1. Introduction
In plants, the heat-shock response and some crucial developmental processes are regulated by a gene family of transcription factors known as the heat-shock transcription factors (HSFs). The functional specialization of the multiple plant HSFs is, for the most part, unexplored. Only HSFs from a few plant species have been functionally analyzed [
1,
2]. Our lab has characterized HSFs, which, in sunflower (
Helianthus annuus L.), contribute to longevity, thermotolerance, and desiccation tolerance of seeds. HaHSFA9 (A9;
Helianthus annuus Heat Stress Factor A9) is a peculiar Class A HSF that, in sunflower, is expressed only in seeds [
3]. The gaining of function upon the overexpression of A9 in transgenic tobacco has indicated its involvement in thermotolerance, seed longevity, and tolerance to extreme desiccation [
4,
5]. A9 activates a genetic program—the “A9-programme”—that includes subsets of genes encoding Heat Shock Proteins (HSP) normally expressed during zygotic embryogenesis in seeds. Furthermore, the photosynthetic apparatus and green organs of 35S:A9 seedlings (constitutively overexpressing A9) showed an unusual resistance to extreme conditions of dehydration and oxidative stress [
6].
In connection with seed longevity, we reported some requirements and consequences of loss-of-function of A9. Different modified forms of A9, expressed under the seed-specific DS10 promoter, were analyzed in transgenic tobacco seeds. Transcription-inactive forms of A9 (as A9M1) were inefficient compared to an active repressor form (A9 fused to SRDX, A9M3). Thus, using only A9M3, we observed a substantial reduction in seed longevity [
7]. This strongly indicates that A9 is not the sole Class A HSF involved in transcriptional activation of the “A9-programme” in developing seeds. Subsequently, HaHSFA4a (A4a;
Helianthus annuus Heat Stress Factor A4a) was identified as one of such accessory HSFs [
8]. Interestingly, both A4a and A9 were repressed by the auxin/Indole-3-Acetic Acid (aux/IAA) protein HaIAA27, which revealed a connection between seed longevity and auxin signaling: aux/IAA proteins reduced seed longevity by interfering the A9-A4a synergic interaction [
8,
9]. A9 and A4a coactivate the same genetic program involving specific sHSP target genes. This has been confirmed by observing enhanced seed longevity in DS10:A4a and DS10:A9/A4a transgenic tobacco lines, which specifically overexpress A4a, or A4a with A9, in seeds [
10]. Similar analyses with 35S:A4a and 35S:A9/A4a lines revealed enhanced tolerance to vegetative severe dehydration and oxidative stress in young transgenic seedlings, furthermore showing that A4a strictly requires A9 to cause the enhanced stress resistance [
10].
Plants use sunlight as an important developmental cue. Chloroplast biogenesis starts, for the first time, during plant embryogenesis, normally halts during seed development, and continues after germination. Embryos in seeds contain immature plastids (proplastids) that, during dark germination, develop into partially assembled plastids that completely transform into chloroplasts only after photomorphogenesis is induced by light [
11]. Light perception by different receptors is crucial for the initiation and progression of photomorphogenic development. This includes the receptors for far-red (FR) and red (R) light, which are Phytochrome A (PHYA), and Phytochrome B (PHYB), respectively (see the reviews [
12,
13,
14]). The FR and R wavelengths of white light suffice—in separate—for photomorphogenesis. However, plants also use different receptors for blue light, including Cryptochromes (CRY) and Phototropins (PHOT), respectively reviewed in [
15,
16]. A recent publication from our lab also demonstrated a functional link between A9 and the initiation of seedling photomorphogenesis [
17]. This link is active under darkness immediately after seed germination, and also upon exposure to light, partially operating through direct and indirect effects on the PHYA and PHYB photoreceptors. In transgenic tobacco plants, A9 thus causes complex effects, resulting in accelerated photomorphogenesis. This adds to the enhanced drought, heat, and oxidative stress tolerance also conferred by A9, as revealed by our former studies [
4,
5,
6]. However, it has not yet been explored whether A4a coactivates the photomorphogenic effects induced by A9 in a similar way to that reported for the effects of A9 on seed longevity. In this work, we performed comparative RNA-seq analyses of the overexpression effects of A9 and A4a. We also reported comparative loss-of-function analyses of seed longevity and seedling greening, performed using dominant-negative and inactive forms of A9. Collectively, our results support a model whereby A9 enhances seedling photomorphogenesis without the concourse of additional seed HSFs as A4a.
2. Results
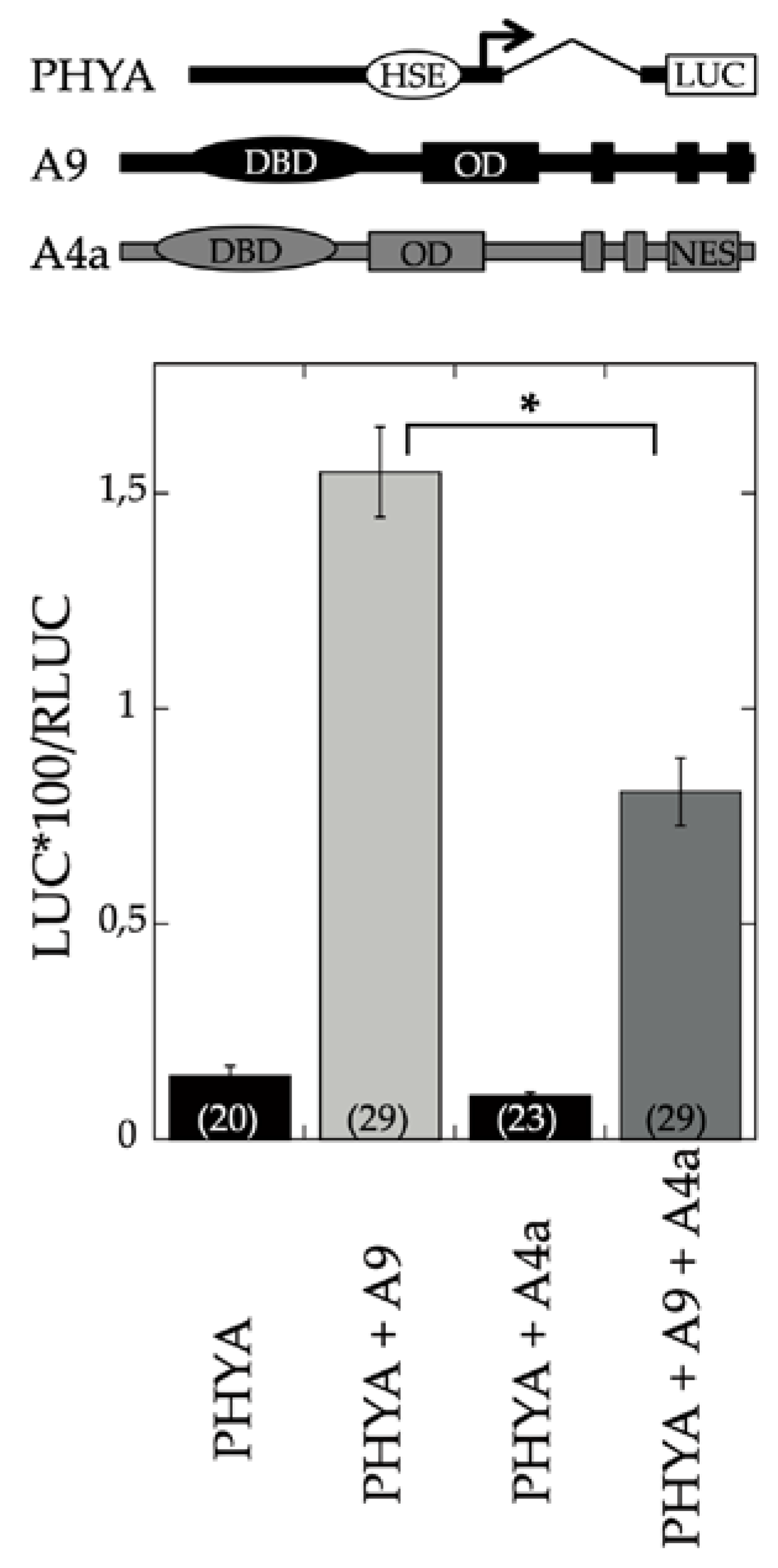
A9 is a seed-specific transcriptional activator [
3] that enhances gene expression connected both with seed longevity [
4] and seedling photomorphogenesis [
17]. To investigate whether the regulation by A9 is similar or different in both cases, we first analyzed the effect of A4a on transient transcriptional activation of the PHYA promoter, a photoreceptor that is directly activated by A9 [
17]. The results depicted in
Figure 1 showed that A4a attenuated the transcriptional activation of PHYA by A9. Additional experiments demonstrated the inhibitory effects of A4a on seedling photomorphogenesis in the A9 genetic background, for example, in experiments of early greening under white light (
Figure 2). These results contrast the published effects of A4a- on A9-induced seed longevity and on promoters of genes (as sHSP) involved in seed longevity, where A4a functions as an A9-dependent synergic transcriptional coactivator [
10], which enhances seed longevity, as determined by controlled-deterioration assays [
4]. The results in
Figure 1 and
Figure 2 indicate that the A9 effects on seed longevity and seedling greening were differentially regulated.
2.1. Transcriptomic Analysis of the Effects of A9 and A4a
The differential regulation indicated by the results of
Figure 1 and
Figure 2 was further investigated by exploratory (pooled, single-replicated; see Materials and Methods for details) RNA-seq analyses of transcript accumulation induced by A9 or A4a overexpression in transgenic tobacco. Each transgene in the various lines was integrated at different single locations (as detailed in Materials and Methods). For RNA-seq, we used the transgenic A9 lines and sibling nontransgenic (NT) segregant lines that were described by our lab in precedent works [
4,
5]. For A9-induced (A9up) transcripts, we identified mRNAs that were consistently upregulated in both seedlings of 35S:A9 lines and in imbibed seeds of DS10:A9 lines (by comparison with the respective NT sibling lines in each case). The effects of A4a were determined using imbibed seeds (therefore expressing endogenous A9) from the sibling DS10:A4a and NT lines [
10] because we previously determined that A4a was not able to activate transcription in absence of A9, for example, in the vegetative organs of 35S:A4a plants [
10].
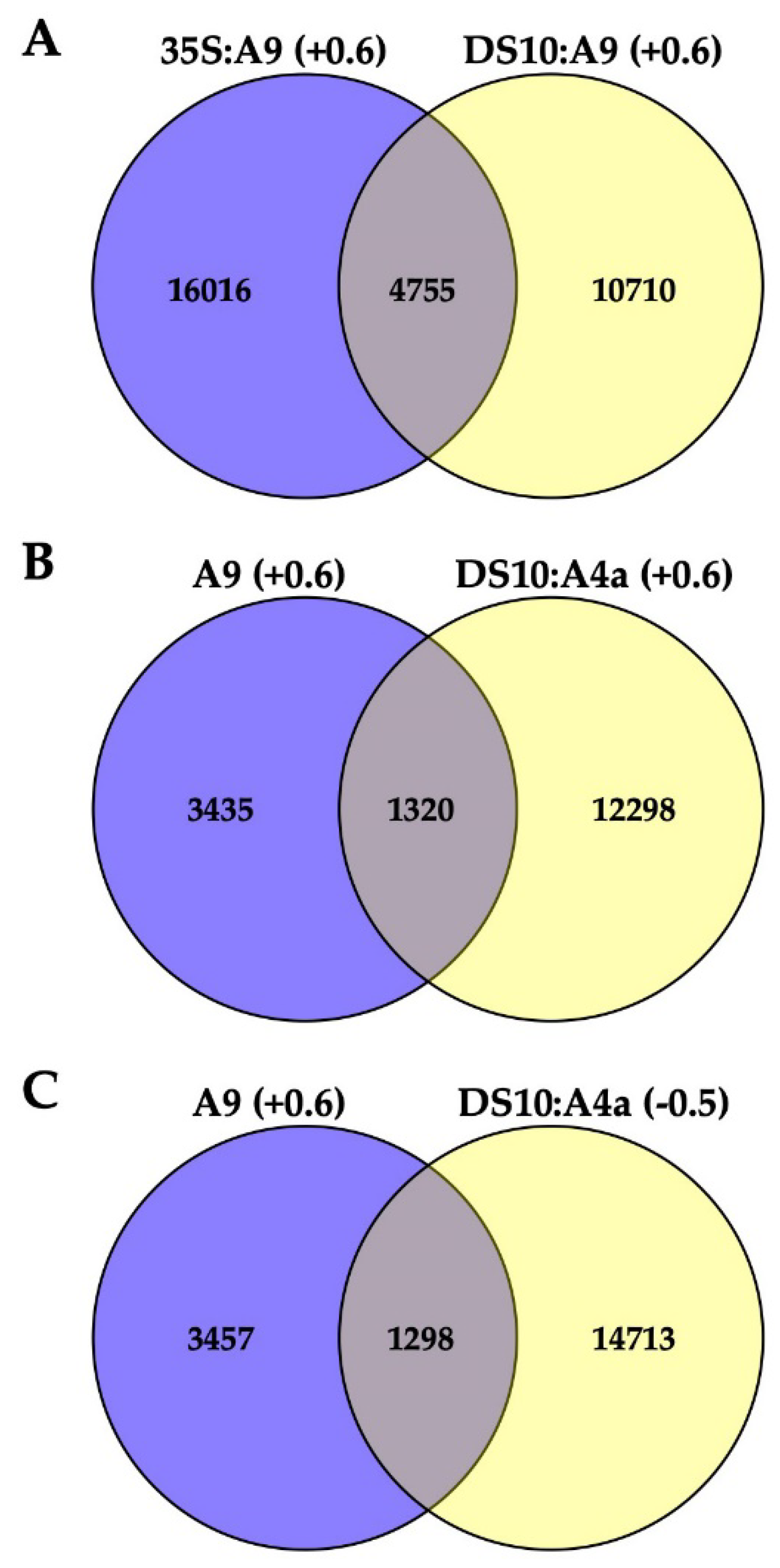
Table S1 includes a total of 4755 identified genes consistently upregulated by A9 at least 1.5-fold (Log2 fold ≥ 0.6; the A9up set) in both the 35S:A9 and DS10:A9 RNA-seq experiments (
Figure 3A). The A9up set included the target genes of A9, which, in connection with seed longevity (for example, multiple sHSP genes), or photomorphogenesis (for example, PHYA, PHYB, and ELONGATED HYPOCOTYL 5, HY5) were confirmed by RT-qPCR and with other different analyses in our precedent publications [
4,
5,
7,
9,
10,
17]. This supports the quality of our exploratory RNA-seq.
Table S2 outlines some examples of these important genes. The sufficient quality of the RNA-seq analyses used in
Figure 3 for the inference of differential expression and regulation of putative A9 target genes is also supported by functional verification of predictions from
Figure 3 (see below and the Discussion section).
Figure 3B,C represent data of the DS10:A4a RNA-seq experiment.
Figure 3B shows that the set of 13,618 A4a-upregulated genes (listed in
Table S3) comprehended a subset of the A9up set (the 1320 genes listed in
Table S4) that included genes known from our previous studies to be coactivated by A9 and A4a, for example, seed sHSP genes expressed in connection to longevity [
4,
9,
10], namely HSP18.2 CI, HSP22.0 CIV, and HSP26.5 M (see
Table S2).
Figure 3C shows that the set of 16011 A4a-downregulated genes (listed in
Table S5) included 1298 A9up genes. This subset of 1298 A9up genes (listed in
Table S6) included A9 targets connected to the published PHYA- and PHYB-dependent photomorphogenic responses enhanced by A9 [
17]. Among these genes, we outline HY5, a transcription factor acting as the regulatory hub for the responses to visible (far-red, red, blue) and UV-B light. Coactivation by A9 and A4a of sHSP genes involved the simultaneous binding to noncanonical HSE (Heat Shock
cis Element) of the two factors, most likely bound as an A9-A4a heteromultimer [
8,
18]. The 1298 A9up genes identified in the downregulated set might represent genes (such as HY5) activated by A9 alone, which bound to different noncanonical HSE as homomultimers (see the Discussion section). The results of
Figure 1 and
Figure 2 and the RNA-seq results summarized in
Figure 3B,C encouraged us to perform additional experiments for the functional confirmation of our hypothesis that A9 target genes involved in enhancing seedling greening would be regulated mainly by A9 alone in contrast to the reported coactivation by A9 and A4a of the A9 target genes involved in seed longevity [
10].
2.2. A9 Enhances Responses to Blue Light Mediated by CRY1
The A9up set genes allowed us to predict the additional effects of A9, such as the enhancement of cryptochrome-dependent blue-light responses indicated by the presence of numerous CRY1/HY5 targets, for example, the sigma factor SIG1, 9-cis-epoxycarotenoid dioxygenases (NCED), chalcone synthase (CHS), dihydroflavonol-4-reductase (DFR), and leucoanthocyanidin dioxygenase (LDOX), However, the A9up set did not include CRY1 itself (see
Table S1 and the gene examples listed in
Table S2). Precedents in the literature showed that polyclonal antibodies against a 17 kDa C-terminal fragment of the
Arabidopsis thaliana CRY1 protein specifically detected the
Nicotiana tabacum CRY1 protein [
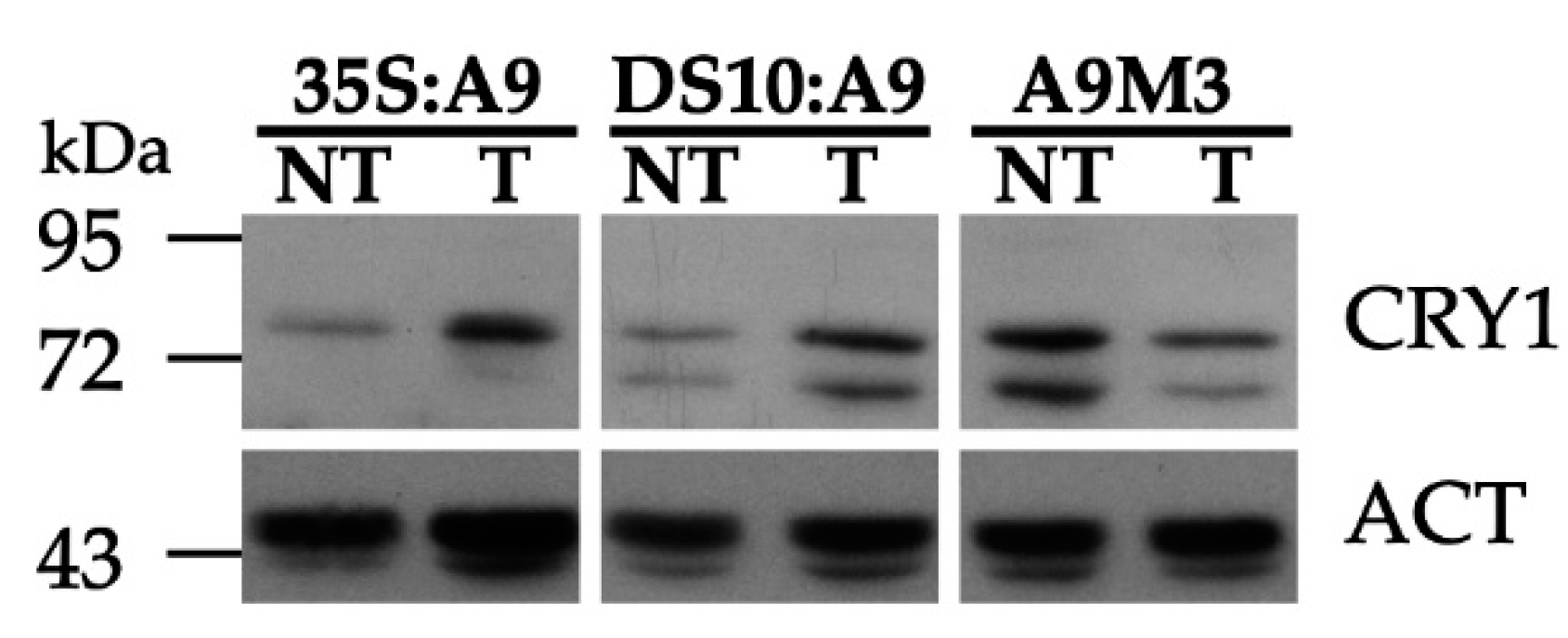
19]. We therefore used these antibodies to investigate whether A9 enhanced CRY1 protein accumulation in our transgenic A9 lines. The results in
Figure 4 indeed show that A9 enhanced the accumulation of a protein band matching the expected mobility of the
Nicotiana tabacum CRY1 protein. This was observed with samples prepared from dark-imbibed DS10:A9 seeds, and also with samples from dark-grown 35S:A9 seedlings. These results suggest that A9 might increase CRY1 protein accumulation. The enhancement by A9 of CRY1 protein accumulation was confirmed by similar analyses performed with samples of imbibed seeds from loss-of-function DS10:A9M3 lines. The expression of a dominant-negative form of A9 in these lines caused the converse effect, decreasing the accumulation of the proteins detected with the anti-CRY1 antibodies (see also
Figure 4).
The A9-enhanced perception of blue light mediated by CRY1 (as inferred from
Figure 4) was finally confirmed with functional hypocotyl-elongation assays. In the experiments summarized in
Figure 5, monochromatic blue light was used at a fluence, which, in previous studies, was shown to mainly affect light perception by CRY1 [
20]. Under these blue-light illumination conditions, the expression of A9 in the 35S:A9 seedlings caused a significant reduction of hypocotyl growth compared to that of NT sibling seedlings. Therefore, A9 enhanced the reduction of hypocotyl growth in response to blue light in addition to the similarly demonstrated enhancement of responses to red and far-red light [
17]. As discussed in our former study [
17], the expression window in the A9 loss-of-function lines did not allow their use for confirmation of the results in hypocotyl-elongation assays.
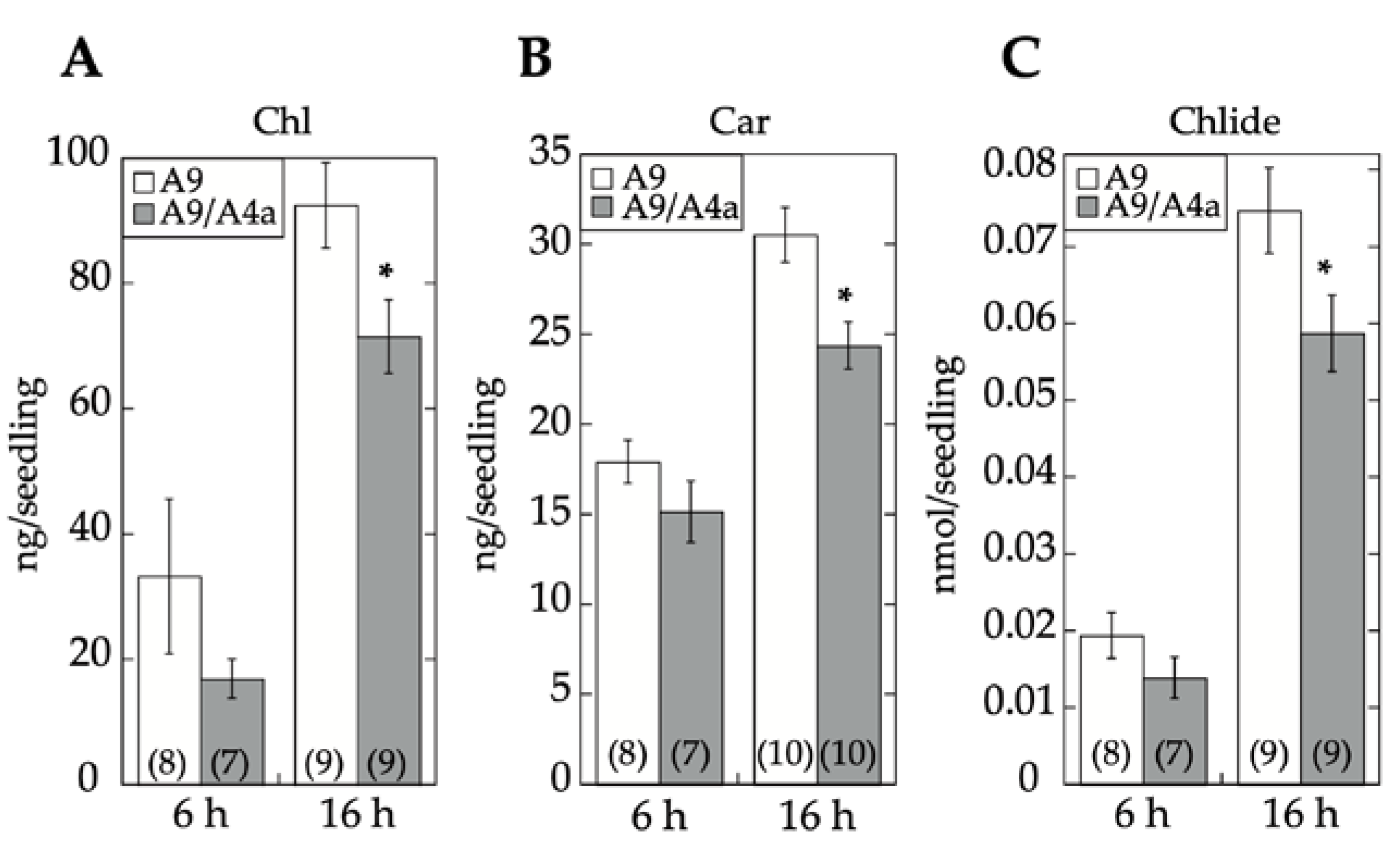
2.3. Comparative Loss-of-Function Effects of A9 in Seed Longevity and Seedling Greening
We investigated the suggested differential regulation by A9 of seed longevity and seedling greening by comparing the effect of loss-of-function caused by two different forms of A9: A9M1 and A9M3. We previously used this strategy to analyze the effect of A9 loss-of-function on seed longevity [
4]. The A9M1 form is transcriptionally inactive because of a deletion in the C-terminal region of A9, which includes three AHA motifs (enriched in aromatic, hydrophobic, and acidic amino-acid residues) that are required for transcriptional activation [
1,
21]. In the A9M3 form, A9 is fused to a potent SRDX repressor domain causing dominant-negative effects [
7,
22]. Seed longevity was assessed by two different protocols of artificial aging: (a) BTA, a fast-deterioration protocol (a seed fast-deterioration protocol based in a Basal Thermotolerance Assay) and (b) by a standard Controlled seed Deterioration Treatment (CDT) [
7]. Regarding longevity, in our former study, we only compared the DS10:A9M1 and DS10:A9M3 transgenic lines with their respective transgenes integrated as single events in heterozygosis. The results of BTA assays showed that a substantial reduction of seed-longevity was observed only with the DS10:A9M3 seeds, compared to much lower effects seen with the DS10:A9M1. Loss-of-function effects on seed-longevity and on gene expression associated with seed longevity were confirmed with homozygous DS10:A9M3 transgenic lines using CDT [
7,
23].
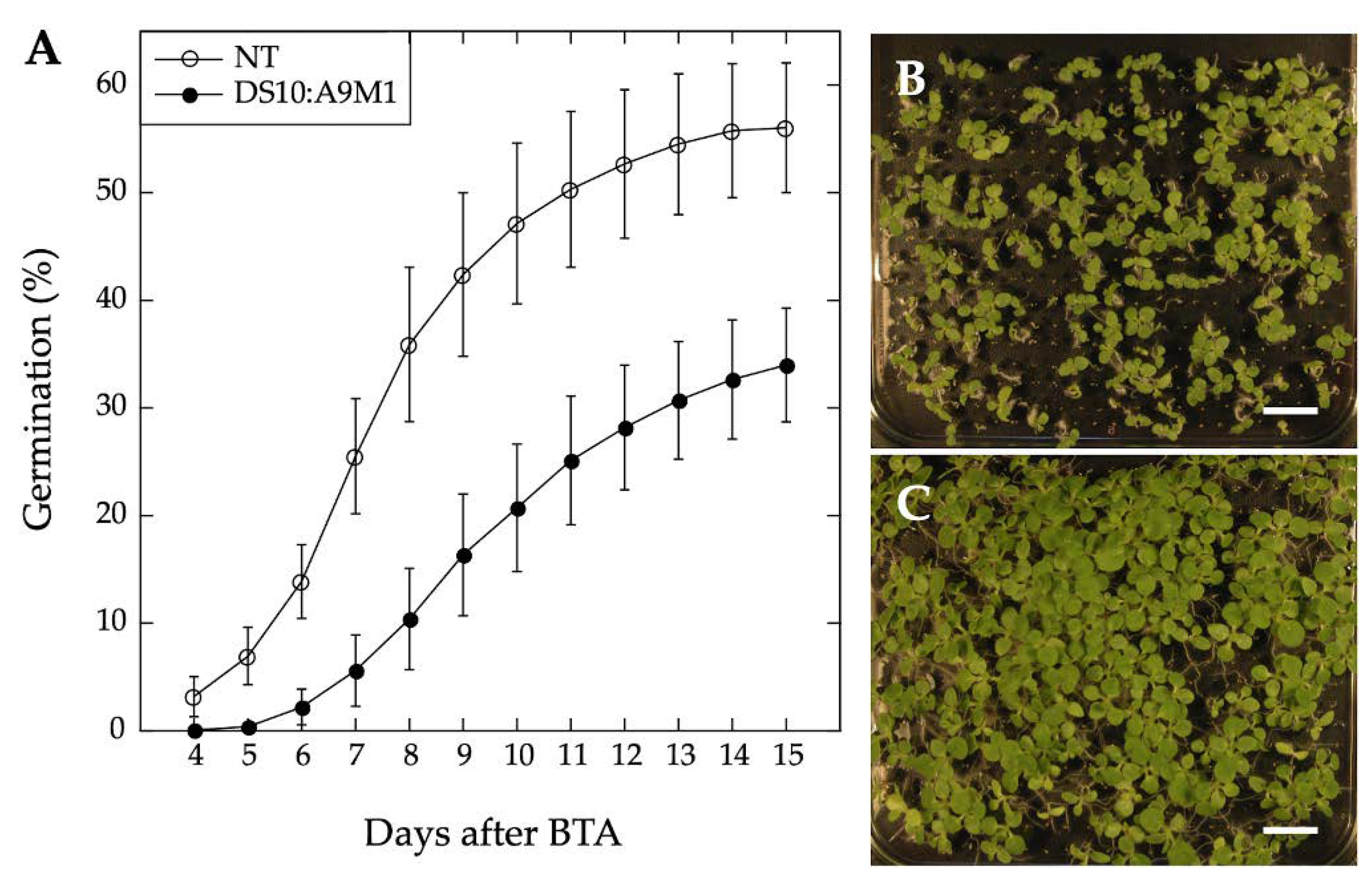
We used homozygous DS10:A9M1 transgenic lines and BTA assays to verify the published loss of function effects in connection with seed longevity. Regarding the A9 effects on seedling greening, we used the DS10:A9M1 and DS10:A9M3 homozygous lines for the comparative loss-of-function. As expected, seed longevity was decreased to some extent in the homozygous DS10:A9M1 seeds. However, these seeds still survived BTA deterioration significantly better than previously reported for homozygous DS10:A9M3 seeds (respectively,
Figure 6 and [
7]). These results confirmed that, regarding longevity, a loss-of-function effect could only be efficiently obtained with A9M3, in which A9 is converted into a very strong active repressor that has dominant-negative transcriptional effects not only on A9, but also on similar class A HSFs, such as A4a [
7,
22]. The loss-of-function of seedling greening enhancement by A9 was analyzed in a similar way, using the white light-induced greening conditions in our previous study [
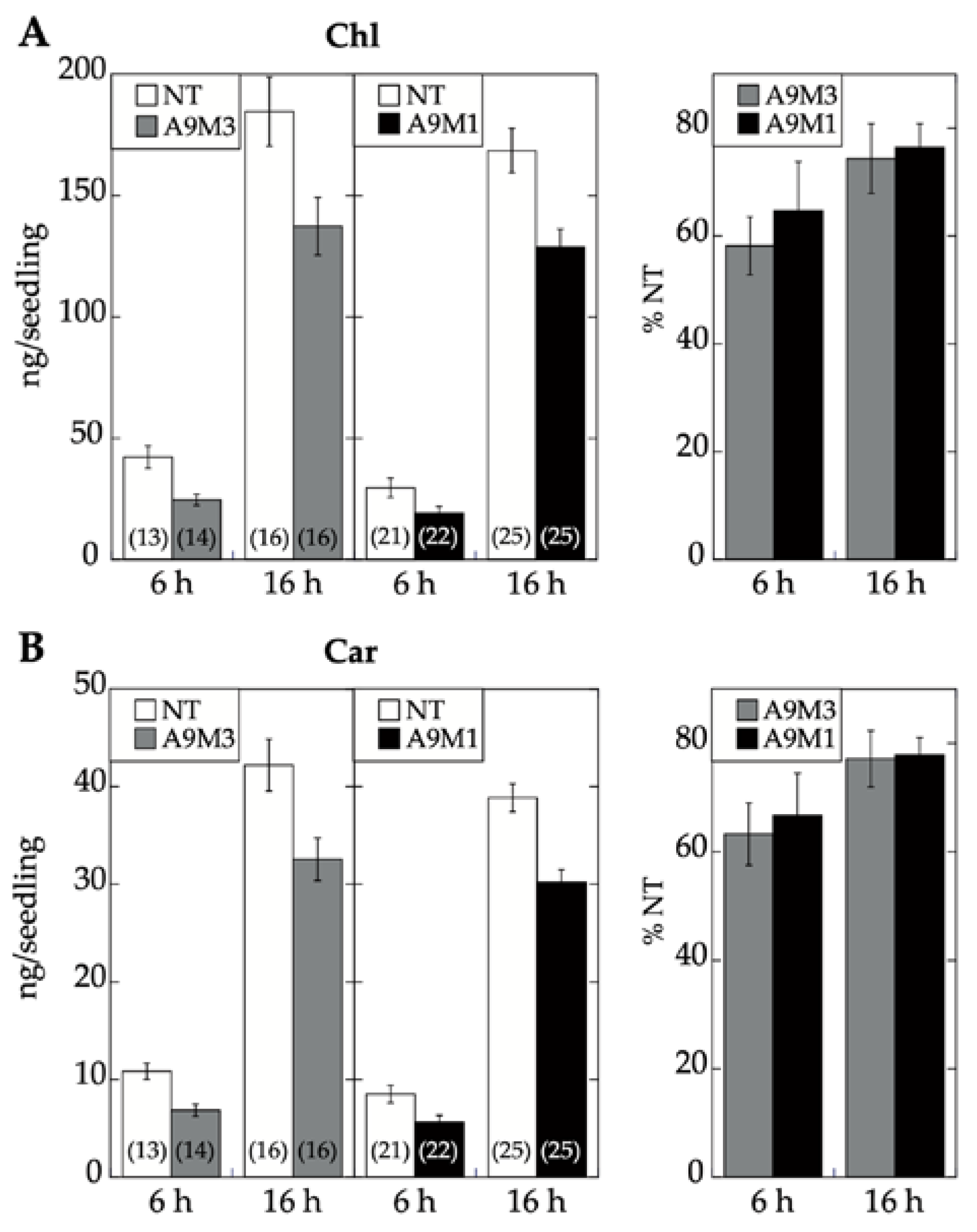
17]. Seedling greening and photomorphogenic development was quantitatively analyzed by chlorophyll and total carotenoid pigment determinations (respectively in
Figure 7A,B). The results, supported by statistical analyses in
Figure 7, clearly showed that regarding seedling greening enhancement determined after 6 h and 16 h of illumination, loss-of-function caused by A9M1 did not significantly differ from that caused by A9M3. Therefore, both forms were similarly efficient. These results contrast the observations regarding loss of function of seed longevity ([
7] and
Figure 6), supporting that the reported enhancement of seedling greening [
17] is caused mainly by A9, thus not requiring additional HSFs (such as A4a for seed longevity, [
4]).
3. Discussion
Seed longevity, desiccation tolerance, and seedling performance are important biological processes targeted by plant biotechnology [
24,
25,
26]. A9, a seed-specific transcription factor, participates in controlling seed longevity and embryo desiccation tolerance [
3,
4,
5,
7,
9]. A9 also contributes to the acceleration of early seedling greening. This greening effect occurs shortly after germination and seedling emergence (before the A9 protein accumulated during seed maturation is degraded) [
17]. Seedling greening acceleration impacts a limiting step for establishment, as it facilitates the transition from heterotrophic (dependent on seed reserves) to autotrophic (photosynthetic) growth [
11,
26]. Collectively, our results support a model where A9 alone enhances seedling photomorphogenesis without the concourse of additional seed HSFs. This contrasts the enhancement of seed longevity, where A4a is also required as a coactivator of A9 [
7,
10]. Our model of differential regulation is strongly supported by differential loss-of-function results, showing that inactive A9 (A9M1) sufficed for a negative effect on greening, but not on longevity (
Figure 6 and
Figure 7, see also [
7]). These results, in turn, agree with the puzzling negative effect of A4a on the activation of PHYA promoter and on greening (
Figure 1 and
Figure 2), and with the inference from the comparative RNA-seq analyses, where the accumulation of a subset of transcripts from genes activated by A9 (including the crucial light-response hub regulator HY5 and blue-light responsive genes as CHS, FLS, LDOX, DFR, and NCED1) are negatively affected by A4a (
Figure 3C,
Tables S2 and S6). Upon A4a overexpression, competition between inactive A4a homomultimers and endogenous A9 might be the cause of the observed transcript downregulation. This interpretation agrees with and also explains the results in
Figure 1 and
Figure 2. Thus, A4a, which, by itself, lacks a transcriptional activity
in planta [
8,
10], would compete for A9, causing all the observed negative effects.
The mostly nonredundant (sufficient) effect of A9 on seedling greening might facilitate some future technological applications, as, for example, the engineering of improved seedling establishment [
26]. For A9 to be used with this purpose in the desired crop(s), only the functional conservation of the crop A9(s) would be required, which might occur, at least, in most dicot plants (but not in monocot plants lacking orthologous A9s [
1,
2]). In contrast, using A9 for engineering-enhanced seed longevity would additionally require the functional conservation of the crop A4a(s). Plant A4a(s) have been shown to have functional differences [
27,
28,
29,
30]. In addition, the lack of intrinsic transcriptional activity
in planta has been reported only for sunflower A4a [
8,
10], which limits the use of A9 for enhancing seed longevity to plants similar to sunflower and tobacco (
Asteridae), where the peculiar A4a properties and a synergic functional interaction with A9 is known to be conserved [
10].
The here reported A9 RNA-seq data (
Figure 3A,
Table S1) allowed, after subsequent molecular (
Figure 4) and functional assay confirmation (
Figure 5), extending the embryonic A9 link to effects on the blue-light receptor CRY1. Thus, A9 would link seed maturation and seedling photomorphogenesis by acting on photoreceptors for light with wavelengths placed at both ends of the visible spectrum: CRY1 (for blue light; as confirmed by the results in
Figure 4 and
Figure 5), in addition to PHYA (for far-red light) and PHYB (for red light) as reported earlier [
17].
Tables S1 and S2 (where some important gene examples are outlined) indicate the potential upregulation of additional light receptors, including different phytochromes (PHYC for red light, [
12,
13,
14,
31]) and phototropins (PHOT2 for blue light, [
16]). In connection with early photomorphogenesis and seedling establishment, responses to blue light mainly mediated by CRY1 (and also by PHOT) receptors are crucial for inducing massive gene expression changes that occur after extensive chromatin and nuclear architecture reorganization [
32,
33]. As A9 indeed enhanced a CRY1-dependent response (
Figure 5), it is perhaps not surprising that
Table S1 includes multiple A9-upregulated genes connected with histone modification and chromatin remodeling: for example, histone chaperones ASF1A and ASF1B, mediator subunit MED18, histone deacetylase HDAC2, and DNA Helicase CHR10 (
Table S2). This suggests a potential impact of A9 on these processes, at least in part mediated by A9 effects on CRY1 and PHOT receptors. This suggestion is currently under investigation in our lab. The results reported here demonstrate that the A9up dataset (
Figure 3A,
Table S1) can be used to predict novel useful effects of A9, and the gene subsets listed in
Tables S4 and S6 can be used to predict a regulation, respectively, involving A9 and A4a (for seed longevity), or mainly A9 (for seedling greening), which, in turn, could have implications on the understanding and future practical application on these novel effects.