Characterization of Glutamate-Mediated Hormonal Regulatory Pathway of the Drought Responses in Relation to Proline Metabolism in Brassica napus L.
Abstract
1. Introduction
2. Results
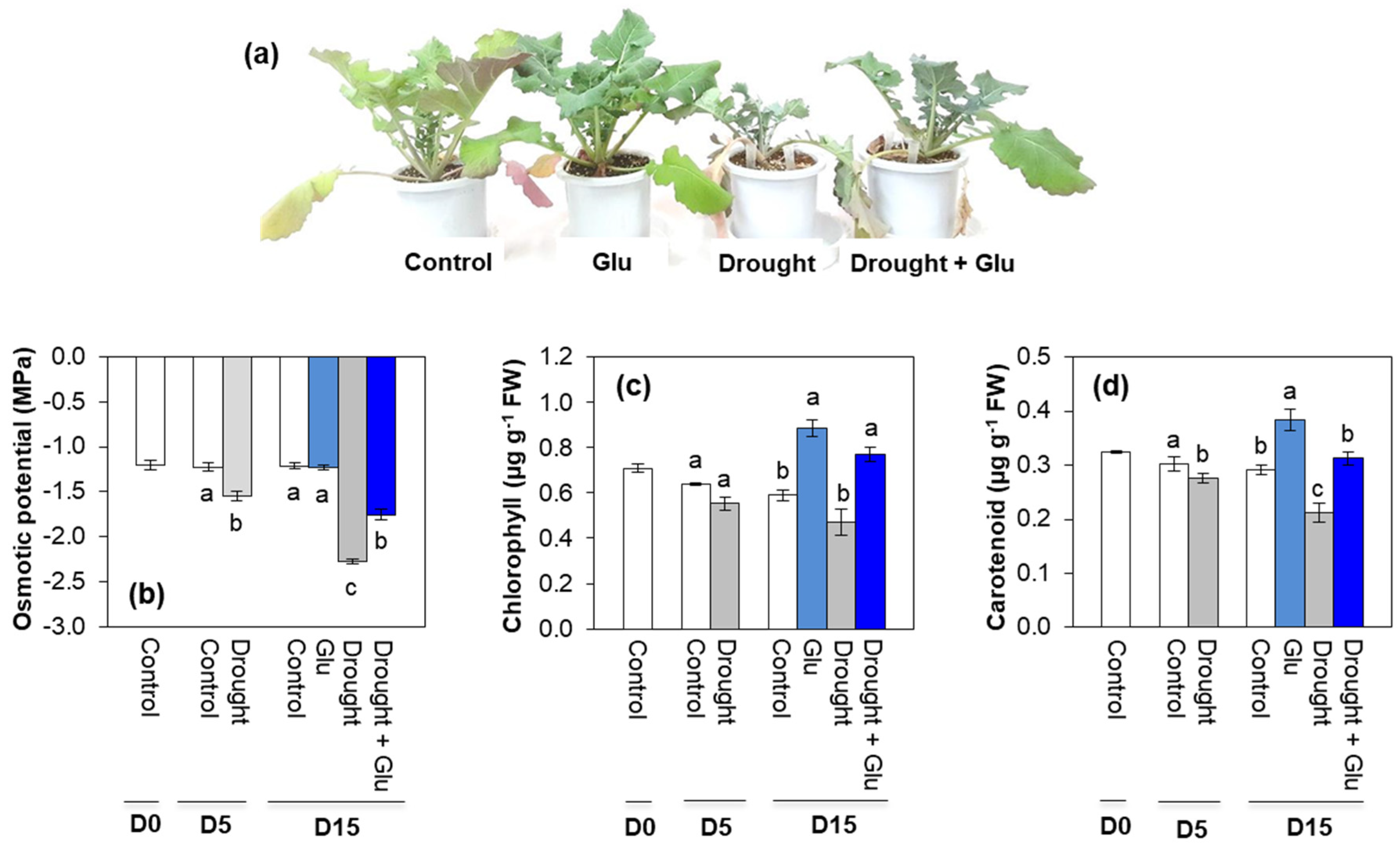
2.1. Physiological Symptoms, Osmotic Potential, and Pigments
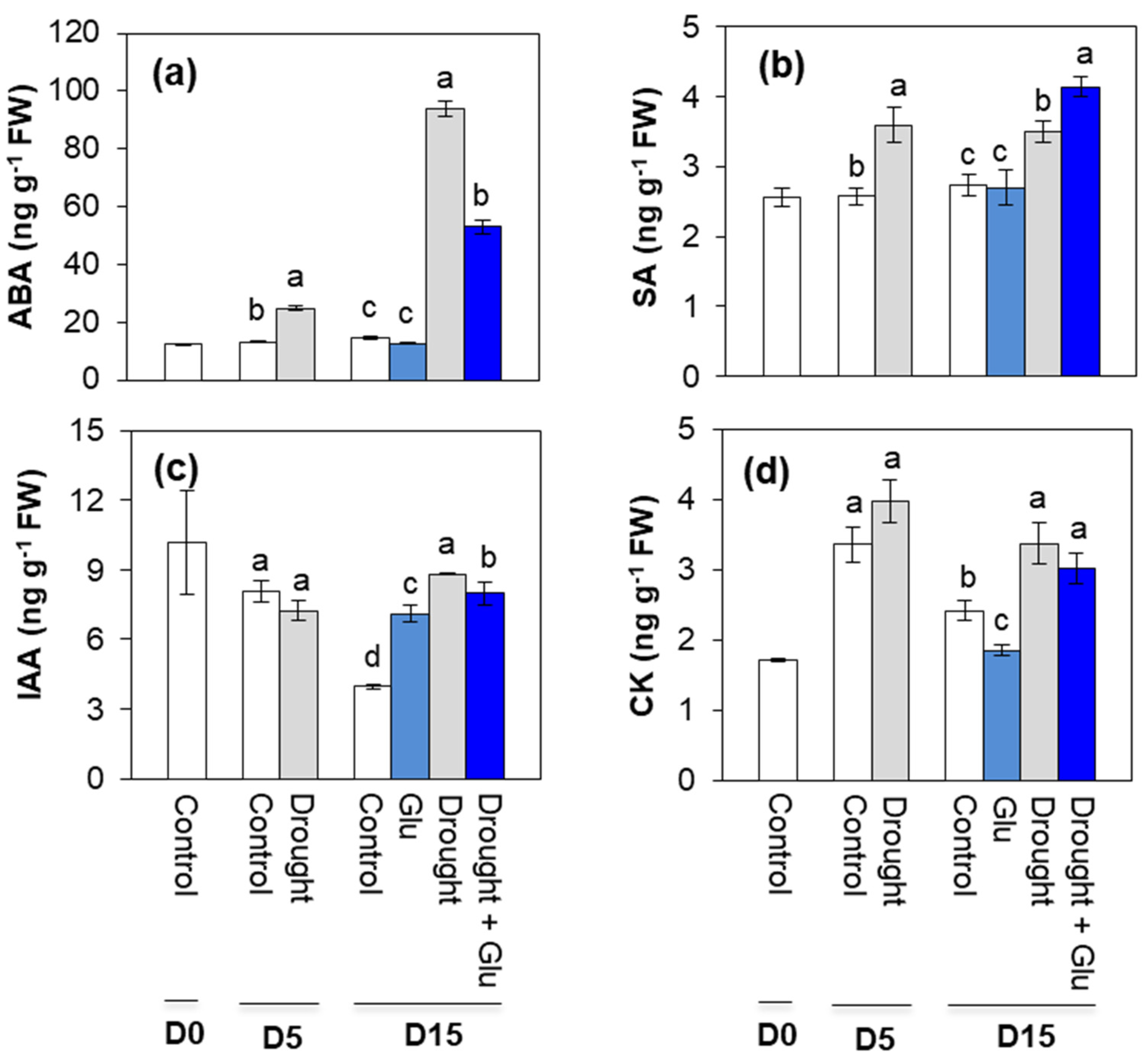
2.2. Phytohormone Content and Related Gene Expression
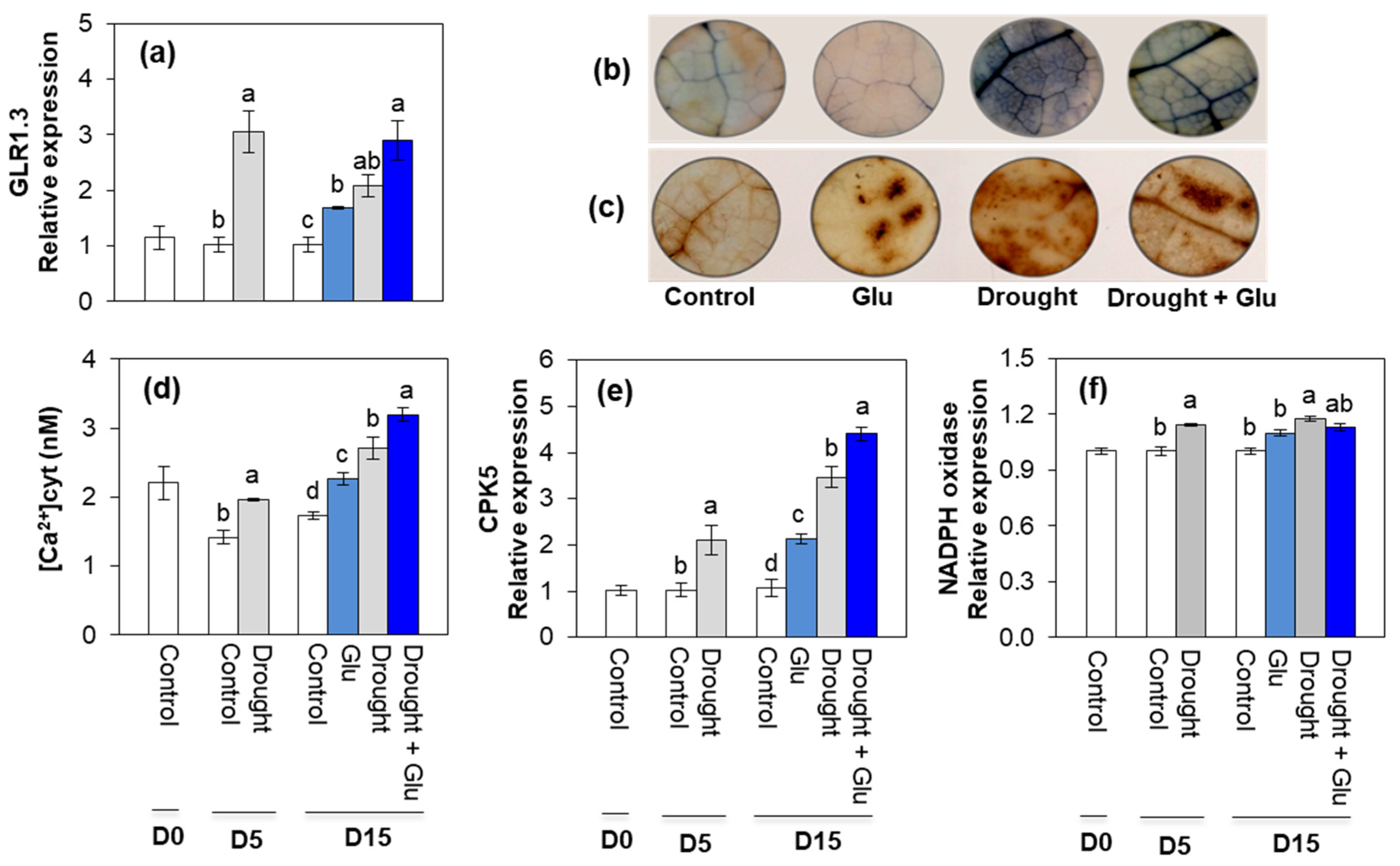
2.3. Glutamate Receptor, ROS, Ca2+ Signaling, and Antioxidant Activity
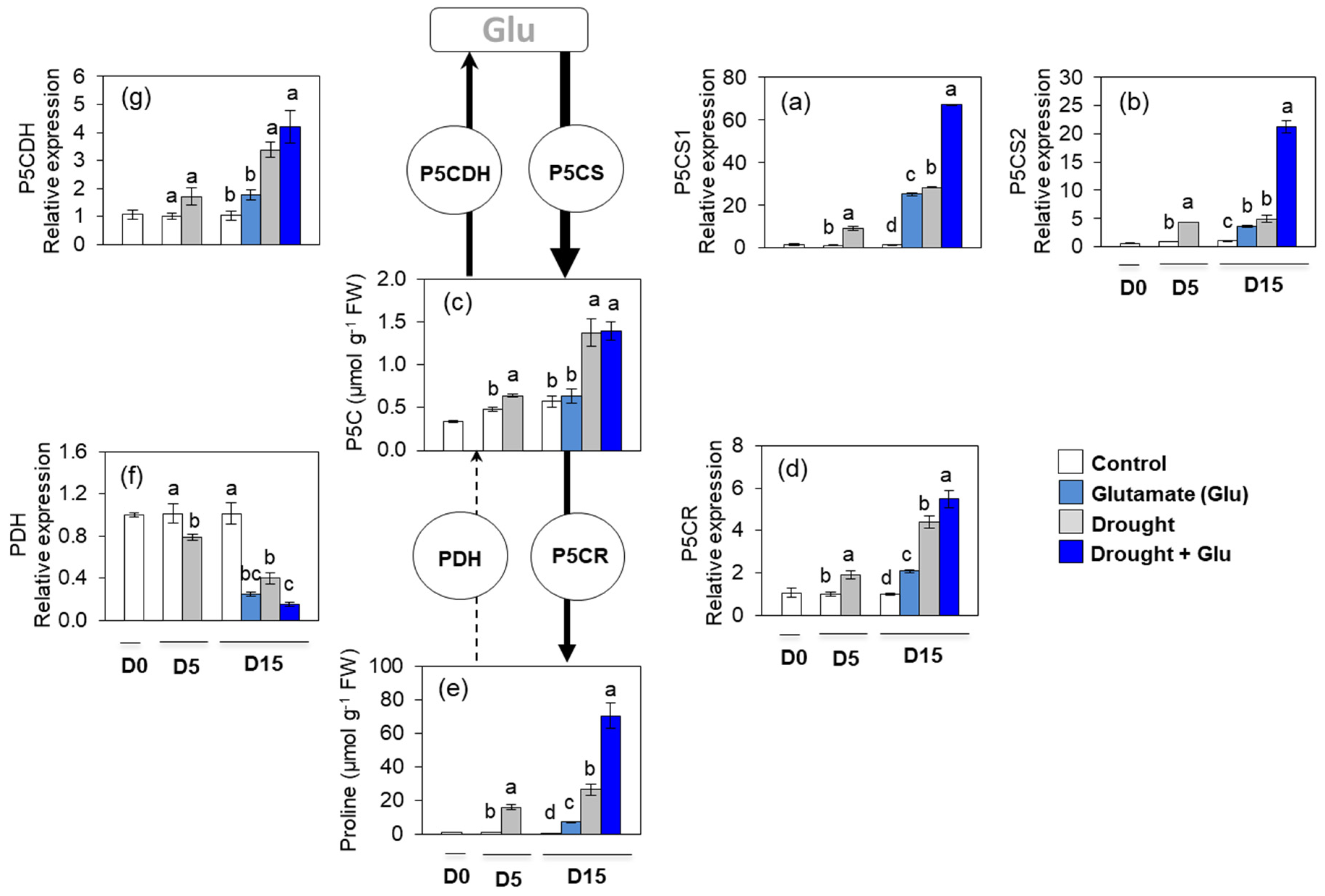
2.4. Proline Metabolism and Transport
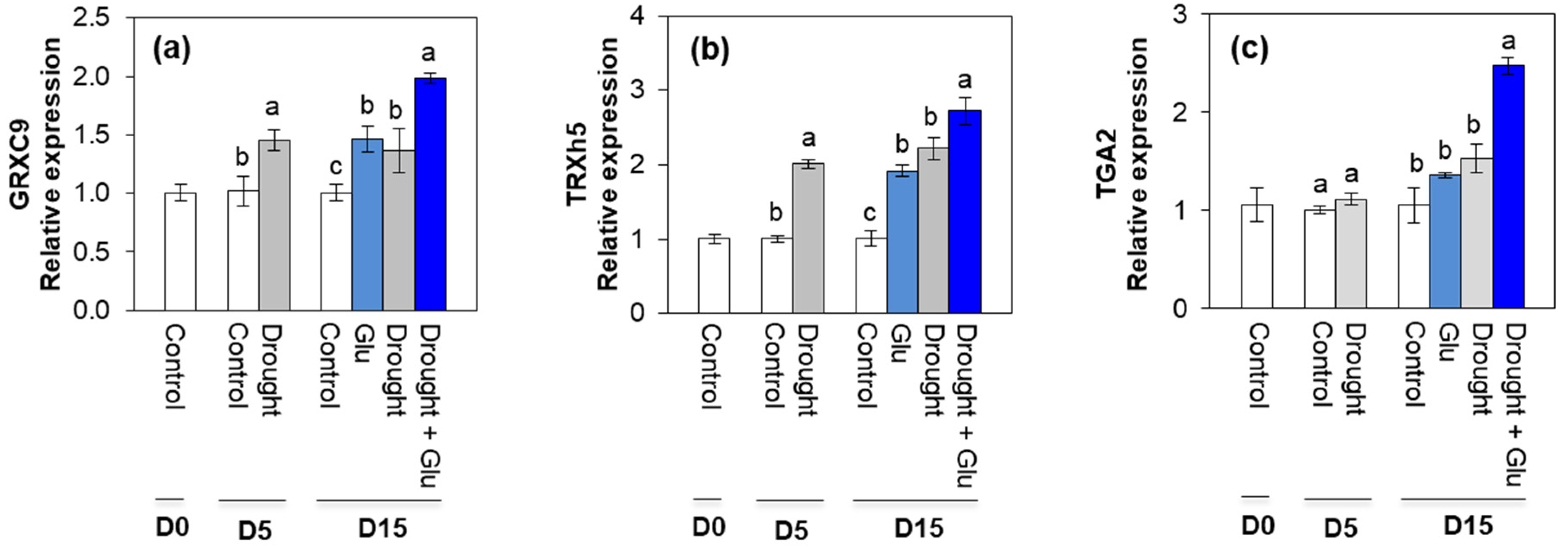
2.5. Redox Status and Redox Signaling Component
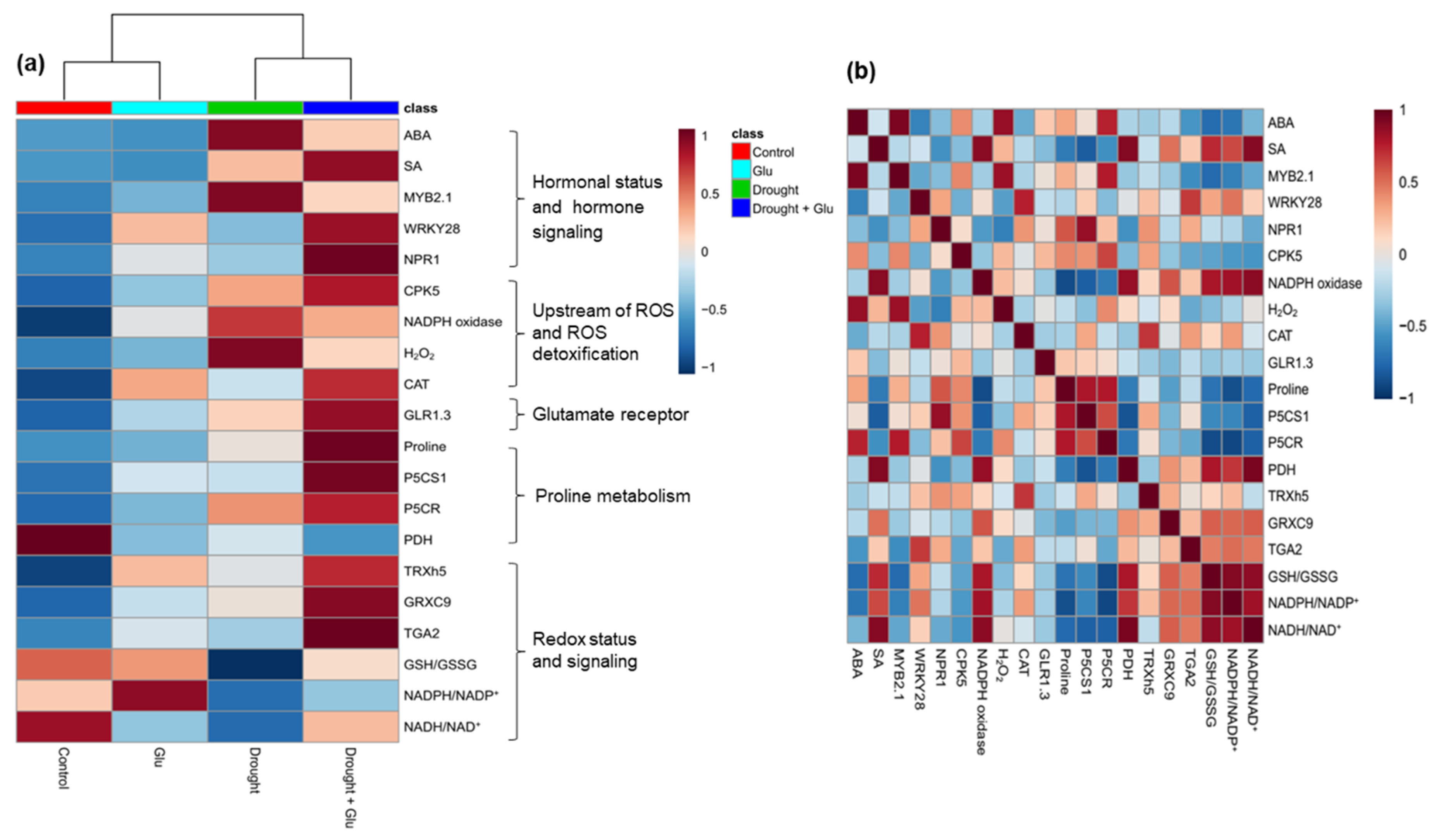
2.6. Heatmap Visualization and Pearson Correlation Analysis for the Metabolites or Gene Expression
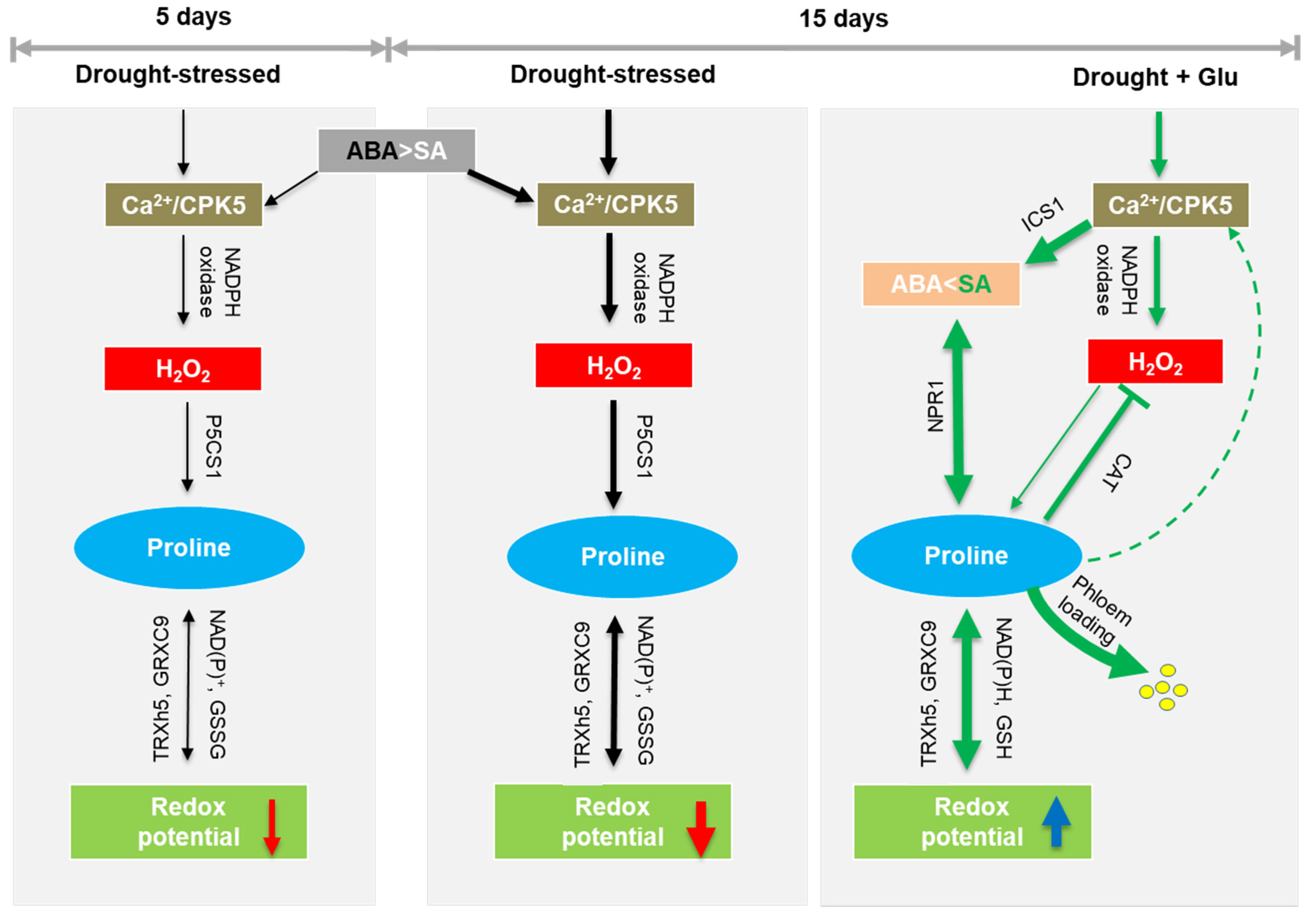
3. Discussion
4. Materials and methods
4.1. Plant Growth and Treatment
4.2. Osmotic Potential and Measurement of Photosynthetic Pigment Content
4.3. Determination of ROS Production and Antioxidative Enzymes Activity
4.4. Measurement of Cytosolic Ca2+ Concentration
4.5. Determination of Proline and Δ1-pyrroline-5-carboxylate Content
4.6. Collection of Phloem Exudate and Xylem Sap
4.7. Measurement of Glutathione and Pyridine Nucleotides
4.8. Phytohormone Analysis
4.9. RNA Extraction and Quantitative Real-Time PCR Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, T.H.; Lee, B.R.; Jung, W.J.; Kim, K.Y.; Avice, J.C.; Qurry, A. De novo protein synthesis in relation to ammonia and proline accumulation in water stressed white clover. Funct. Plant Biol. 2004, 31, 847–855. [Google Scholar] [CrossRef]
- Lee, B.R.; Muneer, S.; Park, S.H.; Zhang, Q.; Kim, T.H. Ammonium-induced proline and sucrose accumulation, and their significance in antioxidative activity and osmotic adjustment. Acta Physiol. Plant. 2013, 35, 2655–2664. [Google Scholar] [CrossRef]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Jin, Y.L.; Park, S.H.; Zaman, R.; Zhang, Q.; Avice, J.C.; Qurry, A.; Kim, T.H. Genotypic variation in N uptake and assimilation estimated by 15N tracing in water deficit-stressed Brassica napus. Environ. Exp. Bot. 2015, 109, 73–79. [Google Scholar] [CrossRef]
- Finiti, I.; Leyva, M.O.; Vicedo, B.; Gómez-Pastor, R.; López-Cruz, J.; García-Agustín, P.; Real, M.D.; González-Bosch, C. Hexanoic acid protects tomato plants against Botrytis cinerea by priming defence responses and reducing oxidative stress. Mol. Plant Pathol. 2014, 15, 550–562. [Google Scholar] [CrossRef]
- Islam, M.T.; Lee, B.R.; Park, S.H.; La, V.H.; Bae, D.W.; Kim, T.H. Cultivar variation in hormonal balance is a significant determinant of disease susceptibility to Xanthomonas campestris pv. campestris in Brassica napus. Front. Plant Sci. 2017, 8, 2121. [Google Scholar] [CrossRef]
- Lee, B.R.; Li, L.S.; Jung, W.J.; Jin, Y.L.; Avice, J.C.; Qurry, A.; Kim, T.H. Water deficit-induced oxidative stress and the activation of antioxidant enzymes in white clover leaves. Biol. Plant. 2009, 53, 505–510. [Google Scholar] [CrossRef]
- Hong, Z.; Lakkineni, K.; Zhang, Z.; Verma, D.P.S. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthestase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000, 122, 1129–1136. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.R.; Islam, M.T.; Park, S.H.; Jung, H.I.; Bae, D.W.; Kim, T.H. Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ. Exp. Bot. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Rejeb, B.K.; Lefebvre-De Vos, D.; Le Disquet, I.; Leprince, A.S.; Bordenave, M.; Maldinev, R.; Jdey, A.; Abdelly, C.; Savouré, A. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytol. 2015, 208, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A mulitfunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanism of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vásquez, A.; Carvallo, L.; Blanco, B.; Tobar, M.; Villarroel-Candia, E.; Vicente-Carbaijosa, J.; Salinas, P.; Holuique, L. Transcriptional control of glutaredoxin GRXC9 expression by a salicylic acid-dependent and NPR1-independent pathway in Arabidopsis. Plant Mol. Biol. Report. 2015, 33, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signalling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Chung, J.S.; Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M.; Shi, H. Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 2008, 53, 554–565. [Google Scholar] [CrossRef]
- Verslues, P.E.; Kim, Y.S.; Zhu, J.K. Altered ABA, proline and hydrogen peroxide in an Arabidopsis glutamate: Glyoxylate aminotransferase mutant. Plant Mol. Biol. 2007, 64, 205–217. [Google Scholar] [CrossRef]
- Seyfferth, C.; Tsuda, K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcription reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Wang, C.; Lü, W.; Jin, J.B.; Hua, X. Proline induces calcium-mediated oxidative burst and salicylic acid signaling. Amino Acids 2011, 40, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.C.; Chung, T.Y.; Wu, H.Y.; Juo, Y.A.; Hsieh, M.H. Exogenous glutamate rapidly induces the expression of genes involved in metabolism and defense response in rice roots. BMC Genom. 2017, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Jin, Y.L.; Avice, J.C.; Cliquet, J.B.; Ourry, A.; Kim, T.H. Increased proline loading to phloem and its effects on nitrogen uptake and assimilation in water-stressed white clover (Trifolium repens). New Phytol. 2009, 182, 654–663. [Google Scholar] [CrossRef]
- Stael, S.; Kmiecik, P.; Willems, P.; Van der Kelen, K.; Coll, N.S.; Teige, M.; Breusegem, F.V. Plant innate immunity-sunny side up? Trends Plant Sci. 2015, 20, 3–11. [Google Scholar] [CrossRef]
- Prodhan, M.Y.; Munemasa, S.; Nahar, M.N.E.N.; Nakamura, Y.; Murata, Y. Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol. 2018, 178, 441–450. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit required for defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Asano, T.; Hayashi, N.; Kobayashi, M.; Aoki, N.; Miyao, A.; Mitsuhara, I.; Ichikawa, H.; Komatsu, S.; Hirochika, H.; Kikuchi, S.; et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012, 69, 26–36. [Google Scholar] [CrossRef]
- Wang, L.; Tsuka, K.; Truman, W.; Sato, M.; Nguyen, L.V.; Katagiri, F.; Glazebrook, J. CBP60g and SARD1 play partially redundant critical roles in salicylic acid signaling. Plant J. 2011, 67, 1029–1041. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Ding, P.; Wang, D.; Cheng, Y.T.; He, J.; Gao, M.; Xu, F.; Li, Y.; Zhu, Z.; et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18220–18225. [Google Scholar] [CrossRef]
- Elizabeth, M.; Zhang, S. Calcium-independent activation of salicylic acid –induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol. 2000, 122, 1355–1363. [Google Scholar]
- Straus, M.R.; Rietz, S.; van Themaat, E.; Bartsch, M.; Parker, J.E. Salicylic acid antagonism of EDS1-driven cell death is important for immune and oxidative stress responses in Arabidopsis. Plant J. 2010, 62, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Fan, W.; Dong, X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef]
- Szymanski, D.B.; Liao, B.; Zielinski, R.E. Calmodulin isoforms differentially enhance the binding of cauliflower nuclear proteins and recombinant TGA3 to a region derived from the Arabidopsis Cam-3 promoter. Plant Cell 1996, 8, 1069–1077. [Google Scholar]
- Kneeshaw, S.; Gelinau, S.; Tada, Y.; Loake, G.J.; Spoel, S.H. Selective protein denitrosylation activity of thioredoxin-h5 modulate plant immunity. Mol. Cell 2014, 56, 153–162. [Google Scholar] [CrossRef]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational charges of NPR1 via S-Nitrosylation and Thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef]
- Sharma, S.; Villamor, J.G.; Verslues, P.E. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Graeme, P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Tanaka, K.; Swanson, S.J.; Gilroy, S.; Stacey, G. Extracellular nucleotides elicit cytosolic free calcium oscillation in Arabidopsis. Plant Physiol. 2010, 154, 705–719. [Google Scholar] [CrossRef]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Cold calcium signaling in Arabidopsis involves tow cellular pools and a change in calcium signature after acclimation. Plant Cell 1996, 8, 489–503. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mezl, V.A.; Knox, W.E. Properties and analysis of a stable derivative of pyrroline-5-carboxylic acid for use in metabolic studies. Anal. Biochem. 1976, 74, 430–440. [Google Scholar] [CrossRef]
- Kotov, A.A.; Kotova, L.M. Role of acropetal water transport in regulation of cytokinin levels in stems of pea seedlings. Russ. J. Plant Physiol. 2015, 62, 390–400. [Google Scholar] [CrossRef]
- Pan, X.Q.; Welti, R.; Wang, W.M. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Livak, J.K.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Treatments | Days after Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 15 | |||||||
| Reduced | NADPH | NADH | GSH | NADPH | NADH | GSH | NADPH | NADH | GSH |
| Control | 2.65 ± 0.83 | 5.39 ± 0.48 | 61.51 ± 4.81 | 2.62 ± 0.17 b | 5.58 ± 0.44 a | 58.58 ± 2.01 a | 2.32 ± 0.25 c | 4.44 ± 0.33 c | 53.20 ± 3.26 a |
| Glu | - | - | - | - | - | - | 5.28 ± 0.39 a | 5.07 ± 0.40 bc | 60.35 ± 5.00a |
| Drought | - | - | - | 3.48 ± 0.25 a | 5.79 ± 0.12 a | 15.77 ± 0.21 b | 3.78 ± 0.10 b | 5.37 ± 0.03 b | 7.27 ± 0.12 c |
| Drought + Glu | - | - | - | - | - | - | 4.84 ± 0.04 b | 6.56 ± 0.03 a | 38.67 ± 1.87 b |
| Oxidized | NADP+ | NAD+ | GSSG | NADP+ | NAD+ | GSSG | NADP+ | NAD+ | GSSG |
| Control | 8.86 ± 0.06 | 6.81 ± 0.06 | 2.30 ± 0.05 | 9.62 ± 0.59 b | 6.82 ± 0.30 b | 2.62 ± 0.05 b | 7.23 ± 1.05 d | 6.01 ± 0.22 c | 2.45 ± 0.09 a |
| Glu | - | - | - | - | - | - | 14.36 ± 0.61 c | 8.53 ± 0.25 b | 3.10 ± 0.25 a |
| Drought | - | - | - | 17.80 ± 1.00 a | 9.10 ± 0.17 a | 3.23 ± 0.13 a | 24.57 ± 0.30 a | 9.89 ± 0.07 a | 2.69 ± 0.22 a |
| Drought + Glu | - | - | - | - | - | - | 21.36 ± 0.52 b | 9.51 ± 0.17 a | 2.47 ± 0.22 a |
| Ratios | NADPH/ NADP+ | NADH/ NAD+ | GSH/ GSSG | NADPH/ NADP+ | NADH/ NAD+ | GSH/ GSSG | NADPH/ NADP+ | NADH/ NAD+ | GSH/ GSSG |
| Control | 0.41 ± 0.02 | 0.79 ± 0.07 | 26.65 ± 1.98 | 0.26 ± 0.00 a | 0.81 ± 0.03 a | 25.94 ± 1.02 a | 0.27 ± 0.02 b | 0.74 ± 0.06 a | 21.70 ± 0.67 a |
| Glu | - | - | - | - | - | - | 0.37 ± 0.02 a | 0.60 ± 0.04 ab | 19.58 ± 1.20 ab |
| Drought | - | - | - | 0.21 ± 0.01 a | 0.64 ± 0.02 b | 4.92 ± 0.25 b | 0.15 ± 0.03 c | 0.54 ± 0.00 b | 2.75 ± 0.21 c |
| Drought + Glu | - | - | - | - | - | - | 0.21 ± 0.00 bc | 0.69 ± 0.01 ab | 15.96 ± 1.36 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, V.H.; Lee, B.-R.; Islam, M.T.; Mamun, M.A.; Park, S.-H.; Bae, D.-W.; Kim, T.-H. Characterization of Glutamate-Mediated Hormonal Regulatory Pathway of the Drought Responses in Relation to Proline Metabolism in Brassica napus L. Plants 2020, 9, 512. https://doi.org/10.3390/plants9040512
La VH, Lee B-R, Islam MT, Mamun MA, Park S-H, Bae D-W, Kim T-H. Characterization of Glutamate-Mediated Hormonal Regulatory Pathway of the Drought Responses in Relation to Proline Metabolism in Brassica napus L. Plants. 2020; 9(4):512. https://doi.org/10.3390/plants9040512
Chicago/Turabian StyleLa, Van Hien, Bok-Rye Lee, Md. Tabibul Islam, Md. Al Mamun, Sang-Hyun Park, Dong-Won Bae, and Tae-Hwan Kim. 2020. "Characterization of Glutamate-Mediated Hormonal Regulatory Pathway of the Drought Responses in Relation to Proline Metabolism in Brassica napus L." Plants 9, no. 4: 512. https://doi.org/10.3390/plants9040512
APA StyleLa, V. H., Lee, B.-R., Islam, M. T., Mamun, M. A., Park, S.-H., Bae, D.-W., & Kim, T.-H. (2020). Characterization of Glutamate-Mediated Hormonal Regulatory Pathway of the Drought Responses in Relation to Proline Metabolism in Brassica napus L. Plants, 9(4), 512. https://doi.org/10.3390/plants9040512








