Natural Products, Traditional Uses and Pharmacological Activities of the Genus Biebersteinia (Biebersteiniaceae)
Abstract
1. Introduction
2. Data Collections
3. Natural Products Isolated from Bieberstrinia
3.1. Flavonoids
3.2. Guanidines
3.3. Phenylpropanoids
3.4. Terpenoids
3.5. Other Compounds
3.6. Fatty Acids
4. Chemical Compositions of Essential Oils in Biebersteinia Species
5. Applications in Traditional Medicines
6. Pharmacological Activities
6.1. In Vivo Pharmacological Activities
6.1.1. Anti-Inflammatory and Analgesic Effects
6.1.2. Anti-Hypertensive and Hypoglycemic Effects
6.1.3. Anti-Fatigue and Anxiolytic Effects
6.1.4. Hypolipidemic Effect
6.2. In Vitro Pharmacological Activities
6.2.1. Antimicrobial Effects
6.2.2. Antioxidant Activities
6.2.3. Anti-Cancer Effects
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.Q.; Ho, T.N.; Chen, S.L.; Lu, A. Karyomorphology of Biebersteinia Stephan (Geraniaceae) and its systematic and taxonomic significance. Bot. Bull. Acad. Sin. 2001, 42, 61–66. [Google Scholar]
- Bakker, F.T.; Vassiliades, D.D.; Morton, C. Phylogenetic relationships of Biebersteinia Stephan (Geraniaceae) inferred from rbcL and atpB sequence comparisons. Bot. J. Linn. Soc. 1998, 127, 149–158. [Google Scholar] [CrossRef]
- Muellner, A.N.; Vassiliades, D.D.; Renner, S.S. Placing Biebersteiniaceae, a herbaceous clade of Sapindales, in a temporal and geographic context. Plant Syst. Evol. 2007, 266, 233–252. [Google Scholar] [CrossRef][Green Version]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Tan, K.; Perdetzoglou, D.K.; Roussis, V. Biebersteinia orphanidis (Geraniaceae) from southern Greece. Ann. Bot. Fenn. 1997, 34, 41–45. [Google Scholar]
- Yannitsaros, A.G.; Constantinidis, T.A.; Vassiliades, D.D. The rediscovery of Biebersteinia orphanidis Boiss. (Geraniaceae) in Greece. Bot. J. Linn. Soc. 1996, 120, 239–242. [Google Scholar] [CrossRef]
- Jahromy, M.; Mohajer, A.; Adibi, L.; Khakpour, S. Effects of Biebersteinia multifida DC. Root extract on physical stamina in male mice. Food Nutr. Sci. 2015, 6, 326–331. [Google Scholar] [CrossRef][Green Version]
- Doležal, J.; Dvorský, M.; Börner, A.; Wild, J.; Schweingruber, F.H. Anatomical descriptions of dicotyledons. In Anatomy, Age and Ecology of High Mountain Plants in Ladakh, the Western Himalaya; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Abbas, Z.; Khan, S.M.; Alam, J.; Khan, S.W.; Abbasi, A.M. Medicinal plants used by inhabitants of the Shigar Valley, Baltistan region of Karakorum range-Pakistan. J. Ethnobiol. Ethnomed. 2017, 13, 53. [Google Scholar] [CrossRef]
- Amirghofran, Z. Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran J. Immunol. 2010, 7, 65–73. [Google Scholar]
- Zhang, X.F.; Hu, B.L.; Zhou, B.N. Studies on the active constituents of Tibetan herb Biebersteinia heterostemon Maxim. Acta Pharm. Sin. 1995, 30, 211–214. [Google Scholar]
- Wang, W.E.; Zhang, X.F.; Shen, J.W.; Lou, D.J. Chemical constituents in aerial parts of Biebersteinia heterostemon Maxim. Nat. Prod. Res. Develop. 2009, 21, 199–202. [Google Scholar]
- Zhang, P.Z.; Zhong, G.Y.; Xie, W.W.; Zhang, Y.M. Flavonoids from Biebersteinia heterostemon. Chin. Tradit. Herb. Drugs 2016, 47, 3565–3568, (In Chinese with English abstract). [Google Scholar]
- Zhang, Y.H.; Zhang, D.J.; Zhang, B.Y. Chemical structures of alkaloids with hypoglycemic activity and their hypoglycemic mechanisms. Chin. Tradit. Herb. Drugs 2018, 49, 3692–3702, (In Chinese with English abstract). [Google Scholar]
- Greenham, J.; Vassiliades, D.D.; Harborne, J.B.; Williams, C.A.; Eagles, J.; Grayer, R.J.; Veitch, N.C. A distinctive flavonoid chemistry for the anomalous genus Biebersteinia. Phytochemistry 2001, 56, 87–91. [Google Scholar] [CrossRef]
- Bate-Smith, E.C. Chemotaxonomy of Geranium. Bot. J. Linn. Soc. 1973, 67, 347–359. [Google Scholar] [CrossRef]
- Duda, S.C.; Mărghitaş, L.A.; Dezmirean, D.; Duda, M.; Mărgăoan, R.; Bobiş, O. Changes in major bioactive compounds with antioxidant activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: Effect of harvest time and plant species. Ind. Crops Prod. 2015, 77, 499–507. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M.C. Ecological roles and biological activities of specialized metabolites from the genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef]
- Zidorn, C. Secondary metabolites of seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical diversity, bioactivity, and ecological function. Phytochemistry 2016, 124, 5–28. [Google Scholar] [CrossRef]
- Tan, Z. Study on the geographic distribution of Geraniaceae plants in Sichuan. Bull. Bot. Res. 1995, 15, 523–531. [Google Scholar]
- Zhang, B.Y.; Wang, H.; Yang, X.Y.; Shen, J.W.; Zhang, X.F. The determination of total flavonoids of Biebersteinia heterostemon from different regions of Qinghai province. Chin. J. Anal. Lab. 2009, 28 (Suppl. 2), 39–41. [Google Scholar]
- Nabavi, S.F.; Ebrahimzadeh, M.A.; Nabavi, S.M.; Eslami, B.; Dehpour, A. Antihemolytic and antioxidant activities of Biebersteinia multifida. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 823–830. [Google Scholar] [PubMed]
- Monsef-Esfahani, H.R.; Amini, M.; Goodarzi, N.; Saiedmohammadi, F.; Hajiaghaee, R.; Faramarzi, M.A.; Tofighi, Z.; Ghahremani, M.H. Coumarin compounds of Biebersteinia multifida roots show potential anxiolytic effects in mice. DARU J. Pharm. Sci. 2013, 21, 51. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, X.; Zhang, B.Y.; Zhang, D.J. Chemical constituents from Tibetan herbal medicines Biebersteinia heterostemon. Chin. Tradit. Herb. Drugs 2019, 50, 1551–1554, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Wu, H.F.; Zhang, X.F.; Deng, Y.; Pan, L.; Ding, L.S. Studies on chemical constituents from bark of Biebersteinia heterostemon. China J. Chin. Mater. Med. 2007, 32, 2141–2143, (In Chinese with English abstract). [Google Scholar]
- Meng, J.C.; Lu, H.; Li, H.; Yang, L.; Tan, R.X. A new antibacterial sesquiterpene glycoside and other bioactive compounds from Biebersteinia heterostemon. Spectrosc. Lett. 1999, 32, 1005–1012. [Google Scholar] [CrossRef]
- Kurbanov, D.; Zharekeev, B.K. Investigation of the alkaloids of Biebersteinia multifida and Peganum harmala from Karakalpakia. Chem. Nat. Compd. 1974, 10, 715. [Google Scholar] [CrossRef]
- Tzakou, O.; Yannitsaros, A.; Vassiliades, D. Investigation of the C(16:3)/C(18:3) fatty acid balance in leaf tissues of Biebersteinia orphanidis Boiss. (Biebersteiniaceae). Biochem. Syst. Ecol. 2001, 29, 765–767. [Google Scholar] [CrossRef]
- Wang, F.C.; Che, Z.; Qiu, D.; Wei, L. Analysis of fatty acids in Tibetan medicine Biebersteinia heterostemon Maxim. J. Qinghai Norm. Univ. (Nat. Sci. Ed.) 2013, 38–40, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Benn, M.H.; Shustov, G.; Shustova, L.; Majak, W.; Bai, Y.; Fairey, N.A. Isolation and characterization of two guanidines from Galega orientalis Lam. cv. Gale (fodder galega). J. Agric. Food Chem. 1996, 44, 2779–2781. [Google Scholar] [CrossRef]
- Pufahl, K.; Schreiber, K. Isolation of a new guanidine derivative from goat’s rue, Galega officinalis L. Experientia 1961, 17, 302–303. [Google Scholar] [CrossRef]
- Yang, T.C.; Chao, H.F.; Shi, L.S.; Chang, T.C.; Lin, H.C.; Chang, W.L. Alkaloids from Coptis chinensis root promote glucose uptake in C2C12 myotubes. Fitoterapia 2014, 93, 239–244. [Google Scholar] [CrossRef]
- Umezawa, K.; Hiroki, A.; Kawakami, M.; Naka, H.; Takei, I.; Ogata, T.; Kojima, I.; Koyano, T.; Kowithayakorn, T.; Pang, H.S.; et al. Induction of insulin production in rat pancreatic acinar carcinoma cells by conophylline. Biomed. Pharmacother. 2003, 57, 341–350. [Google Scholar] [CrossRef]
- Tiong, S.H.; Looi, C.Y.; Arya, A.; Wong, W.F.; Hazni, H.; Mustafa, M.R.; Awang, K. Vindogentianine, a hypoglycemic alkaloid from Catharanthus roseus (L.) G. Don (Apocynaceae). Fitoterapia 2015, 102, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Yu, S.; Yan, H.; Guo, S.; Xiao, W.; Wang, Z.; Zhang, L.; Ding, A.; Wu, Q.; Li, S.F.Y. A review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules 2017, 22, 1689. [Google Scholar] [CrossRef]
- Xiao, W.; Li, S.; Wang, S.; Ho, C.T. Chemistry and bioactivity of Gardenia jasminoides. J. Food Drug Anal. 2017, 25, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Arifkhodzhaev, A.O.; Arifkhodzhaev, K.A.; Kondratenko, E.S. Polysaccharides of saponin-bearing plants. II. Isolation and characterization of the polysaccharides of Biebersteinia multifida. Chem. Nat. Compd. 1985, 21, 714–716. [Google Scholar] [CrossRef]
- Arifkhodzhaev, A.O.; Rakhimov, D.A. Polysaccharides of saponin-bearing plants. III. Polysaccharides of the epigeal organs of Biebersteinia multifida. Chem. Nat. Compd. 1986, 22, 719–720. [Google Scholar] [CrossRef]
- Arifkhodzhaev, A.O.; Rakhimov, D.A. Polysaccharides of saponin-bearing plants. IV. Structure of glucans A, B, and C of Biebersteinia multifida. Chem. Nat. Compd. 1993, 29, 151–153. [Google Scholar] [CrossRef]
- Arifkhodzhaev, A.O.; Rakhimov, D.A. Polysaccharides of saponin-bearing plants. V. Structural investigation of glucans A, B, and C and their oligosaccharides from Biebersteinia multifida plants. Chem. Nat. Compd. 1994, 30, 655–660. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, A.; Reglero, G.; Ibañez, E. Recent trends in the advanced analysis of bioactive fatty acids. J. Pharm. Biomed. Anal. 2010, 51, 305–326. [Google Scholar] [CrossRef]
- Ahmadzadeh Sani, T.; Golmakani, E.; Mohammadi, A.; Feyzi, P.; Kamali, H. Optimization of pressurized hot water extraction on the extract yield and antioxidant activity from Biebersteinia multifida DC using a modified supercritical fluid extractor. J. Supercrit. Fluid. 2014, 94, 130–137. [Google Scholar] [CrossRef]
- Fakir, H.; Yasar, S.; Erbas, S.; Ozderin, S. Essential oil composition of Biebersteinia orphanidis Boiss. growing in mediterranean region of Turkey. Asian J. Chem. 2011, 23, 3767–3768. [Google Scholar]
- Feyzi, P.; Sani, T.A.; Alesheikh, P.; Kamali, H.; Mohammadi, A. Comparative study of essential oils extracted from Biebersteinia multifida DC using hydro-distillation, microwave and solvent extraction. West Indian Med. J. 2016. [Google Scholar] [CrossRef][Green Version]
- Hamzeh, A. Antioxidant activities of the essential oils and extracts of Biebersteinia multifida DC. Herba Pol. 2009, 55, 59–68. [Google Scholar]
- Akhlaghi, H.; Shafaghat, A.; Mohammadhosseini, M. Chemical composition of the essential oil from leaves of Biebersteinia multifida DC. growing wild in Iran. J. Essent. Oil Bear. Plants 2009, 12, 365–368. [Google Scholar] [CrossRef]
- Javidnia, K.; Miri, R.; Soltani, M.; Khosravi, A.R. Essential oil composition of Biebersteinia multifida DC. (Biebersteiniaceae) from Iran. J. Essent. Oil Res. 2010, 22, 611–612. [Google Scholar] [CrossRef]
- Yang, Y. The Study on Essential Oil of Eleven Tibetan Medicines. Master’s Thesis, Qinghai Univerisity for Nationalities, Xining, China, 2012. [Google Scholar]
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical fluid extraction of plant flavors and fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef]
- Donato, P.; Inferrera, V.; Sciarrone, D.; Mondello, L. Supercritical fluid chromatography for lipid analysis in foodstuffs. J. Sep. Sci. 2017, 40, 361–382. [Google Scholar] [CrossRef]
- Fornari, T.; Vicente, G.; Vazquez, E.; Garcia-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef]
- Hartmann, A.; Ganzera, M. Supercritical fluid chromatography—Theoretical background and applications on natural products. Planta Med. 2015, 81, 1570–1581. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Wrona, O.; Rafinska, K.; Mozenski, C.; Buszewski, B. Supercritical fluid extraction of bioactive compounds from plant materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Li, Y.; Wang, J.J.; Lin, X.Y.; Liu, X.P.; Ren, Y. Pharmacological effect method of different extraction process evaluation about Tibetan medicine Biebersteinia heterostemon Maxim. J. Med. Pharma. Chin. Minorit. 2012, 18, 62–63. [Google Scholar]
- Tang, Y.C. On the Affinities and the Role of the Chinese Flora. Acta Bot. Yunnan. 2000, 22, 1–26. [Google Scholar]
- Farsam, H.; Amanlou, M.; Reza Dehpour, A.; Jahaniani, F. Anti-inflammatory and analgesic activity of Biebersteinia multifida DC. root extract. J. Ethnopharmacol. 2000, 71, 443–447. [Google Scholar] [CrossRef]
- Aboutorabi, H. Ethnobotanic and Phytochemical Study of Plants in Rouin Region. Ph.D. Thesis, Tehran University of Medical Sciences, Tehran, Iran, 2001. [Google Scholar]
- Wang, W.E.; Zhao, W.Y. The hypoglycemic effect of total alkanoids of Biebersteinia heterostemon on streptozotocin-induced diabetic mice. Chin. Tradition. Patent Med. 2011, 33, 1584–1586. [Google Scholar]
- Rios, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, W.S.; Kim, J.J.; Chin, Y.W.; Jeong, H.C.; Choi, J.S.; Min, H.G.; Cha, H.J. Identification of anti-melanogenic natural compounds from Galega officinalis and further drug repositioning. J. Dermatol. Sci 2012, 67, 61–63. [Google Scholar] [CrossRef]
- Patade, G.; Marita, A. Metformin: A Journey from countryside to the bedside. J. Obes. Metab. Res. 2014, 1, 127–130. [Google Scholar] [CrossRef]
- Jeong, S.H.; Han, X.H.; Hong, S.S.; Hwang, J.S.; Hwang, J.H.; Lee, D.; Lee, M.K.; Ro, J.S.; Hwang, B.Y. Monoamine oxidase inhibitory coumarins from the aerial parts of Dictamnus albus. Arch. Pharmacal Res. 2006, 29, 1119–1124. [Google Scholar] [CrossRef]
- Yun, B.S.; Lee, I.K.; Ryoo, I.J.; Yoo, I.D. Coumarins with monoamine oxidase inhibitory activity and antioxidative coumarino-lignans from Hibiscus syriacus. J. Nat. Prod. 2001, 64, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Ahotupa, M. Oxidized lipoprotein lipids and atherosclerosis. Free Radic. Res. 2017, 51, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Orso, E.; Schmitz, G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin. Res. Cardiol. Suppl. 2017, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Aluganti Narasimhulu, C.; Fernandez-Ruiz, I.; Selvarajan, K.; Jiang, X.; Sengupta, B.; Riad, A.; Parthasarathy, S. Atherosclerosis—Do we know enough already to prevent it? Curr. Opin. Pharmacol. 2016, 27, 92–102. [Google Scholar] [CrossRef]
- Bandeali, S.; Farmer, J. High-density lipoprotein and atherosclerosis: The role of antioxidant activity. Curr. Atheroscler. Rep. 2012, 14, 101–107. [Google Scholar] [CrossRef]
- Hu, J.; Xi, D.; Zhao, J.; Luo, T.; Liu, J.; Lu, H.; Li, M.; Xiong, H.; Guo, Z. High-density lipoprotein and inflammation and its significance to atherosclerosis. Am. J. Med. Sci. 2016, 352, 408–415. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Sobenin, I.A. Modified lipoproteins as biomarkers of atherosclerosis. Front Biosci. (Landmark Ed.) 2018, 23, 1422–1444. [Google Scholar] [CrossRef]
- Parhofer, K.G. Increasing HDL-cholesterol and prevention of atherosclerosis: A critical perspective. Atheroscler. Suppl. 2015, 18, 109–111. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Khakpour, S.; Akhlaghdoust, M.; Naimi, S.; Mirlohi, S.M.J.; Abedian, M.; Seyed Forootan, N.S.; Foroughi, F. Effect of Biebersteinia multifida DC. root extract on cholesterol in mice. Zahedan J. Res. Med. Sci. 2013, 15, 49–51. [Google Scholar]
- Raeesi, M.; Eskandari-Roozbahani, N.; Shomali, T. Gastro-protective effect of Biebersteinia multifida root hydro-methanolic extract in rats with ethanol-induced peptic ulcer. Avicenna J. Phytomed. 2019, 9, 410–418. [Google Scholar] [PubMed]
- Lu, Y.Z.; Jing, M.; Liu, J.J.; Lu, N.H.; Chen, H.; Chen, Z.J.; Zhang, Y.X. Inhibition of commonly used traditional Chinese medicine mixed on Chinese Angelica stem nematode disease pathogen. J. Tradit. Chin. Vet. Med. 2017, 36, 38–40. [Google Scholar]
- Ghodrati, N.; Asili, J.; Mohammadi, S.A.; Fazli-Bazzaz, B.S. Evaluation of antibacterial activities of different roots extracts of Biebersteinia multifida DC. J. North Khorasan Univ. Med. Sci. 2013, 4, 149–154. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Bertonha, A.F.; Takaki, M.; Rodriguez, J.P.G. The chemistry and biology of guanidine natural products. Nat. Prod. Rep. 2017, 34, 1264–1301. [Google Scholar] [CrossRef]
- Coqueiro, A.; Regasini, L.O.; Stapleton, P.; da Silva Bolzani, V.; Gibbons, S. In vitro antibacterial activity of prenylated guanidine alkaloids from Pterogyne nitens and synthetic analogues. J. Nat. Prod. 2014, 77, 1972–1975. [Google Scholar] [CrossRef]
- De Oliveira, N.K.S.; Almeida, M.R.S.; Pontes, F.M.M.; Barcelos, M.P.; de Paula da Silva, C.H.T.; Rosa, J.M.C.; Cruz, R.A.S.; da Silva Hage-Melim, L.I. Antioxidant effect of flavonoids present in Euterpe oleracea martius and neurodegenerative diseases: A literature review. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 75–99. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mostofa, M.G.; Rahman, M.A.; Islam, M.R.; Keya, S.S.; Das, A.K.; Miah, M.G.; Kawser, A.; Ahsan, S.M.; Hashem, A.; et al. Acetic acid: A cost-effective agent for mitigation of seawater-induced salt toxicity in mung bean. Sci. Rep. 2019, 9, 15186. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martinez Florez, S.; Agudo Toyos, P.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Zhang, L.; Rasul, A.; Anwar, H.; Sohail, M.U.; Razzaq, A.; Aziz, N.; Shabbir, A.; Ali, M.; Sun, T. Role of plant-derived flavonoids and their mechanism in attenuation of Alzheimer’s and Parkinson’s diseases: An update of recent data. Molecules 2018, 23, 814. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.G.; Otieno, D.; Ha, K. Flavonoids, potential bioactive compounds, and non-shivering thermogenesis. Nutrients 2018, 10, 1168. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Yu, O. Metabolic engineering of flavonoids in plants and microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hou, J.P.; Guo, Y.X.; Ren, J. Antioxidant activities of the extracts of Biebersteinia heterostemon Maxim. Food Ind. 2014, 35, 169–171. [Google Scholar]
- Ko, M.J.; Cheigh, C.I.; Chung, M.S. Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chem. 2014, 143, 147–155. [Google Scholar] [CrossRef]
- Thavamoney, N.; Sivanadian, L.; Tee, L.H.; Khoo, H.E.; Prasad, K.N.; Kong, K.W. Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents. J. Food. Sci. Technol. 2018, 55, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes Drummond, E.S.F.; Miralles, B.; Hernandez-Ledesma, B.; Amigo, L.; Iglesias, A.H.; Reyes Reyes, F.G.; Netto, F.M. Influence of protein-phenolic complex on the antioxidant capacity of flaxseed (Linum usitatissimum L.) products. J. Agric. Food Chem. 2017, 65, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Fuentes, I.; Vega-Galvez, A.; Aranda, M. Evaluation of phenolic profiles and antioxidant capacity of maqui (Aristotelia chilensis) berries and their relationships to drying methods. J. Sci. Food Agric. 2018, 98, 4168–4176. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Jojart, R.; Fonad, P.; Mihaly, R.; Palagyi, A. Phenolic composition and antioxidant activity of colored oats. Food Chem. 2018, 268, 153–161. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, G.; Jin, M.; Niu, L.X.; Zhang, Y.L. Variation in phenolic content, profile, and antioxidant activity of seeds among different Paeonia ostii cultivated populations in China. Chem. Biodivers. 2018, 15, e1800093. [Google Scholar] [CrossRef]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef]
- Yan, X.; Murphy, B.T.; Hammond, G.B.; Vinson, J.A.; Neto, C.C. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef]
- Dabaghian, F.H.; Entezari, M.; Ghobadi, A.; Hashemi, M. Antimutagenicity and anticancer effects of Biebersteinia multifida DC. Annu. Rev. Res. Biol. 2014, 4, 906–913. [Google Scholar] [CrossRef]
- Golshan, A.; Hassanzadeh, S.; Mojdekanloo, M.; Tayarani-Najaran, Z. Effects of Biebersteinia multifida hydro-ethanol extract on proliferation and apoptosis of human prostate cancer and human embryonic kidney cells. Avicenna J. Phytomed. 2016, 6, 671–677. [Google Scholar] [PubMed]
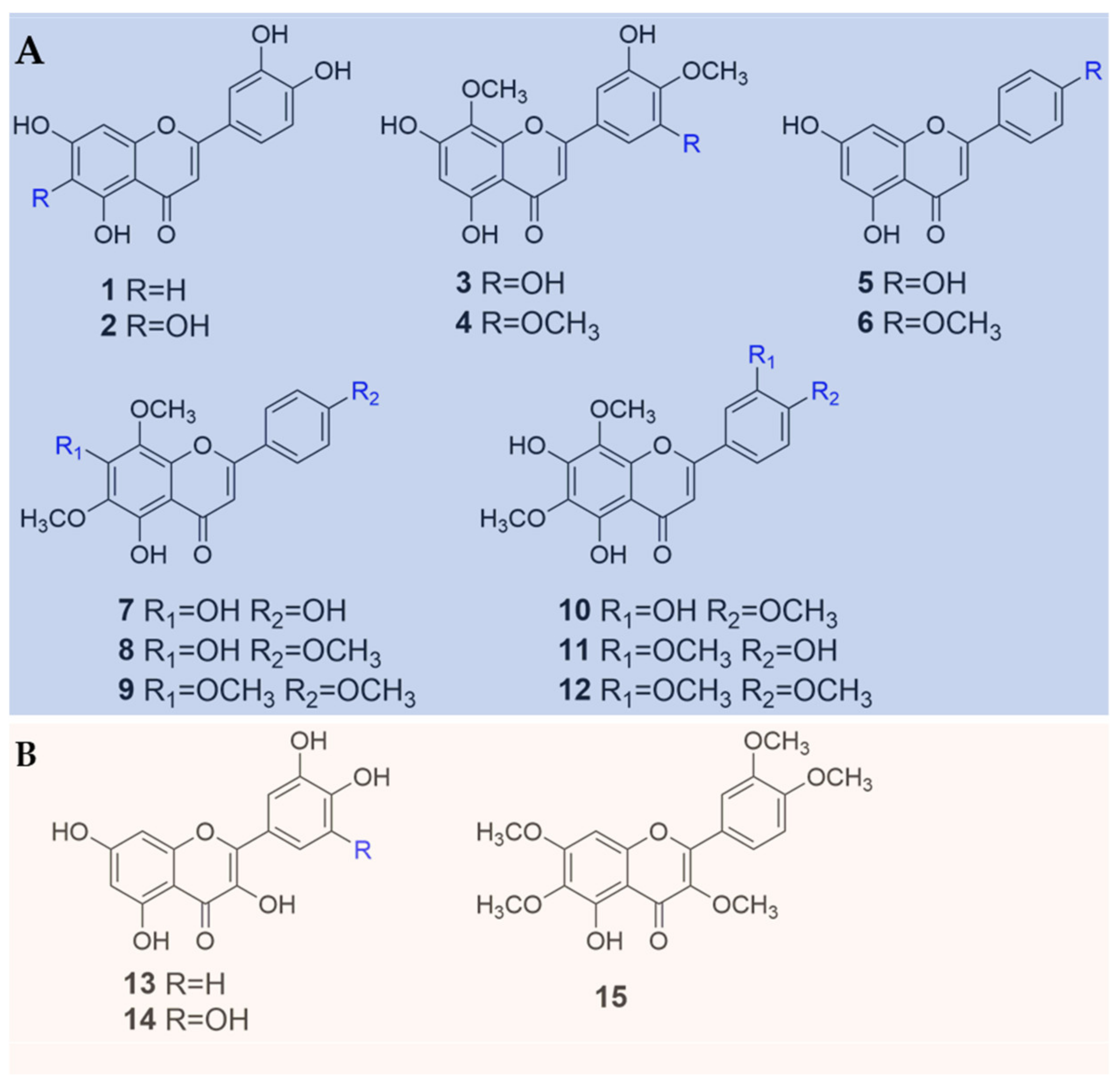
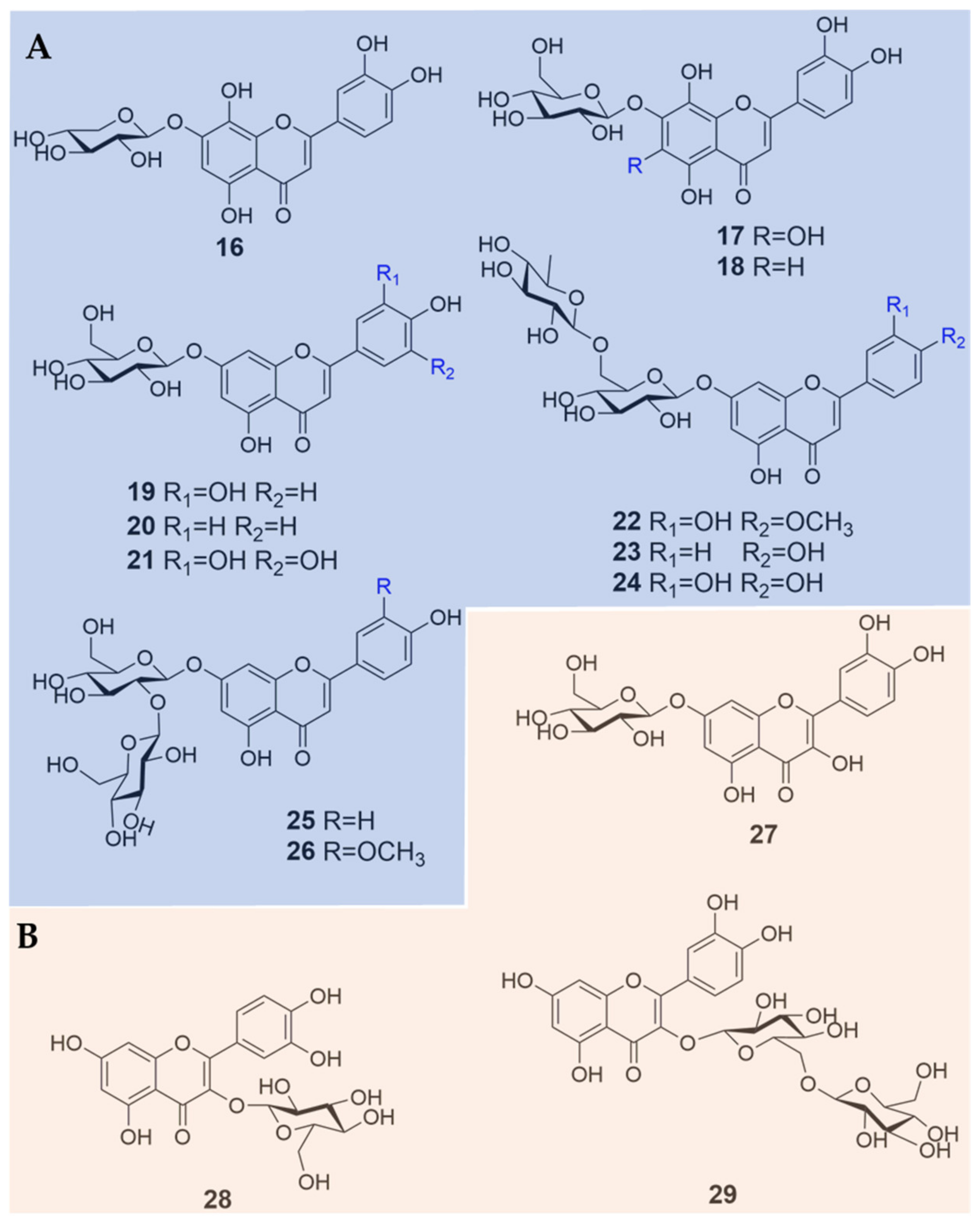
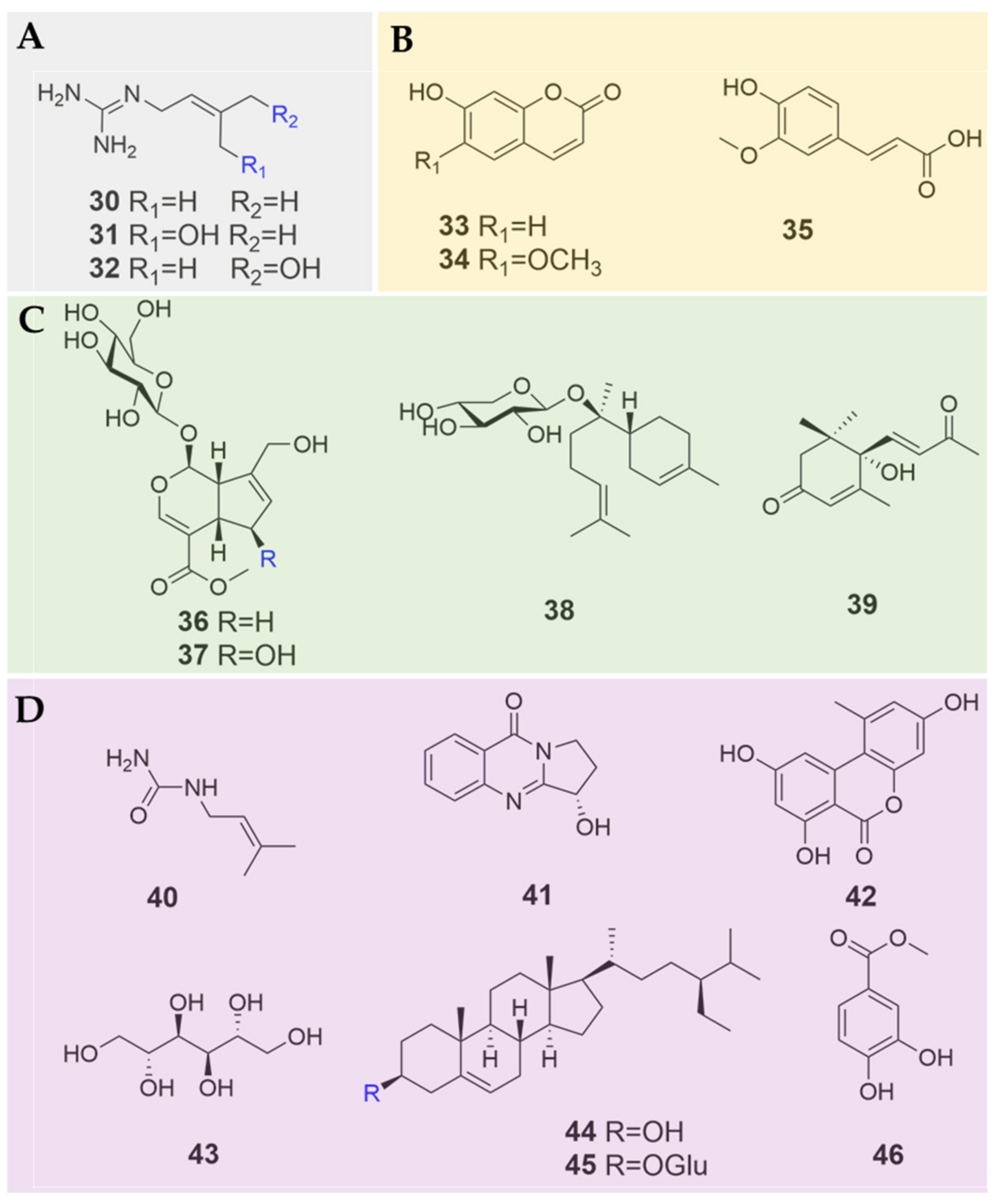

| No. | Compound Name | Sources | References |
|---|---|---|---|
| Flavonoids | |||
| 1 | luteolin | B. heterostemon | [11,13,15] |
| B. multifida | |||
| B. orphanidis | |||
| 2 | 6-hydroxyluteolin | B. heterostemon | [13] |
| 3 | 4′-methoxytricetin | B. heterostemon | [14] |
| 4 | 5,7,3′-trihydroxy-8,4′,5′-trimethoxyflavone | B. heterostemon | [11] |
| 5 | apigenin | B. orphanidis | [15] |
| 6 | acacetin | B. orphanidis | [15] |
| 7 | 5,7,4′-trihydroxy-6,8-dimethoxyflavone | B. orphanidis | [15] |
| 8 | nevadensin | B. orphanidis | [15] |
| 9 | gardenin B | B. orphanidis | [15] |
| 10 | acerosin | B. orphanidis | [15] |
| 11 | sudachitin | B. orphanidis | [15] |
| 12 | hymenoxin | B. orphanidis | [15] |
| 13 | quercetin | B. heterostemon | [12] |
| 14 | myricetin | B. odora | [16] |
| 15 | artemetin | B. heterostemon | [23] |
| 16 | hypolaetin-7-O-β-D-xylopyranoside | B. heterostemon | [12] |
| 17 | hypolaetin-7-O-β-D-glucopyranoside | B. heterostemon | [13] |
| 18 | 3′,4′,5,8-tetrahydroxyflavanone-7-O-β-glucopyranoside | B. heterostemon | [24] |
| 19 | luteolin-7-O-glucoside | B. heterostemon | [11,13,15] |
| B. multifida | |||
| B. orphanidis | |||
| 20 | apigenin-7-O-glucoside | B. orphanidis | [15] |
| 21 | tricetin-7-O-glucoside | B. orphanidis | [15] |
| 22 | diosmin | B. heterostemon | [13] |
| 23 | apigenin-7-O-rutinoside | B. heterostemon | [13,15] |
| B. orphanidis | |||
| 24 | luteolin-7-O-rutinoside | B. heterostemon | [13,15] |
| B. multifida | |||
| 25 | apigenin-7-O-sophoroside | B. heterostemon | [13] |
| 26 | chrysoeriol-7-O-sophoroside | B. heterostemon | [13] |
| 27 | quercetin-7-O-glucoside | B. heterostemon | [11] |
| 28 | quercetin-3-O-β-glucopyranoside | B. heterostemon | [13] |
| 29 | quercetin-3-O-β-D-glucopyranosyl | B. heterostemon | [13] |
| (1→2)-β-D-glucopyranoside | |||
| Guanidines | |||
| 30 | galegine | B. heterostemon | [12,25] |
| 31 | cis-4-hydroxy galegine | B. heterostemon | [25] |
| 32 | trans-4-hydroxy galegine | B. heterostemon | [25] |
| Phenylpropanoids | |||
| 33 | umbelliferone | B. multifida | [23] |
| 34 | scopoletin | B. multifida | [23] |
| 35 | ferulic acid | B. multifida | [23] |
| Terpenoids | |||
| 36 | geniposide | B. heterostemon | [26] |
| 37 | 6β-hydroxy geniposide | B. heterostemon | [26] |
| 38 | (-)-anymol-8-O-β-D-lyxopyranoside | B. heterostemon | [26] |
| Other Types | |||
| 39 | (+)-dehydrovomifoliol | B. heterostemon | [24] |
| 40 | N-3-methyl-2-butenyl urea | B. heterostemon | [11] |
| 41 | vasicinone | B. multifida | [27] |
| 42 | alternariol | B. heterostemon | [24] |
| 43 | mannitol | B. heterostemon | [12] |
| 44 | β-sitosterol | B. heterostemon | [11,12,24] |
| 45 | daucosterol | B. heterostemon | [12] |
| 46 | protocatechuic acid methyl ester | B. heterostemon | [24] |
| Fatty Acids | |||
| 47 | myristic acid | B. orphanidis | [28] |
| 48 | palmitic acid | B. heterostemon | [28,29] |
| B. orphanidis | |||
| 49 | stearic acid | B. heterostemon | [28,29] |
| B. orphanidis | |||
| 50 | arachidic acid | B. heterostemon | [29] |
| 51 | docosanoic acid | B. orphanidis | [28] |
| 52 | tetracosanoic acid | B. orphanidis | [28] |
| 53 | hexacosanoic acid | B. orphanidis | [28] |
| 54 | palmitoleic acid | B. heterostemon | [28,29] |
| B. orphanidis | |||
| 55 | oleic acid | B. heterostemon | [28,29] |
| B. orphanidis | |||
| 56 | eicosenoic acid | B. heterostemon | [28,29] |
| B. orphanidis | |||
| 57 | linoleic acid | B. heterostemon | [28,29] |
| B. orphanidis | |||
| 58 | α-linolenic acid | B. heterostemon | [28,29] |
| B. orphanidis | |||
| 59 | γ-linolenic acid | B. heterostemon | [29] |
| 60 | 7,10,13-hexadecatrienoic acid | B. orphanidis | [28] |
| No. | Compound Name | Molecular Formula | Retention Indices (RI) | Sources | References |
|---|---|---|---|---|---|
| 1 | α-thujene | C10H16 | 920 | B. multifida | [47] |
| 2 | α-pinene | C10H16 | 939 | B. multifida | [44,45,46,48] |
| B. heterostemon | |||||
| 3 | camphene | C10H16 | 946 | B. multifida | [45,48] |
| B. heterostemon | |||||
| 4 | sabinene | C10H16 | 970 | B. multifida | [45] |
| 5 | β-pinene | C10H16 | 978 | B. multifida | [45,48] |
| B. heterostemon | |||||
| 6 | 6-methyl-5-hepten-2-one | C8H14O | 988 | B. multifida | [47] |
| 7 | myrcene | C10H16 | 991 | B. multifida | [45,48] |
| B. heterostemon | |||||
| 8 | α-phellandrene | C10H16 | 1005 | B. multifida | [45] |
| 9 | α-terpinene | C10H16 | 1018 | B. multifida | [45] |
| 10 | p-cymene | C10H14 | 1025 | B. heterostemon | [48] |
| 11 | limonene | C10H16 | 1029 | B. multifida | [45,48] |
| B. heterostemon | |||||
| 12 | 1,8-cineole | C10H18O | 1033 | B. multifida | [45,47,48] |
| B. heterostemon | |||||
| 13 | trans-β-ocimene | C10H16 | 1050 | B. heterostemon | [48] |
| 14 | γ-terpinene | C10H16 | 1062 | B. multifida | [45,48] |
| B. heterostemon | |||||
| 15 | trans-sabinene hydrate | C10H18O | 1064 | B. multifida | [44,45] |
| 16 | linalool | C10H18O | 1099 | B. multifida | [44,45,48] |
| B. heterostemon | |||||
| 17 | nonanal | C9H18O | 1102 | B. multifida | [45] |
| 18 | octyl acetate | C10H20O2 | 1124 | B. multifida | [45] |
| 19 | cis-limonene oxide | C10H16O | 1131 | B. orphanidis | [43] |
| 20 | trans-pinocarveol | C10H16O | 1140 | B. multifida | [45] |
| 21 | camphor | C10H16O | 1143 | B. multifida | [44,47,48] |
| B. heterostemon | |||||
| 22 | (Z)-3-nonenol | C9H18O | 1158 | B. multifida | [47] |
| 23 | pinocarvone | C10H14O | 1164 | B. multifida | [45] |
| 24 | borneol | C10H18O | 1168 | B. multifida | [47] |
| 25 | terpinen-4-ol | C10H18O | 1177 | B. multifida | [45] |
| 26 | α-terpineol | C10H18O | 1189 | B. multifida | [43,45,47] |
| B. orphanidis | |||||
| 27 | myrtenal | C10H14O | 1197 | B. multifida | [45] |
| 28 | decanal | C10H20O | 1204 | B. multifida | [47] |
| 29 | trans-carveol | C10H16O | 1217 | B. multifida | [45] |
| 30 | carvone | C10H14O | 1242 | B. multifida | [45,47,48] |
| B. heterostemon | |||||
| 31 | geraniol | C10H18O | 1255 | B. heterostemon | [48] |
| 32 | linalyl acetate | C12H20O2 | 1257 | B. orphanidis | [43] |
| 33 | isobornyl acetate | C12H20O2 | 1283 | B. multifida | [47] |
| 34 | bornyl acetate | C12H20O2 | 1285 | B. multifida | [45] |
| 35 | thymol | C10H14O | 1290 | B. multifida | [45] |
| 36 | (2E,4Z)-decadienal | C10H16O | 1293 | B. multifida | [47] |
| 37 | carvacrol | C10H14O | 1302 | B. multifida | [47] |
| 38 | (2E,4E)-decadienal | C10H16O | 1316 | B. multifida | [47] |
| 39 | δ-elemene | C15H24 | 1339 | B. heterostemon | [48] |
| 40 | α-longipinene | C15H24 | 1352 | B. heterostemon | [48] |
| 41 | eugenol | C10H12O2 | 1361 | B. multifida | [47] |
| 42 | α-ylangene | C15H24 | 1374 | B. multifida | [47] |
| 43 | geranyl acetate | C12H20O2 | 1381 | B. heterostemon | [48] |
| 44 | β-elemene | C15H24 | 1391 | B. multifida | [43,45,48] |
| B. orphanidis | |||||
| B. heterostemon | |||||
| 45 | tetradecane | C14H30 | 1400 | B. multifida | [46] |
| 46 | isocaryophyllene | C15H24 | 1408 | B. multifida | [47] |
| 47 | cis-caryophyllene | C15H24 | 1409 | B. heterostemon | [48] |
| 48 | α-gurjunene | C15H24 | 1412 | B. orphanidis | [43] |
| 49 | β-caryophyllene | C15H24 | 1416 | B. multifida | [43,44,45,47,48] |
| B. orphanidis | |||||
| B. heterostemon | |||||
| 50 | α-bergamotene | C15H24 | 1418 | B. heterostemon | [48] |
| 51 | β-duprezianene | C15H24 | 1424 | B. multifida | [47] |
| 52 | γ-elemene | C15H24 | 1431 | B. multifida | [45,48] |
| B. heterostemon | |||||
| 53 | α-humulene | C15H24 | 1449 | B. multifida | [45,48] |
| B. heterostemon | |||||
| 54 | β-farnesene | C15H24 | 1457 | B. multifida | [44,45,47,48] |
| B. heterostemon | |||||
| 55 | allo-aromadendrene | C15H24 | 1462 | B. multifida | [44,48] |
| B. heterostemon | |||||
| 56 | α-amorphene | C15H24 | 1480 | B. heterostemon | [48] |
| 57 | germacrene D | C15H24 | 1485 | B. multifida | [45,48] |
| B. heterostemon | |||||
| 58 | (E)-β-ionone | C13H20O | 1486 | B. multifida | [47] |
| 59 | cis-β-guaiene | C15H24 | 1487 | B. heterostemon | [48] |
| 60 | epi-cubebol | C15H26O | 1495 | B. multifida | [47] |
| 61 | bicyclogermacrene | C15H24 | 1495 | B. multifida | [45] |
| 62 | α-selinene | C15H24 | 1498 | B. heterostemon | [48] |
| 63 | germacrene A | C15H24 | 1501 | B. heterostemon | [48] |
| 64 | β-bisabolene | C15H24 | 1505 | B. multifida | [47] |
| 65 | α-farnesene | C15H24 | 1507 | B. multifida | [44] |
| 66 | γ-cadinene | C15H24 | 1512 | B. multifida | [44,47] |
| 67 | δ-cadinene | C15H24 | 1522 | B. multifida | [44,45,47] |
| 68 | d-cadinene | C15H24 | 1525 | B. heterostemon | [48] |
| 69 | guaia-3,9-diene | C15H24 | 1534 | B. heterostemon | [48] |
| 70 | α-cadinene | C15H24 | 1536 | B. multifida | [44,48] |
| B. heterostemon | |||||
| 71 | nerolidol | C15H26O | 1538 | B. multifida | [45,48] |
| B. heterostemon | |||||
| 72 | eudesma-3,7(11)-diene | C15H24 | 1545 | B. heterostemon | [48] |
| 73 | elemol | C15H26O | 1552 | B. multifida | [44] |
| 74 | elixene | C15H24 | 1559 | B. heterostemon | [48] |
| 75 | germacrane B | C15H24 | 1563 | B. orphanidis | [43] |
| 76 | (E)-nerolidol | C15H26O | 1565 | B. multifida | [44,45,46,47] |
| 77 | spathulenol | C15H24O | 1578 | B. multifida | [43,45,47] |
| B. orphanidis | |||||
| 78 | caryophyllene oxide | C15H24O | 1583 | B. multifida | [43,44,45,47,48] |
| B. orphanidis | |||||
| B. heterostemon | |||||
| 79 | viridiflorol | C15H26O | 1594 | B. multifida | [44] |
| 80 | hexadecane | C16H34 | 1600 | B. multifida | [46] |
| 81 | guaiol | C15H26O | 1601 | B. multifida | [47] |
| 82 | humulene epoxide II | C15H24O | 1610 | B. multifida | [44] |
| 83 | β-elemenone | C15H22O | 1612 | B. heterostemon | [48] |
| 84 | dillapiole | C12H14O4 | 1624 | B. multifida | [47] |
| 85 | τ-cadinol | C15H26O | 1635 | B. multifida | [44,45] |
| 86 | epi-α-cadinol | C15H26O | 1642 | B. multifida | [43,47] |
| B. orphanidis | |||||
| 87 | α-eudesmol | C15H26O | 1656 | B. multifida | [44,47] |
| 88 | α-bisabolol oxide B | C15H26O2 | 1658 | B. orphanidis | [43] |
| 89 | bulnesol | C15H26O | 1671 | B. multifida | [44,47] |
| 90 | (Z)-α-santalol | C15H24O | 1674 | B. orphanidis | [43] |
| 91 | cis-β-elemenone | C15H22O | 1678 | B. heterostemon | [48] |
| 92 | α-bisabolol | C15H26O | 1688 | B. multifida | [43,44,47,48] |
| B. orphanidis | |||||
| B. heterostemon | |||||
| 92 | germacrone | C15H22O | 1699 | B. heterostemon | [48] |
| 94 | (E)-nerolidol acetate | C15H26O | 1714 | B. multifida | [44,47] |
| 95 | (2E,6E)-farnesol | C15H26O | 1727 | B. multifida | [44,47] |
| 96 | octadecane | C18H38 | 1800 | B. multifida | [46,47] |
| 97 | neophytadiene | C20H38 | 1836 | B. multifida | [46] |
| 98 | 6,10,14-trimethyl-2-pentadecanone | C18H36O | 1845 | B. multifida | [44,46,47] |
| 99 | nonadecane | C19H40 | 1900 | B. multifida | [47] |
| 100 | farnesyl acetone | C18H30O | 1917 | B. multifida | [44,47] |
| 101 | methyl linolenate | C19H32O2 | 2098 | B. multifida | [47] |
| 102 | phytol | C20H40O | 2124 | B. multifida | [44,47] |
| 104 | mandenol | C20H36O2 | 2148 | B. multifida | [44] |
| 104 | ethyl linolenate | C20H34O2 | 2162 | B. multifida | [44,47] |
| 105 | 10-cyclohexyl-nonadecane | C25H52 | 2312 | B. multifida | [44] |
| 106 | pentacosane | C25H52 | 2517 | B. multifida | [44] |
| 107 | n-heptacosane | C27H56 | 2682 | B. multifida | [44] |
| 108 | octacosane | C28H58 | 2791 | B. multifida | [44] |
| 109 | nonacosane | C29H60 | 2894 | B. multifida | [44] |
| 110 | vitamin E | C29H50O2 | 3138 | B. multifida | [44] |
| 111 | n-docosane | C22H46 | — | B. multifida | [44] |
| 112 | epizonaren | C15H24 | — | B. multifida | [44] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Jin, X.; Yin, H.; Zhang, D.; Zhou, H.; Zhang, X.; Tran, L.-S.P. Natural Products, Traditional Uses and Pharmacological Activities of the Genus Biebersteinia (Biebersteiniaceae). Plants 2020, 9, 595. https://doi.org/10.3390/plants9050595
Zhang B, Jin X, Yin H, Zhang D, Zhou H, Zhang X, Tran L-SP. Natural Products, Traditional Uses and Pharmacological Activities of the Genus Biebersteinia (Biebersteiniaceae). Plants. 2020; 9(5):595. https://doi.org/10.3390/plants9050595
Chicago/Turabian StyleZhang, Benyin, Xiaona Jin, Hengxia Yin, Dejun Zhang, Huakun Zhou, Xiaofeng Zhang, and Lam-Son Phan Tran. 2020. "Natural Products, Traditional Uses and Pharmacological Activities of the Genus Biebersteinia (Biebersteiniaceae)" Plants 9, no. 5: 595. https://doi.org/10.3390/plants9050595
APA StyleZhang, B., Jin, X., Yin, H., Zhang, D., Zhou, H., Zhang, X., & Tran, L.-S. P. (2020). Natural Products, Traditional Uses and Pharmacological Activities of the Genus Biebersteinia (Biebersteiniaceae). Plants, 9(5), 595. https://doi.org/10.3390/plants9050595






