Flavonoid Compounds and Photosynthesis in Passiflora Plant Leaves under Varying Light Intensities
Abstract
1. Introduction
2. Results and Discussion
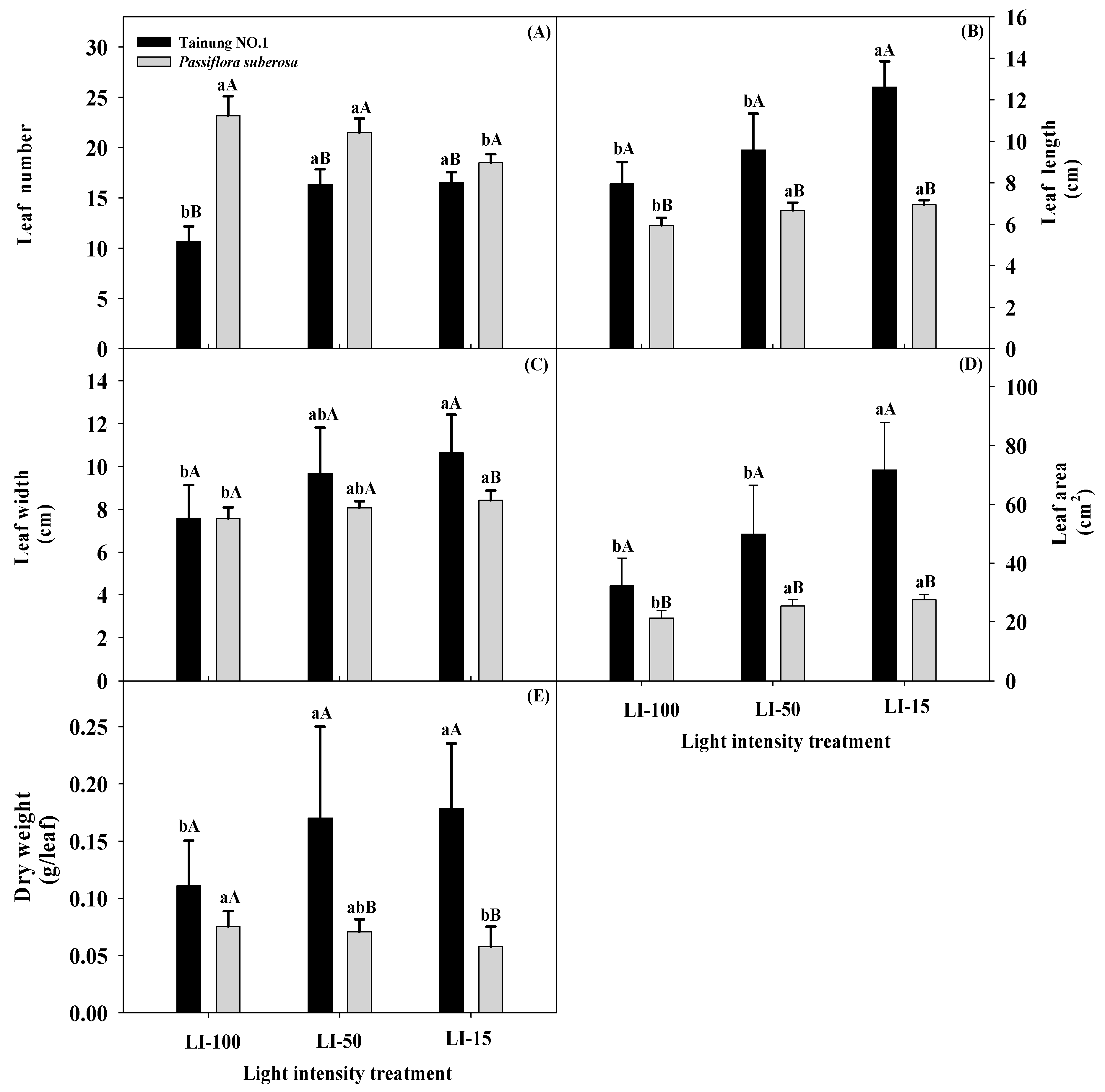
2.1. Light Intensity Effects on Leaf Growth Traits of Passiflora Species
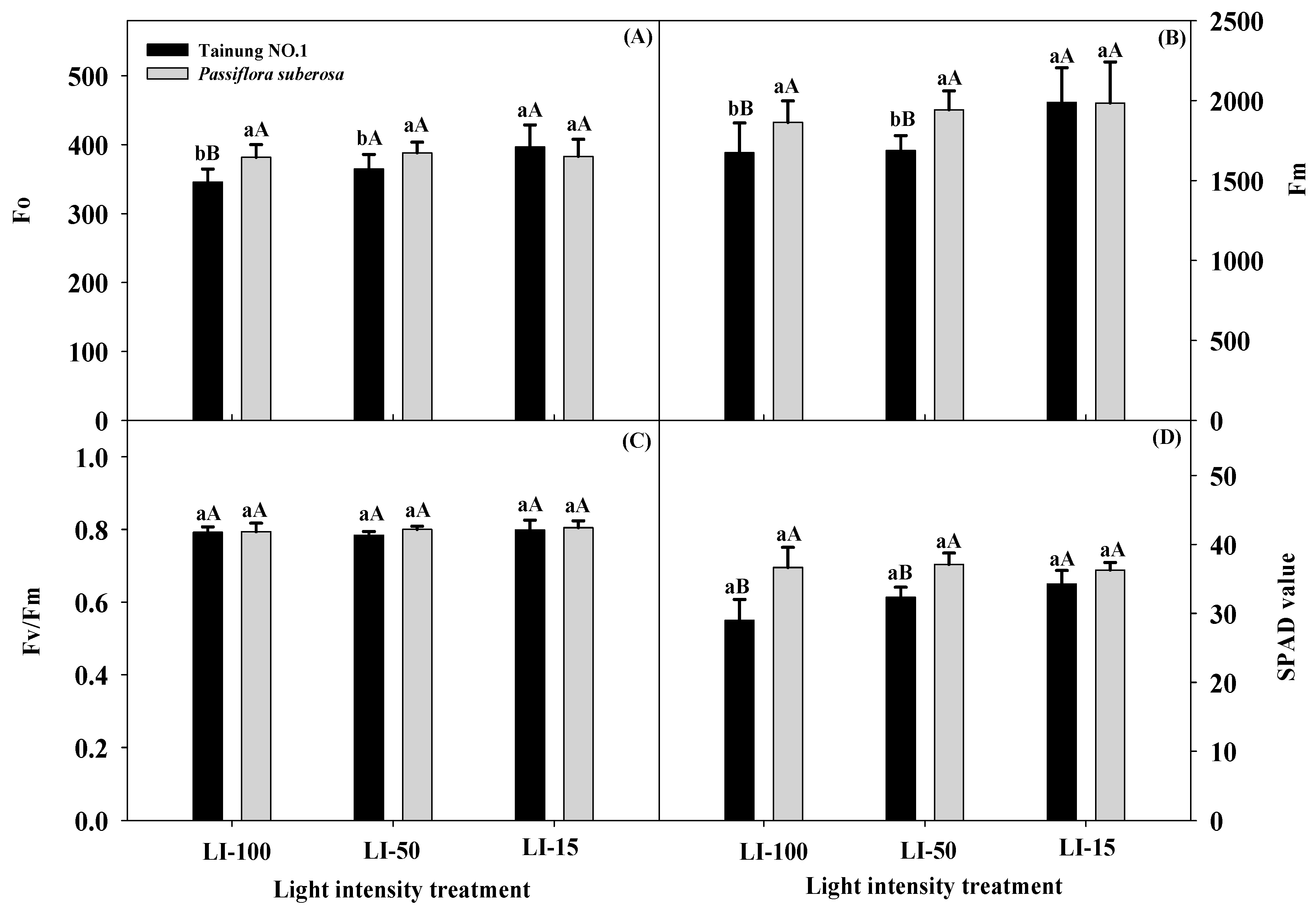
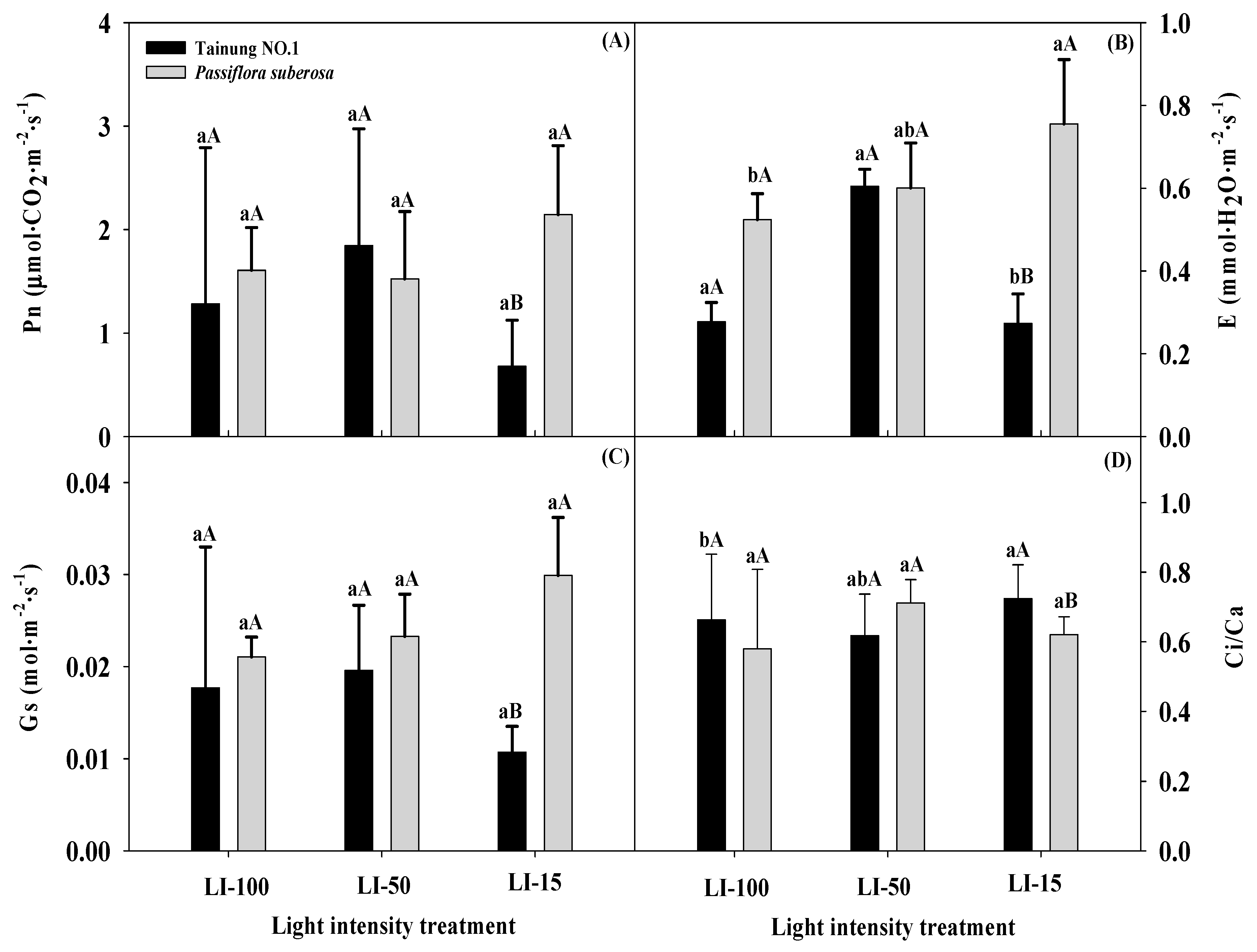
2.2. Effects of Different Light Intensities on Physiological Parameters in Passiflora Species
2.3. Influences of Light Intensity Effects on Secondary Metabolites of Passiflora Species
3. Materials and Methods
3.1. Plant Materials and Cultural Practice
3.2. Light-Intensity Measurements
3.3. Plant Growth Measurements
3.4. Measurement of Photosynthetic Parameters
3.5. Determination of TP and TF Contents and Flavone Concentrations
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ulmer, T.; MacDougal, J.M. Passiflora Passionflowers of the World; Timber Press Co.: Portland, OR, USA, 2004. [Google Scholar]
- Bandara, K.R.V.; Padumadasa, C.; Peiris, D.C. Potent antibacterial, antioxidant and toxic activities of extracts from Passiflora suberosa L. leaves. PeerJ. 2018. [Google Scholar] [CrossRef]
- Zibadi, S.; Watson, R.R. Passion Fruit (Passiflora edulis) Composition, efficacy and safety. Evid. Based Integr. Med. 2004, 1, 183–187. [Google Scholar] [CrossRef]
- Abreu, P.P.; Souza, M.M.; de Almeida, A.F.; Santos, E.A.; de Oliveira, J.C.F.; Figueiredo, A.L. Photosynthetic responses of ornamental passion flower hybrids to varying light intensities. Acta Physiol. Plant. 2014, 36, 1993–2004. [Google Scholar] [CrossRef]
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Givnish, T.J. Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: Dynamic photosynthetic responses. Oecologia 2008, 155, 455. [Google Scholar] [CrossRef]
- Feng, Y.L.; Cao, K.F.; Zhang, J.L. Photosynthetic characteristics, dark respiration, and leaf mass per unit area in seedlings of four tropical tree species grown under three irradiances. Photosynthetica 2004, 42, 431–437. [Google Scholar] [CrossRef]
- Cavichioli, J.C.; Ruggiero, C.; Volpe, C.A.; Paulo, E.M.; Fagundes, J.L.; Kasai, F.S. Flowering and fruiting of yellow passion fruit submitted to artificial lighting, irrigation and shading. Rev. Bras. Frutic. 2006, 28, 92–96. [Google Scholar] [CrossRef]
- Sarijeva, G.; Knapp, M.; Lichtenthaler, H.K. Differences in photosynthetic activity, chlorophyll and carotenoid levels, and in cholorophyll fluorescence parameters in green sun and shade leaves of Ginkgo and Fagus. J. Plant Physiol. 2007, 164, 950–955. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Craine, J.M.; Reich, P.B. Leaf-level light compensation points in shade-tolerant woody seedlings. New Phytol. 2005, 166, 705–708. [Google Scholar] [CrossRef]
- Mouradov, A.; Spangenberg, G. Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; de Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Horticul. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Yang, W.; Shang, X.; Fu, X. Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J. Photochem. Photobio. B: Biol. 2018, 179, 66–73. [Google Scholar] [CrossRef]
- Zoratti, L.; Karppinen, K.; Escobar, A.L.; Haggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar]
- Xu, Y.; Wang, G.; Cao, F.; Zhu, C.; Wang, G.; El-Kassaby, Y.A. Light intensity affects the growth and flavonol biosynthesis of ginkgo (Ginkgo biloba, L.). New For. 2014, 45, 765–776. [Google Scholar] [CrossRef]
- Yuan, M.; Jia, X.; Ding, C.; Zeng, H.; Du, L.; Yuan, S. Effect of fluorescence light on phenolic compounds and antioxidant activities of soybeans (Glycine max L. Merrill) during germination. Food Sci. Biotech. 2015, 24, 1859–1865. [Google Scholar] [CrossRef]
- Ma, X.; Song, L.; Yu, W.; Hu, Y.; Liu, Y.; Wu, J.; Ying, Y. Growth, physiological, and biochemical responses of Camptotheca acuminata seedlings to different light environments. Front. Plant Sci. 2015, 6, 321. [Google Scholar] [CrossRef]
- Pan, J.; Guo, B. Effects of light intensity on the growth, photosynthetic characteristics, and flavonoid content of Epimedium pseudowushanense B.L. Guo. Molecules 2016, 21, 1475. [Google Scholar] [CrossRef]
- Li, A.; Li, S.; Wu, X.; Zhang, J.; He, A.; Zhao, G. Effect of light intensity on leaf photosynthetic characteristics and accumulation of flavonoids in Lithocarpus litseifolius (Hance) Chun. (Fagaceae). Open J. 2016, 6, 445–459. [Google Scholar] [CrossRef]
- Yoneda, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Effects of light intensity and photoperiod on improving steviol glycosides content in Stevia rebaudiana (bertoni) bertoni while conserving light energy consumption. J. Appl. Res. Med Aro. Plants. 2017, 7, 64–73. [Google Scholar] [CrossRef]
- Dębski, H.; Wiczkowski, W.; Szawara-Nowak, D.; Bączek, N.; Szwed, M.; Horbowicz, M. Enhanced light intensity increases flavonol and anthocyanin concentrations but reduces flavone levels in the cotyledons of common buckwheat seedlings. Cereal Res. Commu. 2017, 45, 225–233. [Google Scholar] [CrossRef]
- Arena, M.E.; Postemsky, P.D.; Curvetto, N.R. Changes in the phenolic compounds and antioxidant capacity of Berberis microphylla G. Forst. berries in relation to light intensity and fertilization. Sci. Hort. 2017, 218, 63–71. [Google Scholar] [CrossRef]
- Riachi, L.G.; Simas, D.L.R.; Coelho, G.C.; Marcellini, P.S.; da Silva, A.J.R.; de Maria, C.A.B. Effect of light intensity and processing conditions on bioactive compounds in maté extracted from yerba mate (Ilex paraguariensis A. St.-Hil.). Food Chem. 2018, 266, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Linatoc, A.C.; Muhammad, S.M.; Aliyu, A.M.; Bakar, M.F.A. Effect of light intensity on the total flavonoid and total phenolic contents of Mikania micrantha and Tridax procumbens. J. Sci. Tech. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. Hortscience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Idris, A.; Linatoc, A.C.; Muhammad, S.M.; Takai, Z.I. An effect of light intensity on the total flavonoid and phenolic content of Moringa Oleifera. J. Tomogra. Syst. Sensor Appl. 2019, 2, 19–24. [Google Scholar]
- Liu, Y.Y.; Chen, X.R.; Wang, J.P.; Cui, W.Q.; Xing, X.X.; Chen, X.Y. Transcriptomic analysis reveals flavonoid biosynthesis of Syringa oblata Lindl. in response to different light intensity. Bmc Plant Biol. 2019, 19, 487. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Zhang, Y.; Chen, W. Response of total phenols, flavonoids, minerals, and amino acids of four edible fern species to four shading treatments. PeerJ 2020, 8, 8354. [Google Scholar] [CrossRef]
- Matsui, K.; Walker, A.R. Biosynthesis and regulation of flavonoids in buckwheat. Breed. Sci. Preview 2019, 19041. [Google Scholar] [CrossRef]
- Coleta, M.; Batista, M.T.; Campos, M.G.; Carvalho, R.; Cotrim, M.D.; de Lima, T.C.M. Neuro pharmacological evaluation of the putative anxiolytic effects of Passiflora edulis Sims, its sub-fractions and flavonoid constituents. Phytothe. Res. 2006, 20, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A Review on medicinal properties of orientin. Adva. Pharmac. Sci. 2016, 4104595. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Min, J.W.; Kong, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, S.F.; Lima, I.M.; Modolo, L.V. Phenolic content and antioxidant activity of parts of Passiflora edulis as a function of plant developmental stage. Acta Bot. Bras. 2019. [Google Scholar] [CrossRef]
- Pires, M.V.; Almeida, A.F.; Figueiredo, A.L.; Gomes, F.P.; Souza, M.M. Photosynthetic characteristics of ornamental passion flowers grown under different light intensities. Photosynthetica 2011, 49, 593–602. [Google Scholar] [CrossRef]
- Rahbarian, R.; Khavari-Nejad, R.; Ganjeali, A.; Bagheri, A.; Najafi, F. Drought stress effects on photosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible chickpea (Cicer Arietinum L.) genotypes. Acta Biolo Cracovi Ser. Bot. 2011, 53, 47–56. [Google Scholar] [CrossRef]
- Dabrowski, P.; Baczewska-Dabrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M. Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in perennial ryegrass. Senssons 2019, 19, 2736. [Google Scholar] [CrossRef]
- Mauro, R.P.; Occhipinti, A.; Longo, A.M.G.; Mauromicale, G. Effects of shading on chlorophyll content, chlorophyll fluorescence and photosynthesis of subterranean clover. J. Agro. Crop. Sci. 2011, 197, 57–66. [Google Scholar] [CrossRef]
- Reed, S.; Schnell, R.; Moore, J.M.; Dunn, C. Chlorophyll a+b content and chlorophyll fluorescence in avocado. J. Agric. Sci. 2012, 4, 29–36. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, K.; Chen, L. Response of photosynthetic plasticity of Paeonia suffruticosa to changed light environments. Env. Exp. Bot. 2003, 49, 121–133. [Google Scholar] [CrossRef]
- Ambrosio, N.D.; Arena, C.; De Santo, A.V. Temperature response of photosynthesis, excitation energy dissipation and alternative electron sinks to carbon assimilation in Beta vulgaris L. Environ. Exp. Bot. 2006, 55, 248–257. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef]
- Lu, N.; Takagaki, M.; Yamori, W.; Kagawa, N. Flavonoid productivity optimized for green and red forms of Perilla frutescens via environmental control technologies in plant factory. J. Food Qual. 2018, 2018, 4270279. [Google Scholar] [CrossRef]
- Hikosaka, S.; Iwamoto, N.; Goto, E.; Ching-Hui, C. Effects of supplemental lighting on growth and medicinal compounds of Japanese Honysuckle Lonicera japonica Thunb. Env. Control. Biol. 2017, 55, 71–76. [Google Scholar] [CrossRef][Green Version]
- Habibi, G. Effects of mild and severe drought stress on the biomass, phenolic compounds production and photochemical activity of Aloe vera (L.) Burm.f. Acta Agric Slov. 2018, 111, 463–476. [Google Scholar] [CrossRef]
- Liu, F.Y.; Peng, Y.L.; Chang, Y.S. Effects of temperature and ethylene response inhibitors on growth and flowering of passion fruit. Kor. J. Hort. Sci. Technol. 2015, 33, 356–363. [Google Scholar] [CrossRef]
- Wu, S.H.; Hsieh, C.F.; Rejmanek, M. Catalogue of the Naturalized Flora of Taiwan. Taiwania. 2004, 49, 16–31. [Google Scholar]
- Porcar-Castell, A.; Pfündel, E.; Korhonen, J.F.J.; Juurola, E. A new monitoring PAM fluorometer (Mini-Pam) to study the short- and long-term acclimation of photosystem II in field conditions. Photosyn. Res. 2008, 96, 173–179. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Saravanan, S.; Parimelazhagan, T. Total phenolic content, free radical scavenging and antimicrobial activities of Passiflora subpeltata seeds. J. Appl. Pharmac. Sci. 2013, 4, 67–72. [Google Scholar]
- Pereira, C.A.M.; Yariwake, J.H.; McCullagh, M. Distinction of the C-glycosylflavone isomer pairs orientin/isoorientin and vitexin/isovitexin using HPLC-MS exact mass measurement and in-source CID. Phytochem. Anal. 2005, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]

| Source of Variation | Significance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf Number | Leaf Length (cm) | Leaf Width (cm) | Leaf Area (cm2) | Dry Weight (g/Leaf) | Fo | Fm | Fv/Fm | SPAD Value | Pn | E | Gs | Ci/Ca | |
| Variety (V) | *** | *** | ** | *** | NS | NS | * | NS | * | NS | NS | NS | NS |
| Light intensity (L) | ** | *** | ** | *** | *** | * | * | NS | *** | * | ** | * | * |
| L × V | *** | *** | NS | ** | NS | * | NS | NS | * | NS | NS | NS | NS |
| Source of Variation | Significance | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Phenol Concentration (μg GAE (g DW)−1) | Total Phenol Content (μg GAE Per Plant) | Total Flavonoid Concentration (μg RE (g DW)−1) | Total Flavonoid Content (μg RE Per Plant) | Orientin Concentration (μg (g DW)−1) | Orientin Content (μg Per Plant) | Isovitexin Concentration (μg (g DW)−1) | Isovitexin Content (μg Per Plant) | |
| Variety (V) | NS | *** | *** | *** | *** | *** | *** | *** |
| Light intensity (L) | *** | ** | *** | *** | *** | *** | *** | *** |
| L × V | * | ** | * | *** | *** | *** | *** | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Y.-W.; Lin, K.-H.; Chen, K.-H.; Wu, C.-W.; Chang, Y.-S. Flavonoid Compounds and Photosynthesis in Passiflora Plant Leaves under Varying Light Intensities. Plants 2020, 9, 633. https://doi.org/10.3390/plants9050633
Ni Y-W, Lin K-H, Chen K-H, Wu C-W, Chang Y-S. Flavonoid Compounds and Photosynthesis in Passiflora Plant Leaves under Varying Light Intensities. Plants. 2020; 9(5):633. https://doi.org/10.3390/plants9050633
Chicago/Turabian StyleNi, Yu-Wan, Kuan-Hung Lin, Kai-Hsien Chen, Chun-Wei Wu, and Yu-Sen Chang. 2020. "Flavonoid Compounds and Photosynthesis in Passiflora Plant Leaves under Varying Light Intensities" Plants 9, no. 5: 633. https://doi.org/10.3390/plants9050633
APA StyleNi, Y.-W., Lin, K.-H., Chen, K.-H., Wu, C.-W., & Chang, Y.-S. (2020). Flavonoid Compounds and Photosynthesis in Passiflora Plant Leaves under Varying Light Intensities. Plants, 9(5), 633. https://doi.org/10.3390/plants9050633






