Water Influx through the Wetted Surface of a Sweet Cherry Fruit: Evidence for an Associated Solute Efflux
Abstract
1. Introduction
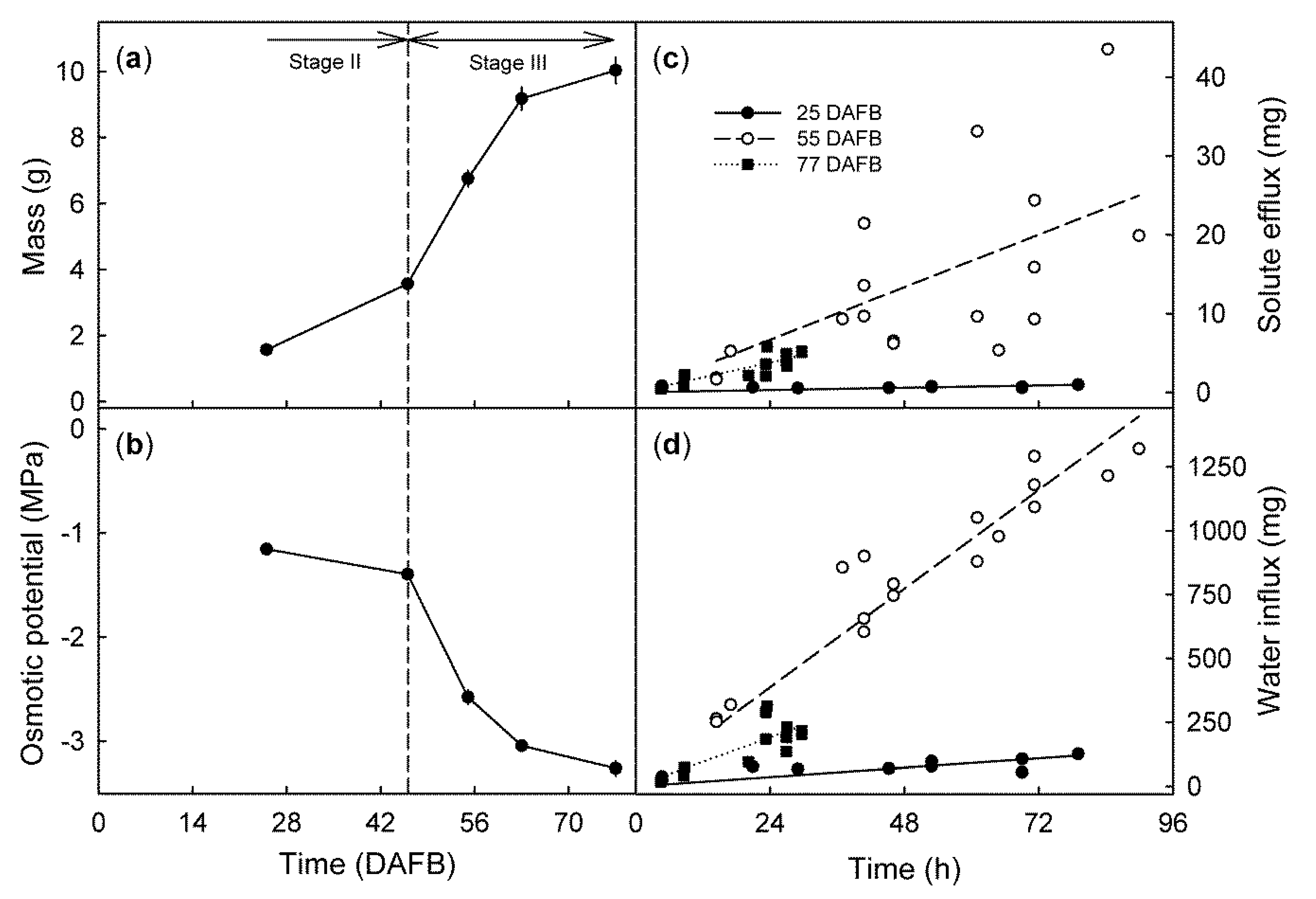
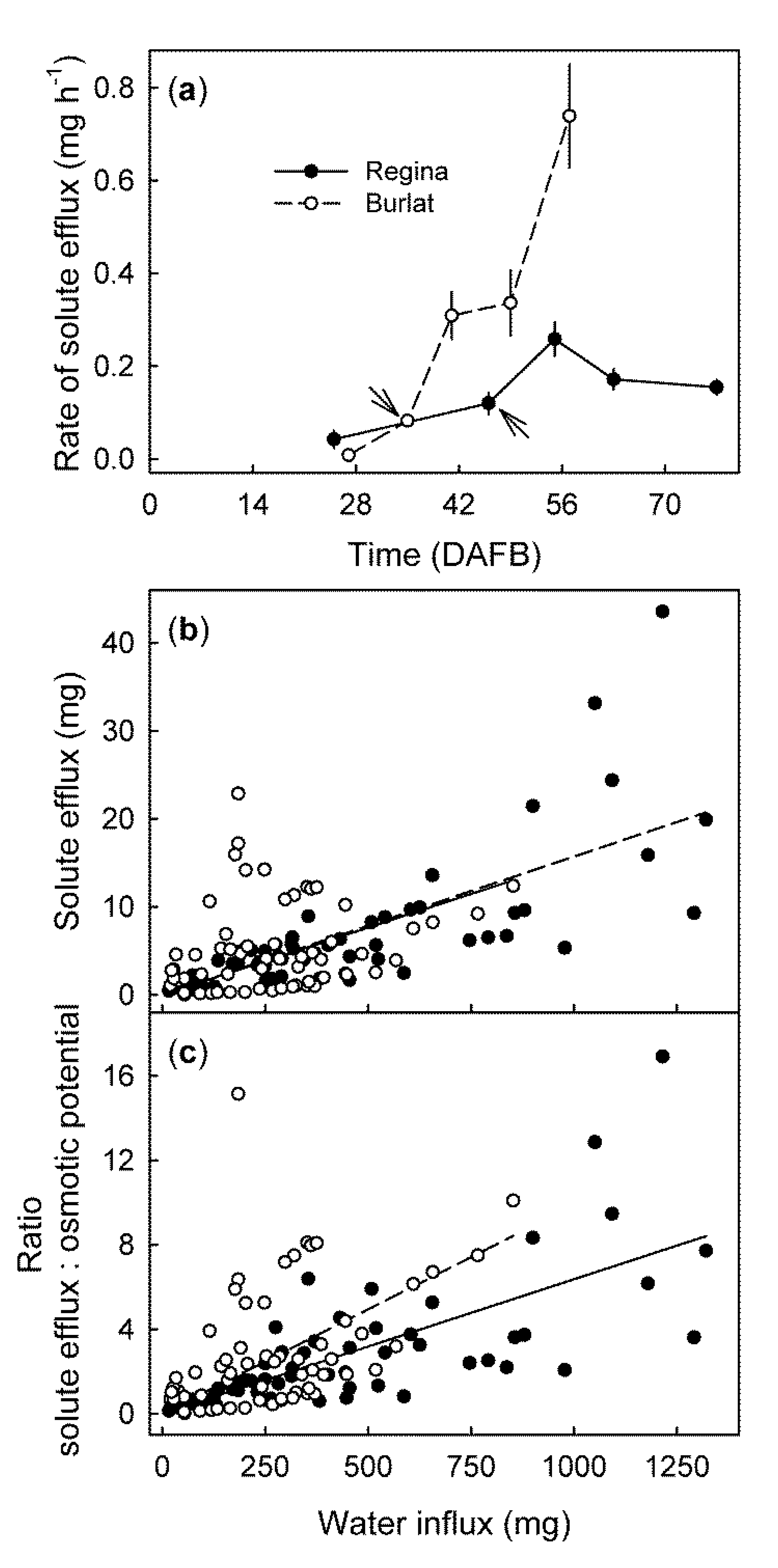
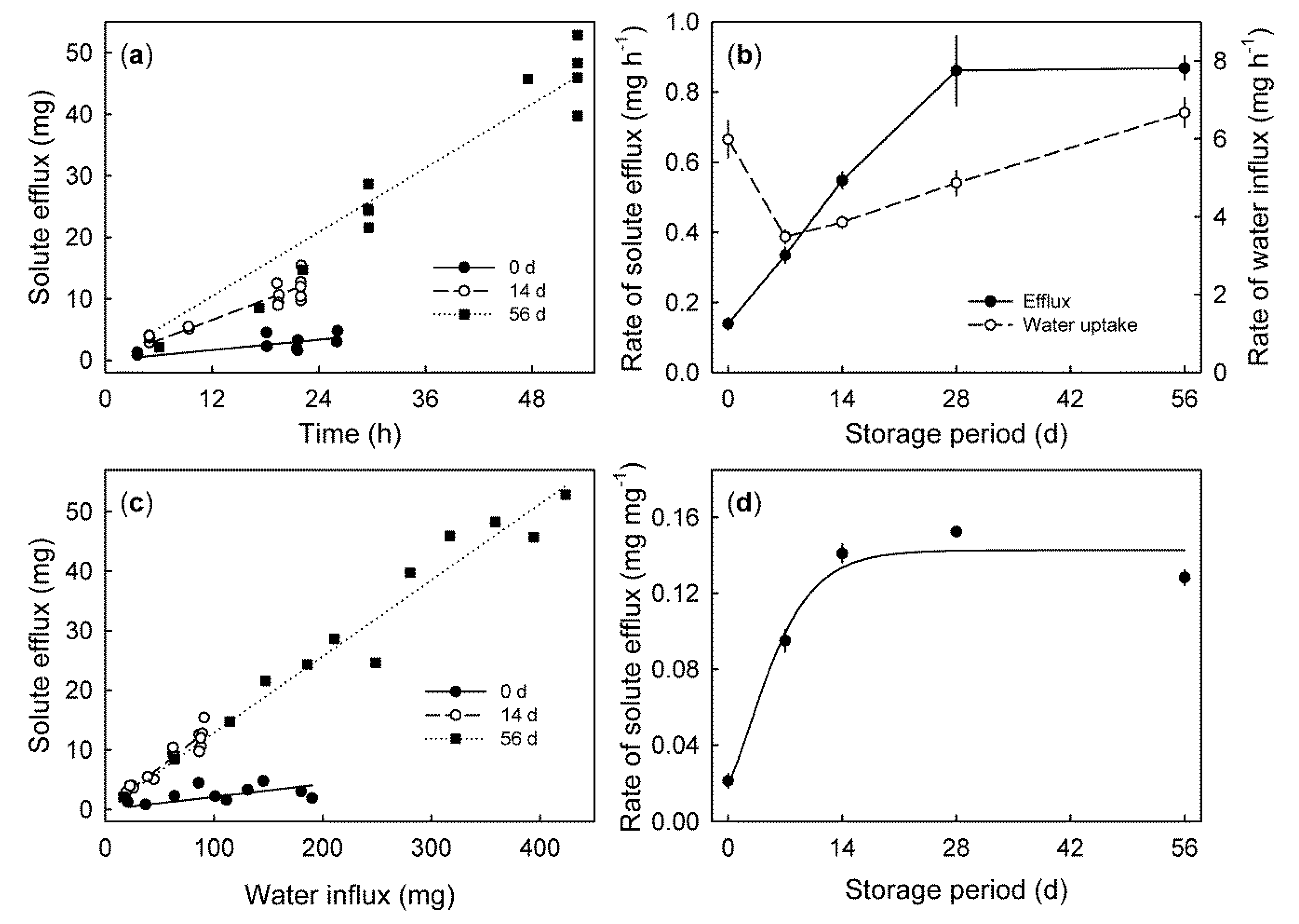
2. Results
3. Discussion
3.1. Source of Solutes
3.2. Mechanism of the Solute Efflux
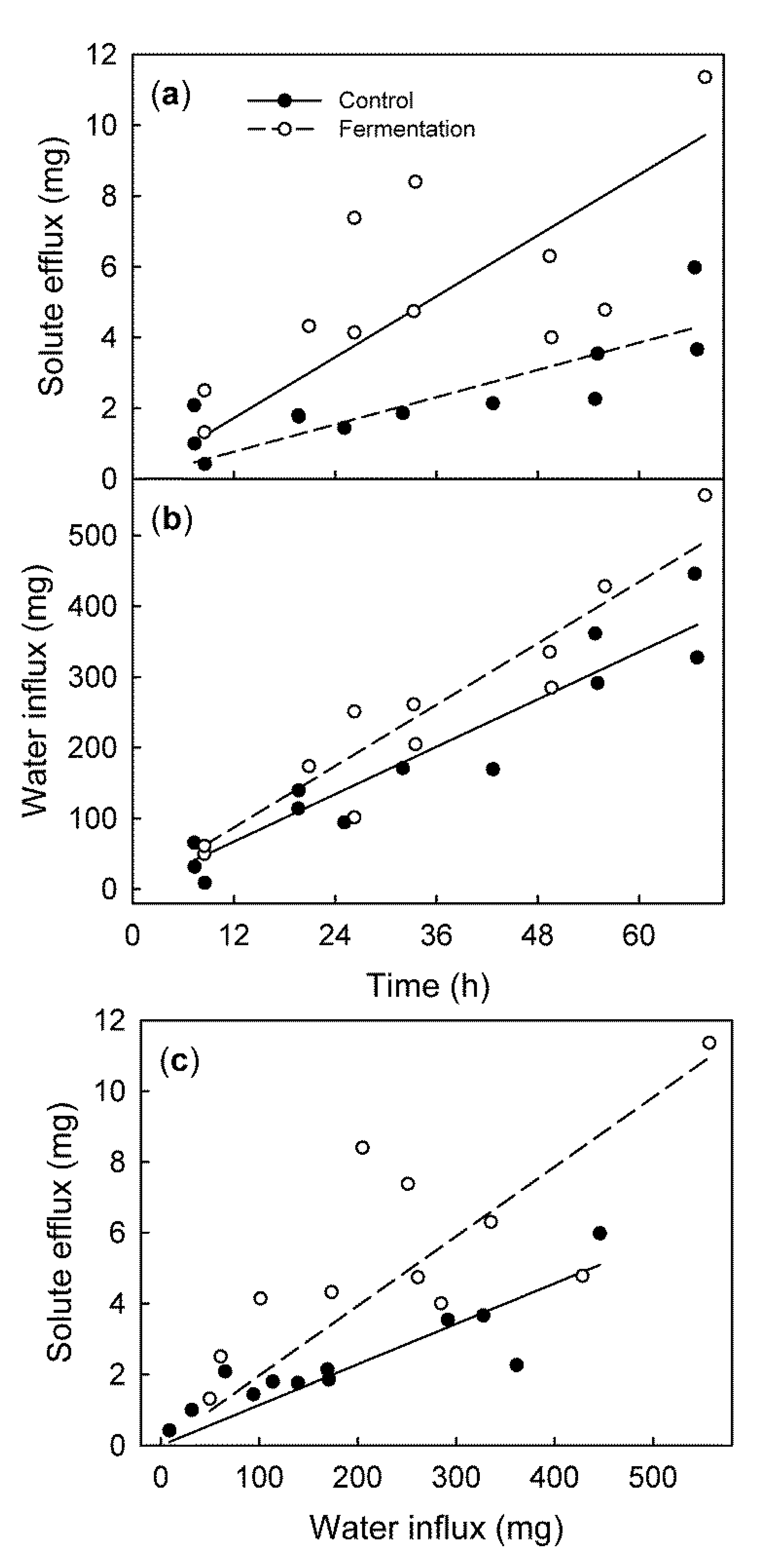
- (a)
- When a fruit is incubated in deionized water, water influx occurs through the cuticle and also through any cuticular microcracks. Here, the microcracks represent the sites of preferential influx, because the water can now bypass the cuticle, whose barrier function has been breached [17]. Following entry into the fruit, the influx of water is partitioned into the parenchyma cells of the flesh, just under the skin. Compared with the skin cells, the parenchyma cells are (1) larger, (2) have thinner walls, and (3) contain higher concentrations of osmotically-active solutes. The epidermal cells are smaller, thicker-walled, and their content is less concentrated [18]. For all three reasons, the parenchyma cells are the first to burst. As they burst, they release solutes and anthocyanins into the apoplast [19]. These solutes also include organic acids (malate) that increase the permeability of the membranes of the neighboring cells and also weaken their cell walls [20]. This process initiates a chain reaction that propagates through the flesh and into the skin like a ‘zipper’ in clothing. The chain reaction soon leads to a macroscopic crack [3,21]. As calcium is involved in the maintenance of membrane permeability and in the cross-linking of cell walls [22,23,24,25], calcium may play a role in this process. The stability of cell walls is positively correlated with its Ca-content [25,26]. Thus, cell walls with higher Ca-contents are less likely to crack than cell walls with lower Ca-contents.
- (b)
- The second process causing solutes to be released from the cells into the apoplast is the last stage of fruit development—ripening and senescence. In general, senescence is accompanied by increases in membrane permeability and, thus, increases in leakage of all cytoplasmic solutes, including ions [27]. In grape berries, experimental evidence indicates a general breakdown of compartmentation [11]. This is likely also to occur in mature sweet cherry, where the regions of the flesh close to the pit appear to be entirely degenerated (Winkler and Knoche, unpublished data). Loss of membrane semipermeability quickly results in cell death. The production of reactive oxygen species (ROS) also causes cell death [28]. In sweet cherry, ROS result from increased activities during the storage of the enzymes superoxide dismutase, ascorbate peroxidase, lipoxygenase, guaiacol peroxidase, and polyphenol oxidase. Meanwhile, the activity of catalase as a part of the radical scavenging capacity in sweet cherry decreases. Additionally, the content of malondialdehyde as an indicator of membrane permeability increased [29]. All these processes are likely to contribute to increases in solute efflux during fruit storage.
3.3. Pathways for Efflux
3.4. Practical Implications
4. Materials and Methods
4.1. Plant Material
4.2. Quantifying Efflux
4.3. Experiments
4.4. HPLC Analysis of Carbohydrates
4.5. Data Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christensen, J.V. Rain-induced cracking of sweet cherries: Its causes and prevention. In Cherries: Crop Physiology, Production and Uses; Webster, A.D., Looney, N.E., Eds.; CAB International: Wallingford, UK, 1996; pp. 297–327. [Google Scholar]
- Knoche, M.; Winkler, A. Rain-induced cracking of sweet cherries. In Cherries: Botany, Production and Uses; Quero-García, J., Iezzoni, A., Puławska, J., Lang, G., Eds.; CAB International: Wallingford, UK, 2017; pp. 140–165. [Google Scholar]
- Winkler, A.; Peschel, S.; Kohrs, K.; Knoche, M. Rain cracking in sweet cherries is not due to excess water uptake but to localized skin phenomena. J. Am. Soc. Hortic. Sci. 2016, 141, 653–660. [Google Scholar] [CrossRef]
- Knoche, M.; Peschel, S.; Hinz, M.; Bukovac, M.J. Studies on water transport through the sweet cherry fruit surface: Characterizing conductance of the cuticular membrane using pericarp segments. Planta 2000, 212, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Grimm, E.; Knoche, M. Sweet cherry fruit: Ideal osmometers? Front. Plant Sci. 2019, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K. Inhaltsstoffe von Obst und Gemüse; Ulmer: Stuttgart, Germany, 2001. [Google Scholar]
- Knoche, M.; Grimm, E.; Schlegel, H.J. Mature sweet cherries have low turgor. J. Am. Soc. Hortic. Sci. 2014, 139, 3–12. [Google Scholar] [CrossRef]
- Schumann, C.; Schlegel, H.J.; Grimm, E.; Knoche, M.; Lang, A. Water potential and its components in developing sweet cherry. J. Am. Soc. Hortic. Sci. 2014, 139, 349–355. [Google Scholar] [CrossRef]
- Wada, H.; Matthews, M.A.; Shackel, K.A. Seasonal pattern of apoplastic solute accumulation and loss of cell turgor during ripening of Vitis vinifera fruit under field conditions. J. Exp. Bot. 2009, 60, 1773–1781. [Google Scholar] [CrossRef]
- Wada, H.; Shackel, K.A.; Matthews, M.A. Fruit ripening in Vitis vinifera: Apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta 2008, 227, 1351–1361. [Google Scholar] [CrossRef]
- Lang, A.; Düring, H. Partitioning control by water potential gradient: Evidence for compartmentation breakdown in grape berries. J. Exp. Bot. 1991, 42, 1117–1122. [Google Scholar] [CrossRef]
- Tilbrook, J.; Tyerman, S.D. Cell death in grape berries: Varietal differences linked to xylem pressure and berry weight loss. Funct. Plant Biol. 2008, 35, 173–184. [Google Scholar] [CrossRef]
- Lilleland, O.; Newsome, L. A growth study of the cherry fruit. Proc. Am. Soc. Hortic. Sci. 1934, 32, 291–299. [Google Scholar]
- Tukey, H.B. Growth of the embryo, seed, and pericarp of the sour cherry (Prunus cerasus) in relation to season of fruit ripening. Proc. Am. Soc. Hortic. Sci. 1934, 31, 125–144. [Google Scholar]
- Wink, M. The plant vacuole: A multifunctional compartment. J. Exp. Bot. 1993, 44, 231–246. [Google Scholar]
- Moing, A.; Carbonne, F.; Zipperlin, B.; Svanella, L.; Gaudillére, J.P. Phloem loading in peach: Symplastic or apoplastic? Physiol. Plant. 1997, 101, 489–496. [Google Scholar] [CrossRef]
- Knoche, M.; Peschel, S. Water on the surface aggravates microscopic cracking of the sweet cherry fruit cuticle. J. Am. Soc. Hortic. Sci. 2006, 131, 192–200. [Google Scholar] [CrossRef]
- Grimm, E.; Knoche, M. Sweet cherry skin has a less negative osmotic potential than the flesh. J. Am. Soc. Hortic. Sci. 2015, 140, 472–479. [Google Scholar] [CrossRef]
- Simon, E.W. Leakage from fruit cells in water. J. Exp. Bot. 1977, 23, 1147–1152. [Google Scholar] [CrossRef]
- Winkler, A.; Ossenbrink, M.; Knoche, M. Malic acid promotes cracking of sweet cherry fruit. J. Am. Soc. Hortic. Sci. 2015, 140, 280–287. [Google Scholar] [CrossRef]
- Schumann, C.; Winkler, A.; Brüggenwirth, M.; Köpcke, K.; Knoche, M. Crack initiation and propagation in sweet cherry skin: A simple chain reaction causes the crack to ‘run’. PLoS ONE 2019, 14, e0219794. [Google Scholar] [CrossRef]
- Van Steveninck, R.F.M. The significance of calcium on the apparent permeability of cell membranes and the effects of substitution with other divalent ions. Physiol. Plant. 1965, 18, 54–69. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; Leopold, A.C. Deferral of leaf senescence with calcium. Plant Physiol. 1973, 52, 236–239. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Diffuse growth of plant cell walls. Plant Physiol. 2018, 176, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Demarty, M.; Morvan, C.; Thellier, M. Calcium and the cell wall. Plant Cell Environ. 1984, 7, 441–448. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Palma, T.; Stanley, D.W. Membrane effects in postharvest physiology. Postharvest Biol. Technol. 1996, 7, 193–217. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transduction in plants. Curr. Sci. 2005, 89, 1113–1121. [Google Scholar]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Weichert, H.; Knoche, M. Studies on water transport through the sweet cherry fruit surface. 10. Evidence for polar pathways across the exocarp. J. Agric. Food Chem. 2006, 54, 3951–3958. [Google Scholar] [CrossRef]
- Schönherr, J. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. J. Exp. Bot. 2006, 57, 2471–2491. [Google Scholar] [CrossRef]
- Schönherr, J. Calcium chloride penetrates plant cuticles via aqueous pores. Planta 2000, 212, 112–118. [Google Scholar] [CrossRef]
- Beyer, M.; Peschel, S.; Knoche, M.; Knörgen, M. Studies on water transport through the sweet cherry fruit surface: IV. Regions of preferential uptake. HortScience 2002, 37, 637–641. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Baldoni, G. Factors influencing anthocyanin content in red cabbage (Brassica oleracea var capitata L f rubra (L) Thell). J. Sci.Food Agric. 2002, 82, 1504–1509. [Google Scholar] [CrossRef]
- Anthony, R.M.; Sutheimer, C.A.; Sunshine, I. Acetaldehyde, methanol, and ethanol analysis by headspace gas chromatography. J. Anal. Toxicol. 1980, 4, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; McCormick, R.; Xuan, H.; Streif, J. Distribution of sugar and organic acid components within the KOB heritage apple cultivar collection. Acta Hortic. 2010, 858, 89–97. [Google Scholar] [CrossRef]

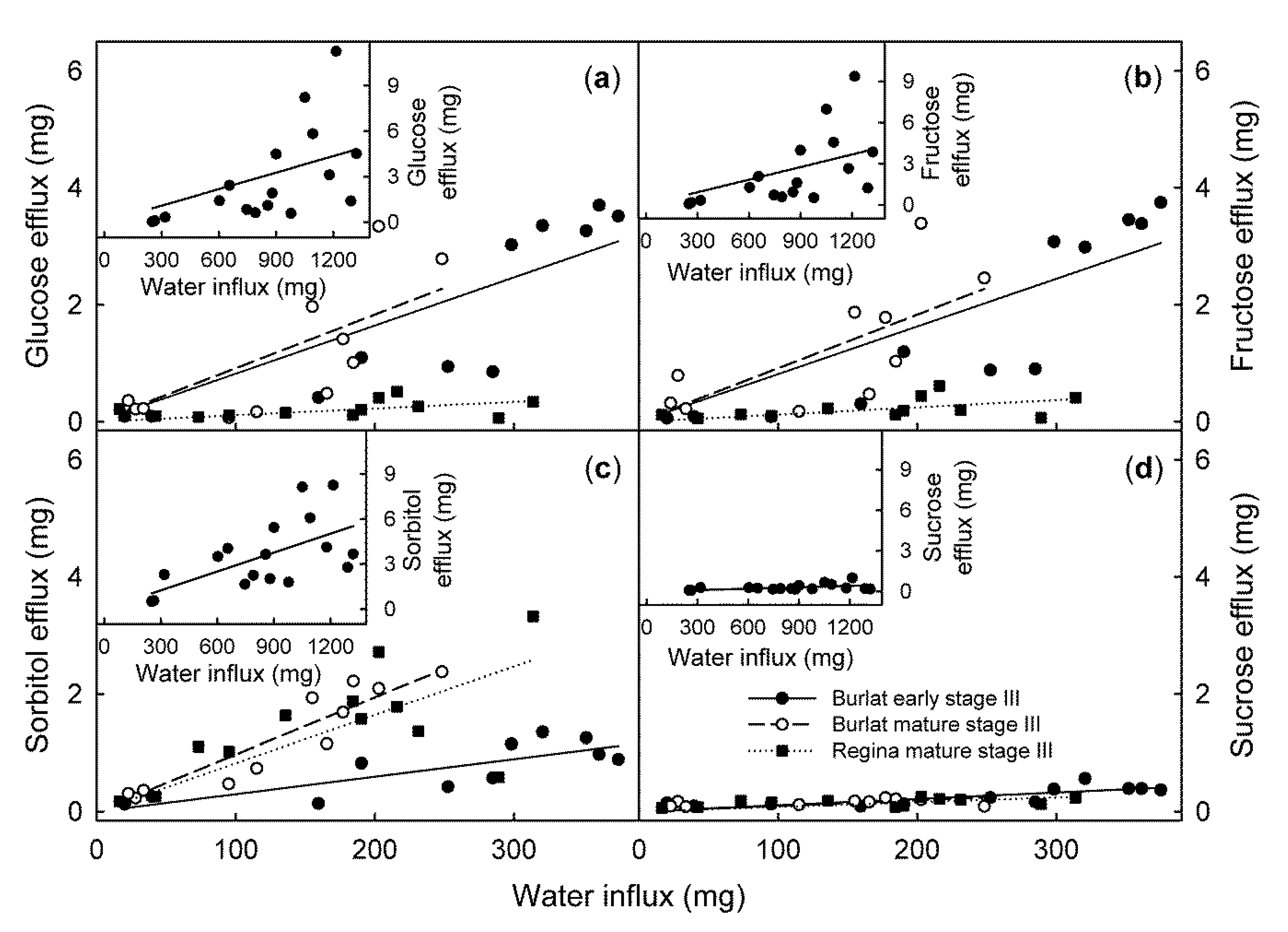

| Relative Content of the Sugars (%) | ||||||
|---|---|---|---|---|---|---|
| Cultivar | Developmental Stage | Sample | Glucose | Fructose | Sorbitol | Sucrose |
| Burlat | early stage III | Fruit juice | 47.4 | 39.7 | 8.3 | 4.7 |
| Solute efflux | 35.7 ** | 33.9 | 20.6 * | 9.8 | ||
| mature stage III | Fruit juice | 47.9 | 40.4 | 9.3 | 2.4 | |
| Solute efflux | 25.0 *** | 28.7 * | 39.0 *** | 7.3 * | ||
| Regina | early stage III | Fruit juice | 50.0 | 35.8 | 11.4 | 2.8 |
| Solute efflux | 24.6 *** | 21.9 *** | 48.9 *** | 4.5 | ||
| mid stage III | Fruit juice | 46.1 | 38.0 | 13.6 | 2.3 | |
| Solute efflux | 8.3 *** | 10.6 *** | 73.9 *** | 7.3 *** | ||
| mature stage III | Fruit juice | 46.8 | 37.1 | 14.3 | 1.8 | |
| Solute efflux | 12.4 *** | 11.0 *** | 67.3 *** | 9.2 ** | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, A.; Riedel, D.; Neuwald, D.A.; Knoche, M. Water Influx through the Wetted Surface of a Sweet Cherry Fruit: Evidence for an Associated Solute Efflux. Plants 2020, 9, 440. https://doi.org/10.3390/plants9040440
Winkler A, Riedel D, Neuwald DA, Knoche M. Water Influx through the Wetted Surface of a Sweet Cherry Fruit: Evidence for an Associated Solute Efflux. Plants. 2020; 9(4):440. https://doi.org/10.3390/plants9040440
Chicago/Turabian StyleWinkler, Andreas, Deborah Riedel, Daniel Alexandre Neuwald, and Moritz Knoche. 2020. "Water Influx through the Wetted Surface of a Sweet Cherry Fruit: Evidence for an Associated Solute Efflux" Plants 9, no. 4: 440. https://doi.org/10.3390/plants9040440
APA StyleWinkler, A., Riedel, D., Neuwald, D. A., & Knoche, M. (2020). Water Influx through the Wetted Surface of a Sweet Cherry Fruit: Evidence for an Associated Solute Efflux. Plants, 9(4), 440. https://doi.org/10.3390/plants9040440





