Are Reproductive Traits Related to Pollen Limitation in Plants? A Case Study from a Central European Meadow
Abstract
1. Introduction
2. Results
2.1. Seed Production and Seed Weight
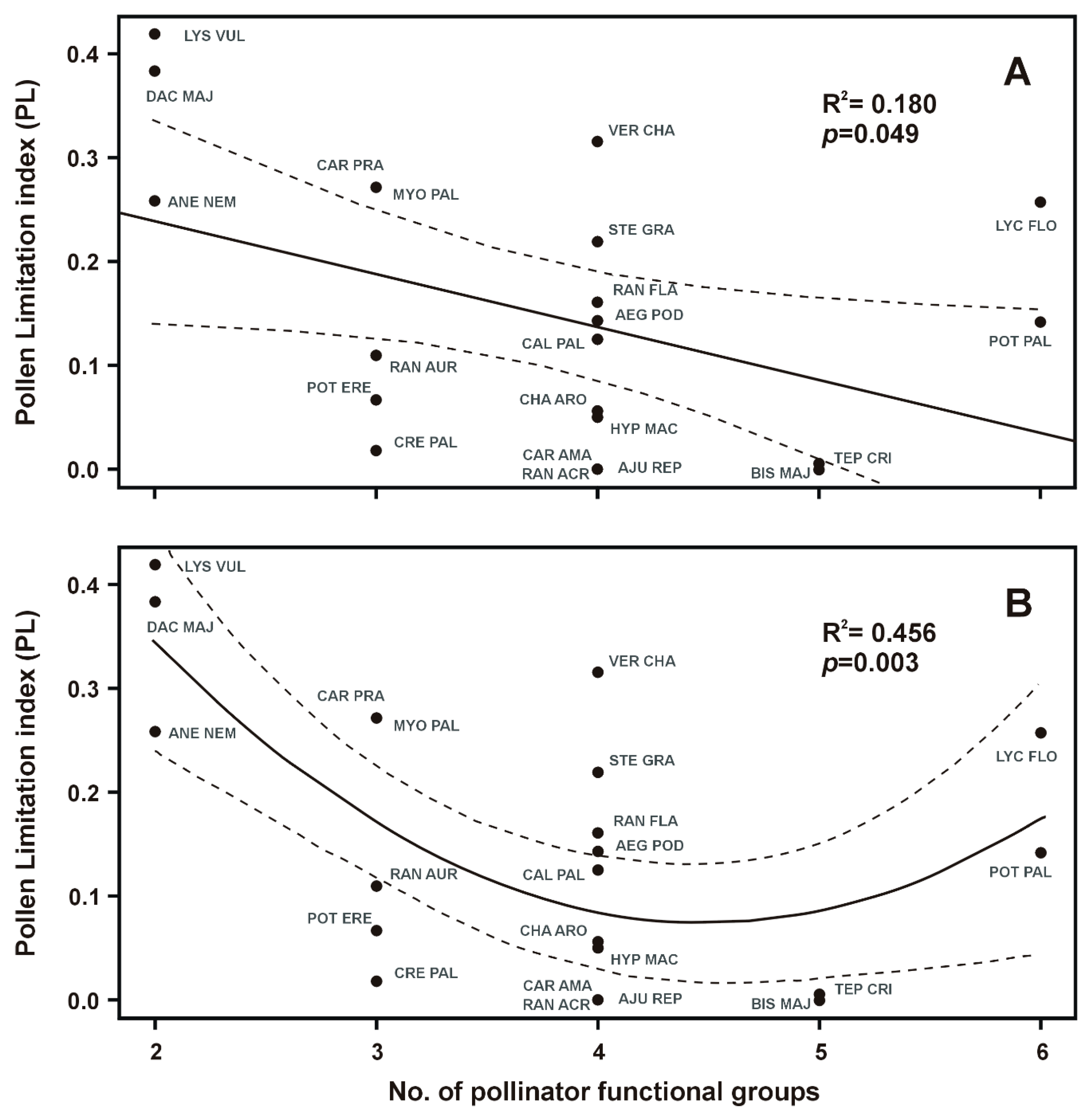
2.2. Traits Correlations
3. Discussion
- (1)
- Effect of sampling size and experimental design. Using power tests (via simulation) for pollen supplementation experiments, Thomson [23] illustrated that moderate pollination deficits of up to 15% will usually not be detected with sample sizes of 20 individuals, and even 40 are insufficient for minor deficits. But, unfortunately, lower sampling effort (such as 20–30 individuals in our study) is an inevitable result of various logistic constrains and trade-offs between the data quantity and quality in most community studies [13,18,21,24].
- (2)
- Publication bias. The community approach, where multiple plant species are studied simultaneously, may lead to a better understanding of patterns in pollen limitation. It is because environmental characteristics, such as nutrient levels within a given community, are relatively homogenous in such studies and the role of plant traits in pollen limitation can, therefore, be better assessed. Nevertheless, there have been few studies focused on the relationships between plant traits and pollen limitation across whole communities [18,21,24,25,26]. All these studies recorded lower levels of pollen limitation in natural systems compared to the pollen limitation documented in comprehensive reviews that are mostly based on single-species studies [1,10]. Therefore, the publication bias, favoring statistically significant responses which then become available for further studies, together with the omission of “grey literature” and studies not written in English [27], complicates our understanding of pollen limitation [14].
- (3)
- Effect of pollinator abundance. Hegland and Totland [21] discussed their results of low pollen limitation in the context of a possible higher pollinator abundance in the studied community, which could substantially reduce the quantitative pollen limitation. A partial cause of low pollen limitation in our study could be that the targeted semi-natural locality is situated in a relatively well-preserved and mosaic-like landscape with a limited influence of intensive agriculture. Such semi-natural, diverse, and heterogeneous environments support pollination services [28,29] and thus increase the plant reproductive success, as suggested by Bennett et al. [13] in their Romanian meadow community.
- (4)
- Effect of plant community composition and study species selection. In our investigated community, only a few plant species with morphologically highly specialized flowers, which are expected to be more prone to pollen limitation, were present. Therefore, this community may have a lower pollen limitation than communities with a greater proportion of specialized flowers.
- (5)
- Choice of the pollen limitation measure. An important factor determining the recorded magnitude of pollen limitation may also be the choice of its measure. Knight et al. [14] compared 263 studies working with different measurements of the production component of reproduction and revealed the largest effect for relative fruit set, and the lowest effect for production of seeds/flower and seeds/fruit. However, because the magnitude of pollen limitation was inter-correlated among these response variables, Knight et al. [14] assumed that pollen limitation occurs simultaneously at different stages of the plant reproduction, but with varying intensity. Also in our study the numbers of pollen-limited species varied substantially between the two applied measures, seed production and seed mass. Furthermore, Hegland and Totland [21] pointed out that the two main components of plant reproductive success, seed production and seed mass, are often not included in the same studies.
4. Materials and Methods
4.1. Study Site
4.2. Pollen Limitation
4.3. Plants Traits
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ashman, T.-L.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef]
- Alonso, C.; Vamosi, J.C.; Knight, T.M.; Steets, J.A.; Ashman, T.L. Is reproduction of endemic plant species particularly pollen limited in biodiversity hotspots? Oikos 2010, 119, 1192–1200. [Google Scholar] [CrossRef]
- Castro, S.; Dostálek, T.; van der Meer, S.; Oostermeijer, G.; Münzbergová, Z. Does pollen limitation affect population growth of the endangered Dracocephalum austriacum L.? Popul. Ecol. 2015, 57, 105–116. [Google Scholar] [CrossRef]
- Janečková, P.; Janeček, Š.; Bartoš, M.; Hrázský, Z. Reproductive system of the critically endangered taxon Gentianella praecox subsp. bohemica. Preslia 2019, 91, 77–92. [Google Scholar] [CrossRef]
- Haig, D.; Westoby, M. On limits to seed production. Am. Nat. 1988, 131, 757–759. [Google Scholar] [CrossRef]
- Charnov, E.L. The Theory of Sex Allocation; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Wilson, P.; Thomson, J.D.; Stanton, M.L.; Rigney, L.P. Beyond floral Batemania: Gender biases in selection for pollination success. Am. Nat. 1994, 143, 283–296. [Google Scholar] [CrossRef]
- Burd, M. Bateman’s principle and plant reproduction: The role of pollen limitation in fruit and seed set. Bot. Rev. 1994, 60, 83–139. [Google Scholar] [CrossRef]
- Larson, B.M.; Barrett, S.C. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc. 2000, 69, 503–520. [Google Scholar] [CrossRef]
- Knight, T.M.; Steets, J.A.; Vamosi, J.C.; Mazer, S.J.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mitchell, R.J.; Ashman, T.L. Pollen limitation of plant reproduction: Pattern and process. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 467–497. [Google Scholar] [CrossRef]
- Bennett, J.M.; Thompson, A.; Goia, I.; Feldmann, R.; Ştefan, V.; Bogdan, A.; Rakosy, D.; Beloiu, M.; Biro, I.-B.; Bluemel, S.; et al. A review of European studies on pollination networks and pollen limitation, and a case study designed to fill in a gap. AoB Plants 2018, 10, ply068. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.M.; Steets, J.A.; Ashman, T.L. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am. J. Bot. 2006, 93, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Burd, M.; Fan, Z. Resource allocation and seed size selection in perennial plants under pollen limitation. Am. Nat. 2017, 190, 430–441. [Google Scholar] [CrossRef]
- Vamosi, J.C.; Knight, T.M.; Steets, J.A.; Mazer, S.J.; Burd, M.; Ashman, T.L. Pollination decays in biodiversity hotspots. Proc. Natl. Acad. Sci. USA 2006, 103, 956–961. [Google Scholar] [CrossRef]
- García-Camacho, R.; Totland, Ø. Pollen limitation in the alpine: A meta-analysis. Arct. Antarct. Alp. Res. 2009, 41, 103–111. [Google Scholar] [CrossRef]
- Lázaro, A.; Lundgren, R.; Totland, Ø. Pollen limitation, species’ floral traits and pollinator visitation: Different relationships in contrasting communities. Oikos 2015, 124, 174–186. [Google Scholar] [CrossRef]
- Brys, R.; Geens, B.; Beeckman, T.; Jacquemyn, H. Differences in dichogamy and herkogamy contribute to higher selfing in contrasting environments in the annual Blackstonia perfoliata (Gentianaceae). Ann. Bot. 2013, 111, 651–661. [Google Scholar] [CrossRef]
- Vallejo-Marín, M.; O’Brien, H.E. Correlated evolution of self-incompatibility and clonal reproduction in Solanum (Solanaceae). New Phytol. 2007, 173, 415–421. [Google Scholar] [CrossRef]
- Hegland, J.S.; Totland, Ø. Is the magnitude of pollen limitation in a plant community affected by pollinator visitation and plant species specialisation levels? Oikos 2008, 117, 883–891. [Google Scholar] [CrossRef]
- Burd, M. The Haig-Westoby model revisited. Am. Nat. 2008, 171, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.D. Using pollination deficits to infer pollinator declines: Can theory guide us? Conserv. Ecol. 2001, 5, 6. [Google Scholar] [CrossRef]
- Wolowski, M.; Ashman, T.L.; Freitas, L. Community-wide assessment of pollen limitation in hummingbird-pollinated plants of a tropical montane rain forest. Ann. Bot. 2013, 112, 903–910. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Motten, A.F. Pollination ecology of the spring wildflower community of a temperate deciduous forest. Ecol. Monogr. 1986, 56, 21–42. [Google Scholar] [CrossRef]
- González, A.V.; Pérez, F. Pollen limitation and reproductive assurance in the flora of the coastal Atacama Desert. Int. J. Plant Sci. 2010, 171, 607–614. [Google Scholar] [CrossRef]
- Auger, P. Information Sources in Grey Literature; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2017. [Google Scholar]
- Steffan-Dewenter, I.; Westphal, C. The interplay of pollinator diversity, pollination services and landscape change. J. Appl. Ecol. 2008, 45, 737–741. [Google Scholar] [CrossRef]
- Viana, B.F.; Boscolo, D.; Mariano Neto, E.; Lopes, L.E.; Lopes, A.V.; Ferreira, P.A.; Pigozzo, C.M.; Primo, L.M. How well do we understand landscape effects on pollinators and pollination services? J. Pollinat. Ecol. 2012, 7, 31–41. [Google Scholar] [CrossRef]
- Ågren, J.; Fortunel, C.; Ehrlén, J. Selection on floral display in insect-pollinated Primula farinosa: Effects of vegetation height and litter accumulation. Oecologia 2006, 150, 225–232. [Google Scholar] [CrossRef]
- Aizen, M.A.; Harder, L.D. Expanding the limits of the pollen-limitation concept: Effects of pollen quantity and quality. Ecology 2007, 88, 271–281. [Google Scholar] [CrossRef]
- Zimmerman, M.; Pyke, G.H. Reproduction in Polemonium: Assessing the factors limiting seed set. Am. Nat. 1988, 131, 723–738. [Google Scholar] [CrossRef]
- Wesselingh, R.A. Pollen limitation meets resource allocation: Towards a comprehensive methodology. New Phytol. 2007, 174, 26–37. [Google Scholar] [CrossRef]
- Schaeffler, I.; Balao, F.; Dötterl, S. Floral and vegetative cues in oil-secreting and non-oil-secreting Lysimachia species. Ann. Bot. 2012, 110, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Jersáková, J.; Johnson, S.D.; Kindlmann, P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 2006, 81, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Schemske, D.W.; Willson, M.F.; Melampy, M.N.; Miller, L.J.; Verner, L.; Schemske, K.M.; Best, L.B. Flowering ecology of some spring woodland herbs. Ecology 1978, 59, 351–366. [Google Scholar] [CrossRef]
- Wolowski, M.; Ashman, T.L.; Freitas, L. Meta-analysis of pollen limitation reveals the relevance of pollination generalization in the Atlantic forest of Brazil. PLoS ONE 2014, 9, e89498. [Google Scholar] [CrossRef] [PubMed]
- Rodger, J.G.; Ellis, A.G. Distinct effects of pollinator dependence and self-incompatibility on pollen limitation in South African biodiversity hotspots. Biol. Lett. 2016, 12, 20160253. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.M.; Abdelaziz, M.; Lorite, J.; Jesús Muñoz-Pajares, A.; Perfectti, F. Changes in pollinator fauna cause spatial variation in pollen limitation. J. Ecol. 2010, 98, 1243–1252. [Google Scholar] [CrossRef]
- Young, H.J. Differential importance of beetle species pollinating Dieffenbachia longispatha (Araceae). Ecology 1988, 69, 832–844. [Google Scholar] [CrossRef]
- Morris, W.F.; Vázquez, D.P.; Chacoff, N.P. Benefit and cost curves for typical pollination mutualisms. Ecology 2010, 91, 1276–1285. [Google Scholar] [CrossRef]
- Moravec, J.; Balaťová-Tuláčková, E.; Blažková, D.; Hadač, E.; Hejný, S.; Husák, Š.; Jenıík, J.; Kolbek, J.; Krahulec, F.; Kropáč, Z.; et al. Rostlinná společenstva České republiky a jejich ohrožení [Red list of plant communities of the Czech Republic and their endangerment]. Ed. 2. Severočes. Přír. Suppl. 1995, 28, 1–206. [Google Scholar]
- Bartoš, M.; Janeček, Š.; Janečková, P.; Padyšáková, E.; Tropek, R.; Götzenberger, L.; Klomberg, Y.; Jersáková, J. Self-compatibility and autonomous selfing of plants in meadow communities. Plant Biol. 2020, 22, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.; Kühn, I.; Durka, W. BIOFLOR—A database on biological and ecological traits of vascular plants in Germany. Schriftenreihe für Vegetationskunde 2002, 38, 1–334. [Google Scholar]
- Klimešová, J.; Danihelka, J.; Chrtek, J.; de Bello, F.; Herben, T. CLO-PLA: A database of clonal and bud bank traits of Central European flora. Ecology 2017, 98, 1179. [Google Scholar] [CrossRef] [PubMed]
- Morrant, D.S.; Schumann, R.; Petit, S. Field methods for sampling and storing nectar from flowers with low nectar volumes. Ann. Bot. 2009, 103, 533–542. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. Pollen limitation and its effect on seed germination. Seed Sci. Res. 2018, 28, 253–260. [Google Scholar] [CrossRef]
- Young, H.J.; Young, T.P. Alternative outcomes of natural and experimental high pollen loads. Ecology 1992, 73, 639–647. [Google Scholar] [CrossRef]
- Thomson, D.M. Effects of long-term variation in pollinator abundance and diversity on reproduction of a generalist plant. J. Ecol. 2019, 107, 491–502. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.5.3; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Vamosi, J.C.; Steets, J.A.; Ashman, T.L. Drivers of pollen limitation: Macroecological interactions between breeding system, rarity, and diversity. Plant. Ecol. Divers. 2013, 6, 171–180. [Google Scholar] [CrossRef]
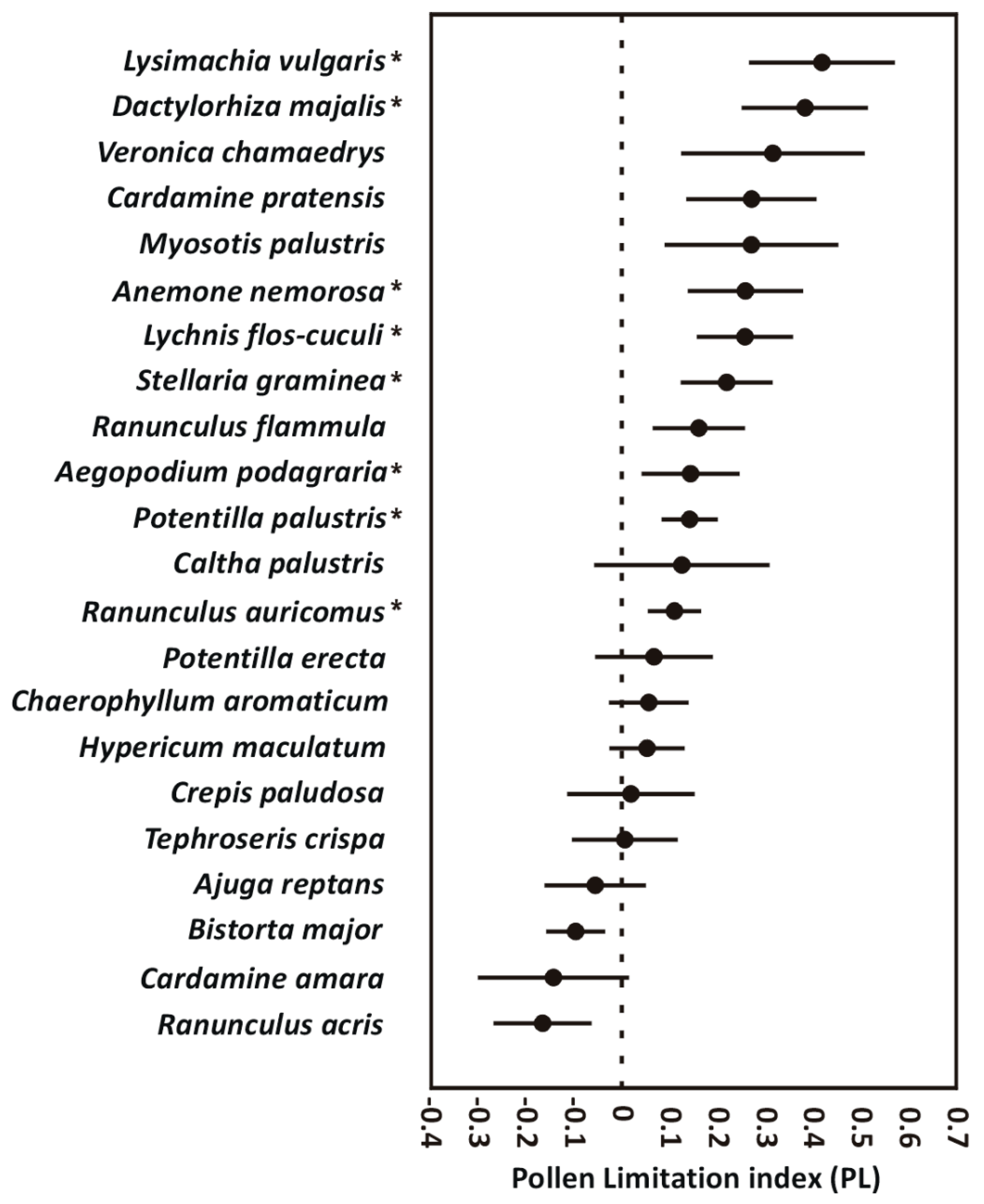
| No. of Seeds | Weight of a Seed (μg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Control | Supplemented | PERMANOVA | Control | Supplemented | PERMANOVA | ||||||
| Species | Mean | (std.dev) | Mean | (std.dev) | Pseudo F | p | Mean | (std.dev) | Mean | (std.dev) | Pseudo F | p |
| Apiaceae | ||||||||||||
| Aegopodium podagraria | 16.76 | (±8.85) | 21.00 | (±8.91) | 3.75 | 0.040 * | 1.64 | (±0.82) | 1.95 | (±0.90) | 1.32 | 0.138 |
| Chaerophyllum aromaticum | 20.63 | (±11.8) | 20.74 | (±9.3) | 0.00 | 0.479 | 2.33 | (±1.06) | 2.45 | (±0.92) | 0.75 | 0.218 |
| Asteraceae | ||||||||||||
| Crepis paludosa | 30.07 | (±13.56) | 30.20 | (±10.77) | 0.00 | 0.487 | 0.37 | (±0.21) | 0.34 | (±0.17) | 0.15 | 0.363 |
| Tephroseris crispa | 55.95 | (±30.02) | 52.60 | (±16.07) | 0.24 | 0.319 | 0.24 | (±0.11) | 0.26 | (±0.11) | 0.44 | 0.269 |
| Boraginaceae | ||||||||||||
| Myosotis palustris | 1.25 | (±1.22) | 1.83 | (±1.34) | 3.01 | 0.063 | 0.23 | (±0.20) | 0.26 | (±0.17) | 0.16 | 0.360 |
| Brassicaceae | ||||||||||||
| Cardamine amara | 9.72 | (±7.05) | 7.94 | (±7.53) | 0.68 | 0.208 | 0.05 | (±0.03) | 0.03 | (±0.03) | 2.46 | 0.065 |
| Cardamine pratensis | 5.89 | (±5.2) | 8.11 | (±5.18) | 2.53 | 0.066 | 0.15 | (±0.09) | 0.19 | (±0.07) | 4.90 | 0.021 * |
| Caryophyllaceae | ||||||||||||
| Lychnis flos-cuculi | 76.32 | (±55.12) | 100.68 | (±46.74) | 5.10 | 0.021 * | 0.08 | (±0.06) | 0.12 | (±0.04) | 8.43 | 0.005 * |
| Stellaria graminea | 7.54 | (±6.00) | 9.57 | (±4.86) | 3.12 | 0.045 * | 0.19 | (±0.11) | 0.22 | (±0.06) | 2.99 | 0.051 |
| Hypericaceae | ||||||||||||
| Hypericum maculatum | 339.10 | (±142.53) | 364.45 | (±161.06) | 1.03 | 0.165 | 0.04 | (±0.01) | 0.04 | (±0.01) | 0.44 | 0.259 |
| Lamiaceae | ||||||||||||
| Ajuga reptans | 3.40 | (±1.12) | 3.13 | (±1.13) | 0.52 | 0.261 | 0.99 | (±0.38) | 1.01 | (±0.34) | 0.02 | 0.431 |
| Orchidaceae | ||||||||||||
| Dactylorhiza majalis * | 0.005 | (±0.00) | 0.01 | (±0.01) | 10.15 | 0.001 * | ||||||
| Plantaginaceae | ||||||||||||
| Veronica chamaedrys | 2.00 | (±2.95) | 3.00 | (±3.42) | 0.68 | 0.208 | 0.08 | (±0.10) | 0.11 | (±0.10) | 0.46 | 0.252 |
| Polygonaceae | ||||||||||||
| Bistorta major | 79.50 | (±45.13) | 69.85 | (±36.36) | 2.16 | 0.080 | 5.21 | (±1.50) | 5.20 | (±1.53) | 0.00 | 0.477 |
| Primulaceae | ||||||||||||
| Lysimachia vulgaris | 12.65 | (±16.24) | 39.05 | (±36.66) | 9.29 | 0.003 * | 0.22 | (±0.15) | 0.24 | (±0.13) | 0.35 | 0.281 |
| Ranunculaceae | ||||||||||||
| Anemone nemorosa | 7.11 | (±4.69) | 11.61 | (±7.01) | 10.71 | 0.002 * | 2.13 | (±0.99) | 2.32 | (±0.84) | 0.33 | 0.283 |
| Caltha palustris | 30.82 | (±39.43) | 32.82 | (±27.1) | 0.03 | 0.429 | 0.43 | (±0.23) | 0.47 | (±0.20) | 0.31 | 0.308 |
| Ranunculus acris | 19.33 | (±7.08) | 15.44 | (±8.12) | 3.43 | 0.041 | 1.23 | (±0.23) | 1.20 | (±0.45) | 0.06 | 0.397 |
| Ranunculus auricomus | 10.40 | (±3.28) | 11.80 | (±2.91) | 3.64 | 0.041 * | 2.01 | (±0.48) | 2.00 | (±0.38) | 0.01 | 0.460 |
| Ranunculus flammula | 31.89 | (±16.94) | 37.83 | (±12.67) | 2.23 | 0.076 | 0.34 | (±0.15) | 0.38 | (±0.06) | 0.75 | 0.213 |
| Rosaceae | ||||||||||||
| Potentilla erecta | 5.55 | (±3.14) | 6.15 | (±3.34) | 0.56 | 0.238 | 0.54 | (±0.24) | 0.52 | (±0.24) | 0.12 | 0.369 |
| Potentilla palustris | 219.11 | (±80.41) | 253.16 | (±54.14) | 4.05 | 0.029 * | 0.26 | (±0.08) | 0.28 | (±0.11) | 0.37 | 0.276 |
| Traits | F-Statistic | DF | p-Value |
|---|---|---|---|
| Specialization (No. of pollinator functional groups) | 4.39 | 20 | 0.049 * |
| Clonality | 1.24 | 20 | 0.278 |
| Dichogamy | 0.30 | 20 | 0.586 |
| Sugar content | 1.29 | 20 | 0.268 |
| No. of open flowers | 0.66 | 20 | 0.426 |
| Self-compatibility | 0.70 | 20 | 0.411 |
| Autonomous selfing | 0.17 | 20 | 0.681 |
| Family | Nectar Sugars (mg) | ||
|---|---|---|---|
| Species | Flower/Day | std.dev | |
| Apiaceae | |||
| Aegopodium podagraria | 0.0149 | ± | 0.007 |
| Chaerophyllum aromaticum | 0.0167 | ± | 0.0124 |
| Asteraceae | |||
| Crepis paludosa | 0.0099 | ± | 0.0123 |
| Tephroseris crispa | 0.0216 | ± | 0.0269 |
| Boraginaceae | |||
| Myosotis palustris | 0.0039 | ± | 0.0076 |
| Brassicaceae | |||
| Cardamine amara | 0.0184 | ± | 0.0196 |
| Cardamine pratensis | 0.0193 | ± | 0.0343 |
| Caryophyllaceae | |||
| Lychnis flos-cuculi | 0.2666 | ± | 0.1266 |
| Stellaria graminea | 0.1185 | ± | 0.0594 |
| Hypericaceae | |||
| Hypericum maculatum | 0.0005 | ± | 0.0003 |
| Lamiaceae | |||
| Ajuga reptans | 0.2150 | ± | 0.0671 |
| Orchidaceae | |||
| Dactylorhiza majalis | 0.0014 | ± | 0.0011 |
| Plantaginaceae | |||
| Veronica chamaedrys | 0.1540 | ± | 0.0548 |
| Polygonaceae | |||
| Bistorta major | 0.0598 | ± | 0.0152 |
| Primulaceae | |||
| Lysimachia vulgaris | 0.0009 | ± | 0.0017 |
| Ranunculaceae | |||
| Anemone nemorosa | 0.0004 | ± | 0.0004 |
| Caltha palustris | 0.0006 | ± | 0 |
| Ranunculus acris | 0.0526 | ± | 0.0375 |
| Ranunculus auricomus | 0.0144 | ± | 0.0074 |
| Ranunculus flamula | 0.0314 | ± | 0.0175 |
| Rosaceae | |||
| Potentilla erecta | 0.0804 | ± | 0.0722 |
| Potentilla palustris | 3.2997 | ± | 1.0182 |
| Family | Ants | Beetles | Bumblebees | Butterflies | Honey Bees | Hoverflies | Long-Tonged Flies | Moths | Other Bees | Other Flies | Other Hymenopterans | Total Visits by Plants |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | ||||||||||||
| Apiaceae | ||||||||||||
| Aegopodium podagraria | 1 | 49 | 2 | 4 | 2 | 13 | 52 | 123 | ||||
| Asteraceae | ||||||||||||
| Crepis paludosa | 1 | 1 | 25 | 8 | 15 | 1 | 1 | 52 | ||||
| Tephroseris crispa | 3 | 5 | 2 | 1 | 32 | 4 | 19 | 66 | ||||
| Boraginaceae | ||||||||||||
| Myosotis palustris | 9 | 12 | 29 | 50 | ||||||||
| Brassicaceae | ||||||||||||
| Cardamine amara | 9 | 12 | 29 | 50 | ||||||||
| Cardamine pratensis | 2 | 1 | 9 | 1 | 3 | 45 | 61 | |||||
| Caryophyllaceae | ||||||||||||
| Lychnis flos-cuculi | 3 | 1 | 47 | 76 | 11 | 8 | 4 | 2 | 152 | |||
| Stellaria graminea | 1 | 5 | 1 | 1 | 53 | 5 | 31 | 97 | ||||
| Hypericaceae | ||||||||||||
| Hypericum maculatum | 1 | 7 | 2 | 129 | 55 | 1 | 4 | 199 | ||||
| Lamiaceae | ||||||||||||
| Ajuga reptans | 4 | 22 | 3 | 7 | 1 | 1 | 38 | |||||
| Orchidaceae | ||||||||||||
| Dactylorhiza majalis | 1 | 1 | 1 | 3 | 1 | 1 | 3 | 11 | ||||
| Plantaginaceae | ||||||||||||
| Veronica chamaedrys | 3 | 1 | 7 | 5 | 11 | 27 | ||||||
| Polygonaceae | ||||||||||||
| Bistorta major | 5 | 1 | 1 | 21 | 4 | 3 | 7 | 42 | ||||
| Primulaceae | ||||||||||||
| Lysimachia vulgaris | 1 | 11 | 12 | 24 | ||||||||
| Ranunculaceae | ||||||||||||
| Anemone nemorosa | 2 | 1 | 4 | 1 | 5 | 13 | ||||||
| Caltha palustris | 1 | 1 | 15 | 77 | 3 | 41 | 138 | |||||
| Ranunculus acris | 7 | 1 | 1 | 1 | 20 | 1 | 10 | 20 | 61 | |||
| Ranunculus auricomus | 1 | 5 | 20 | 1 | 5 | 32 | ||||||
| Ranunculus flamula | 16 | 19 | 5 | 16 | 56 | |||||||
| Rosaceae | ||||||||||||
| Potentilla erecta | 1 | 94 | 7 | 9 | 2 | 113 | ||||||
| Potentilla palustris | 2 | 9 | 7 | 3 | 52 | 1 | 9 | 18 | 101 | |||
| Total visits by groups | 12 | 122 | 46 | 69 | 279 | 511 | 1 | 17 | 101 | 345 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoš, M.; Janeček, Š.; Janečková, P.; Chmelová, E.; Tropek, R.; Götzenberger, L.; Klomberg, Y.; Jersáková, J. Are Reproductive Traits Related to Pollen Limitation in Plants? A Case Study from a Central European Meadow. Plants 2020, 9, 640. https://doi.org/10.3390/plants9050640
Bartoš M, Janeček Š, Janečková P, Chmelová E, Tropek R, Götzenberger L, Klomberg Y, Jersáková J. Are Reproductive Traits Related to Pollen Limitation in Plants? A Case Study from a Central European Meadow. Plants. 2020; 9(5):640. https://doi.org/10.3390/plants9050640
Chicago/Turabian StyleBartoš, Michael, Štěpán Janeček, Petra Janečková, Eliška Chmelová, Robert Tropek, Lars Götzenberger, Yannick Klomberg, and Jana Jersáková. 2020. "Are Reproductive Traits Related to Pollen Limitation in Plants? A Case Study from a Central European Meadow" Plants 9, no. 5: 640. https://doi.org/10.3390/plants9050640
APA StyleBartoš, M., Janeček, Š., Janečková, P., Chmelová, E., Tropek, R., Götzenberger, L., Klomberg, Y., & Jersáková, J. (2020). Are Reproductive Traits Related to Pollen Limitation in Plants? A Case Study from a Central European Meadow. Plants, 9(5), 640. https://doi.org/10.3390/plants9050640





