailing in Poland. Int. J Phytoremed. 2018, 20, 709–720. [Google Scholar] [CrossRef]
- Turnau, K.; Ostachowicz, B.; Wojtczak, G.; Anielska, T.; Sobczyk, Ł. Metal uptake by xerothermic plants introduced into Zn-Pb industrial wastes. Plant Soil. 2010, 337, 299–311. [Google Scholar] [CrossRef]
- Ashfaque, F.; Inam, A.; Sahay, S.; Iqbal, S. Influence of heavy metal toxicity on plant growth, metabolism and its alleviation by phytoremediation-a promising technology. J. Agric. Ecol. Res. Int. 2016, 6, 1–19. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Kral, A. Ecotype-specific pathways of reactive oxygen species deactivation in facultative metallophyte Silene vulgaris (Moench) Garcke treated with heavy metals. Antioxidants 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shen, C.; Wang, Y.; Huang, C.; Shi, J. New insights into regulation of proteome and polysaccharide in cell wall of Elsholtzia splendens in response to copper stress. PLoS ONE 2014, 9, e109573. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Garrido, I.; Casimiro, I.; Espinosa, F. Effects of antimony on redox activities and antioxidant defense systems in sunflower (Helianthus annuus L.) plants. PLoS ONE 2017, 12, e0183991. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kärkönen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry. 2015, 112, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Labudda, M.; Różańska, E.; Hanus-Fajerska, E.; Znojek, E. Heavy metal tolerance in contrasting ecotypes of Alyssum montanum. Ecotoxicol. Environ. Saf. 2018, 161, 305–317. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Wójcik, A.; Tukiendorf, A. Strategy of stress avoidance in resistance of plants to heavy metals. Wiadomości Botaniczne 1995, 39, 33–40. [Google Scholar]
- Krzesłowska, M. The wall cell in plant cell response to trace metals: Polysaccharide remodelling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Vuletic, M.; Hadsi-Taskovic, S.; Sukalovic, V.; Markovic, K.; Kravic, N.; Vucinic, Z.; Maksimovic, V. Differential response of antioxidative systems of maize roots cell walls to osmotic and heavy metal stress. Plant Biol. 2014, 16, 88–96. [Google Scholar] [CrossRef]
- Zhu, X.F.; Shi, Y.Z.; Lei, G.J.; Fry, S.C.; Zhang, B.C.; Zhou, Y.H. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell. 2012, 24, 4731–4747. [Google Scholar] [CrossRef]
- Novaković, L.; Guo, T.; Bacic, A.; Sampathkumar, A.; Johnson, K.L. Hitting the wall-sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants 2018, 7, 89. [Google Scholar] [CrossRef]
- Pajuelo, E.; Carrasco, J.A.; Romero, L.C.; Chamber, M.A.; Gotor, C. Evaluation of the metal phytoextraction potential of crop legumes. Regulation of the expression of O-acetylserine (thiol) lyase under metal stress. Plant Biol. 2007, 9, 672–681. [Google Scholar] [CrossRef]
- Lafuente, A.; Pajuelo, E.; Caviedes, M.A.; Rodriguez-Llorente, I.D. Reduced nodulation in alfalfa induced by arsenic correlates with altered expression of early nodulins. J. Plant Physiol. 2010, 167, 286–291. [Google Scholar] [CrossRef]
- Escarre, J.; Lefèbvre, C.; Raboyeau, S.; Dossantos, A.; Gruber, W.; Cleyet-Marel, J.C.; Frérot, H.; Noret, N.; Mahieu, S.; Collin, C.; et al. Heavy metal concentration survey in soils and plants of the Les Malines Mining District (Southern France): Implications for soil restoration. Water Air Soil Pollut. 2011, 216, 485–504. [Google Scholar] [CrossRef]
- Bąk, B.; Szeląg, A.; Bojakowska, I.; Kwecko, P.; Tomassi-Morawiec, H.; Wojciechowska, K. Kazimierz Dolny (746). Prepared for the Ministry of Environment. PIG (in polish). 2010. [Google Scholar]
- Seregin, I.V.; Kozhevnikova, A.D. Histochemical methods for detection of trace metals and strontium in the tissues of higher plants. Russ. J. Plant Physiol. 2011, 58, 721–727. [Google Scholar] [CrossRef]
- Sujkowska-Rybkowska, M.; Borucki, W. Pectins esterification in the apoplast of aluminum-treated pea root nodules. J. Plant Physiol. 2015, 184, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, S.S.; Stöckmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Salzer, P.; Corbie‘re, H.; Boller, T. Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradices. Planta 1998, 208, 319–325. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, X.; Hu, K.; Wang, Y.; Wang, X.; Du, S.; Li, Y.; Hu, D.; Cheng, K.; An, B.; et al. Impaired magnesium protoporphyrin ix methyltransferase (chlm) impedes chlorophyll synthesis and plant growth in rice. Front. Plant Sci. 2017, 8, 1694. [Google Scholar] [CrossRef]
- Bestwick, C.S.; Brown, I.R.; Bennett, M.H.; Mansfield, J.W. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell. 1997, 9, 209–221. [Google Scholar]
- Frederick, S.E.; Newcomb, E.H. Cytochemical localization of catalase in leaf microbodies (peroxisomes). J. Cell Biol. 1969, 43, 343–353. [Google Scholar] [CrossRef]
- Olmos, E.; Hernández, J.A.; Sevilla, F.; Hellín, E. Induction of several antioxidant enzymes in the selection of a salt-tolerant cell line of Pisum sativum. J. Plant Physiol. 1994, 144, 594–598. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I. Superoxide-driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem. Int. 1989, 19, 1117–1124. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Lück, H. Peroxidase. In Methoden der enzymatischen Analyse; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1962; pp. 895–897. [Google Scholar]
- Ministry of the Environment. Journal of Laws, item 1395. Regulation of the Ministry of Environment dated 1 September 2016 on assessment procedures for the land surface pollution (in Polish). 2016. [Google Scholar]
- Wani, A.; Khan, S.; Zaidi, A. Impact of zinc-tolerant plant growth-promoting rhizobacteria on lentil grown in zinc-amended soil. Agron. Sustain. Dev. 2008, 28, 449–455. [Google Scholar] [CrossRef]
- Fernandez, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.R.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, North of Spain. J. Geochem. Exp. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Staňová, A.; Ďurišová, E.; Banásová, V.; Gurinová, E.; Nadubinská, M.; Kenderešová, L. Root system morphology and primary root anatomy in natural non-metallicolous and metallicolous populations of three Arabidopsis species differing in trace metal tolerance. Biologia 2012, 67, 505–516. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ. J. Plant Physiol. 2008, 55, 1–22. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil. 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Huguet, S.; Bert, V.; Laboudigue, A.; Barthes, V.; Isaure, M.-P.; Llorens, I.; Schat, H.; Sarret, G. Cd speciation and localization in the hyperaccumulator Arabidopsis halleri. Environ. Exp. Bot. 2012, 82, 54–65. [Google Scholar] [CrossRef]
- Huguet, S.; Soussou, S.; Cleyet-Marel, J.C.; Trcera, N.; Isaure, M.P. Rhizostabilzation of a mine tailing highly contaminated: Previous study of Cd localization and speciation in Anthyllis vulneraria. In Proceedings of the E3S Web of Conferences, Rome, Italy, 23 April 2012. [Google Scholar]
- Karnaukhova, N.A. Anatomo-morphological features of the leaves of Hedysarum theinum (Fabaceae) in Western Altai. Contemp. Prob. Ecol. 2016, 9, 349–354. [Google Scholar] [CrossRef]
- Zhao, F.J.; Lombi, E.; Breedon, T.; McGrath, S. Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ. 2000, 23, 507–514. [Google Scholar] [CrossRef]
- Gomes, M.P.; de Sá e Melo Marques, T.C.L.L.; de Oliveira, M.; Nogueira, G.; de Castro, E.M.; Soares, A.M. Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Sci. Agric. 2011, 68, 566–573. [Google Scholar] [CrossRef]
- Probst, A.; Liu, H.; Fanjul, M.; Liao, B.; Hollande, E. Response of Vicia faba L. to metal toxicity on mine tailing substrate: Geochemical and morphological changes in leaf and root. Environ. Exp. Bot. 2009, 66, 297–308. [Google Scholar] [CrossRef]
- Liepman, A.H.; Wightman, R.; Geshi, N.; Turner, S.R.; Scheller, H.V. Arabidopsis a powerful model system for plant cell wall research. Plant J. 2010, 61, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Plazanet, I. Plant cell wall, a challenge for its characterisation. Adv. Biol. Chem. 2016, 6, 70–105. [Google Scholar] [CrossRef][Green Version]
- Douchiche, O.; Driouich, A.; Morvan, C. Spatial regulation of cell wall structure in response to heavy metal stress: Cadmium-induced alteration of the methyl-esterification pattern of homogalacturonans. Ann. Bot. 2010, 105, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Louvet, R.; Johansen, J.N.; Höfte, H.; Laufs, P.; Pelloux, J.; Mouille, G. Arabidopsis phyllotaxis is controlled by the methylesterification status of cell-wall pectins. Curr. Biol. 2008, 18, 1943–1948. [Google Scholar] [CrossRef]
- Eticha, D.; Stass, A.; Horst, W.J. Cell-wall pectin and its degree of methylation in the maize root apex: Significance for genotypic differences in aluminium resistance. Plant Cell Environ. 2005, 28, 1410–1420. [Google Scholar] [CrossRef]
- Baldwin, T.C.; McCann, M.C.; Roberts, K. A novel hydroxyproline-deficient arabinogalactan protein secreted by suspension-cultured cells of Daucus carota. Purification and partial characterization. Plant Physiol. 1993, 103, 115–123. [Google Scholar] [CrossRef]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-proteins: Key regulators at the cell surface? Plant Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C. An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell. 2013, 25, 270–287. [Google Scholar] [CrossRef]
- Baluska, F.; Samaj, J.; Wojtaszek, P.; Volkmann, D.; Menzel, D. Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol. 2003, 133, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Kjellbom, P.; Snogerup, L.; Stohr, C.; Reuzeau, C.; McCabe, P.F.; Pennell, R.I. Oxidative cross-linking of plasma membrane arabinogalactan proteins. Plant J. 1997, 12, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Roberts, K. The biology of arabinogalactan proteins. Ann. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Kenjebaeva, S.; Yamamoto, Y.; Matsumoto, H. The impact of aluminium on the distribution of the cell wall glycoproteins of the pea root tip and their Al-binding capacity. Soil Sci. Plant Nutr. 2001, 47, 629–636. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Pereira, C.S.; Soares, N.C.; Vieira, A.M.; Feijó, J.A.; Jackson, P.A. The contribution of extensin network formation to rapid, hydrogen peroxide-mediated increases in grapevine callus wall resistance to fungal lytic enzymes. J. Exp. Bot. 2006, 57, 2025–2035. [Google Scholar] [CrossRef]
- Fry, S.C. Primary cell wall metabolism: Tracking the careers of wall polymers in living plant cells. New Phytol. 2004, 161, 641–675. [Google Scholar] [CrossRef]
- Nuñez, A.; FisTMan, M.L.; Fortis, L.L.; Cooke, P.H.; Hotchkiss, A.T.J. Identification of extensin protein associated with sugar beet pectin. J. Agric. Food Chem. 2009, 57, 10951–10958. [Google Scholar] [CrossRef]
- Kaftan, D.; Brumfeld, V.; Nevo, R.; Scherz, A.; Reich, Z. From chloroplasts to photosystems: In situ scanning force microscopy on intact thylakoid membranes. EMBO J. 2002, 21, 6146–6153. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Bartoli, C.G.; Beltrano, J.; Guiamet, J.J.; Araus, J.L. Oxidative damage to thylakoid proteins in water stressed leaves of wheat (Triticum aestivum L.). Physiol. Plant. 2000, 108, 398–404. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Young, J. The photoprotective role of carotenoids in higher plants. Physiol Plant. 1991, 83, 702–708. [Google Scholar] [CrossRef]
- Schmidt, É.C.; Felix, M.R.; Kreusch, M.G.; Pereira, D.T.; Costa, G.B.; Simioni, C.; Ouriques, L.C.; Steiner, N.; Chow, F.; Floh, E.S.L.; et al. Profiles of carotenoids and aminoacids and total phenolic compounds of the red alga Pterocladiella capillacea exposed to cadmium and different salinities. J. Appl. Phycol. 2016, 28, 1955–1963. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Bidel, L.P.R.; Urban, L. Carotenoid responses to environmental stimuli: Integrating redox and carbon controls into a fruit model. Plant Cell Environ. 2014, 37, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Meng, L.; Zhang, Y.N.; Mao, P.C.; Tian, X.X.; Li, S.S.; Zhang, L. Antioxidative systems, metal ion homeostasis and cadmium distribution in Iris lactea exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2017, 139, 50–55. [Google Scholar] [CrossRef]
- Gupta, V.; Jatav, P.K.; Verma, R.; Kothari, S.L.; Kachhwaha, S. Nickel accumulation and its effect on growth, physiological and biochemical parameters in millets and oats. Environ. Sci. Pollut. Res. 2017, 24, 23915–23925. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Ranieri, A.; Castagna, A.; Pacini, J.; Baldan, B.; Mensuali Sodi, A.; Soldatini, G.F. Early production and scavenging of hydrogen peroxide in the apoplast of sunflowers plants exposed to ozone. J. Exp. Bot. 2003, 54, 2529–2540. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M. Multiple functional roles of anthocyanins in plant–environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membranes fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation tress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Fukuda, H. Tracheary element differentiation. Plant Cell. 1997, 9, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular mycorrhiza and trace metal tolerance. Phytochemistry 2007, 68, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sujkowska-Rybkowska, M.; Banasiewicz, J.; Rekosz-Burlaga, H.; Stępkowski, T. Anthyllis vulneraria and Lotus corniculatus on calamine heaps form nodules with Bradyrhizobium liaoningense-related strains harboring novel in Europe symbiotic nifD haplotypes. App. Soil Ecol. 2020, 151, 103539. [Google Scholar] [CrossRef]

| M | NM | |||||
|---|---|---|---|---|---|---|
| Shoots | Roots | TF | Shoots | Roots | TF | |
| Zn | 2115.03 a * ± 65.7 | 3857.90 a ± 137.84 | 0.5 | 10.10 b ± 1.04 | 18.87 b ± 2.16 | 0.5 |
| Pb | 75.88 a ± 20.74 | 252.68 a ± 32.45 | 0.3 | 1.23 b ± 0.57 | 2.99 b ± 0.28 | 0.4 |
| Cd | 18.90 a ± 5.10 | 59.50 a ± 0.81 | 0.3 | 0.08 b ± 0.01 | 0.25 b ± 0.02 | 0.3 |
| Analysed Traits | M | NM |
|---|---|---|
| Total thickness (µm) | 206.0 b * ± 22.38 | 273.7 a ± 38.25 |
| Number of palisade cell layers | 2 | 3 |
| Thickness of palisade layer (µm) | 65.1 b ± 8.03 | 102.9 a ± 22.9 |
| Length of palisade parenchyma cells (µm) | 36.2 b ± 1.32 | 45.9 a ± 7.42 |
| Number of spongy cell layers | 2–3 | 3 |
| Thickness of spongy layer (µm) | 54.3 b ± 8.2 | 79.7 a ± 1,29 |
| Length of spongy parenchyma cells (µm) | 22.7 b ± 3.8 | 28.6 a ± 4.89 |
| Thickness of adaxial epidermis (µm) | 23.8 a ± 4.35 | 20.4 b ± 2.27 |
| Stomata number in adaxial epidermis | 4.0 b ± 0.91 | 5.0 a ± 1.13 |
| Thickness of abaxial epidermis (µm) | 22.1 a ± 4.51 | 19.1 b ± 2.24 |
| Stomata number in abaxial epidermis | 2.1 b ± 0.81 | 4.6 a ± 1.14 |
| Parameter | Ecotype | ||
|---|---|---|---|
| Metallicolous | Non-Metallicolous | ||
| Photosynthetic pigments [mg g−1 FW] | Chlorophyll a | 0.76 a * ± 0.146 | 0.49 b ± 0.049 |
| Chlorophyll b | 0.24 a ± 0.012 | 0.14 b ± 0.013 | |
| Chlorophyll a + b | 1.01a ± 0.158 | 0.63 b ± 0.061 | |
| Chlorophyll a/b | 3.10 a ± 0.453 | 3.46 a ± 0.157 | |
| Carotenoids | 0.21 b ± 0.004 | 0.15 b ± 0.012 | |
| Phenolic compounds [mg 100 g−1 FW] | Total phenols | 1294.21 a ± 20.136 | 846.69 b ± 36.304 |
| Phenylpropanoids | 365.59 a ± 6.789 | 249.92 b ± 9.441 | |
| Flavonols | 511.35 a ± 14.452 | 338.94 b ± 13.41 | |
| Anthocyanins | 177.61 a ± 14.78 | 53.42 b ± 9.09 | |
| Radical scavenging activity DPPH [%] | 20.42 a ± 1.54 | 20.67 a ± 0.94 | |
| Lipid peroxidation level [µmol TBARs g– 1 FW] | 73.01 a ± 2.73 | 60.95 b ± 1.12 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujkowska-Rybkowska, M.; Muszyńska, E.; Labudda, M. Structural Adaptation and Physiological Mechanisms in the Leaves of Anthyllis vulneraria L. from Metallicolous and Non-Metallicolous Populations. Plants 2020, 9, 662. https://doi.org/10.3390/plants9050662
Sujkowska-Rybkowska M, Muszyńska E, Labudda M. Structural Adaptation and Physiological Mechanisms in the Leaves of Anthyllis vulneraria L. from Metallicolous and Non-Metallicolous Populations. Plants. 2020; 9(5):662. https://doi.org/10.3390/plants9050662
Chicago/Turabian StyleSujkowska-Rybkowska, Marzena, Ewa Muszyńska, and Mateusz Labudda. 2020. "Structural Adaptation and Physiological Mechanisms in the Leaves of Anthyllis vulneraria L. from Metallicolous and Non-Metallicolous Populations" Plants 9, no. 5: 662. https://doi.org/10.3390/plants9050662
APA StyleSujkowska-Rybkowska, M., Muszyńska, E., & Labudda, M. (2020). Structural Adaptation and Physiological Mechanisms in the Leaves of Anthyllis vulneraria L. from Metallicolous and Non-Metallicolous Populations. Plants, 9(5), 662. https://doi.org/10.3390/plants9050662