Explaining Intricate Morphometric Variability with Environmental Predictors: The Case of Globularia cordifolia Species Complex
Abstract
1. Introduction
2. Results
2.1. Classical Morphometrics
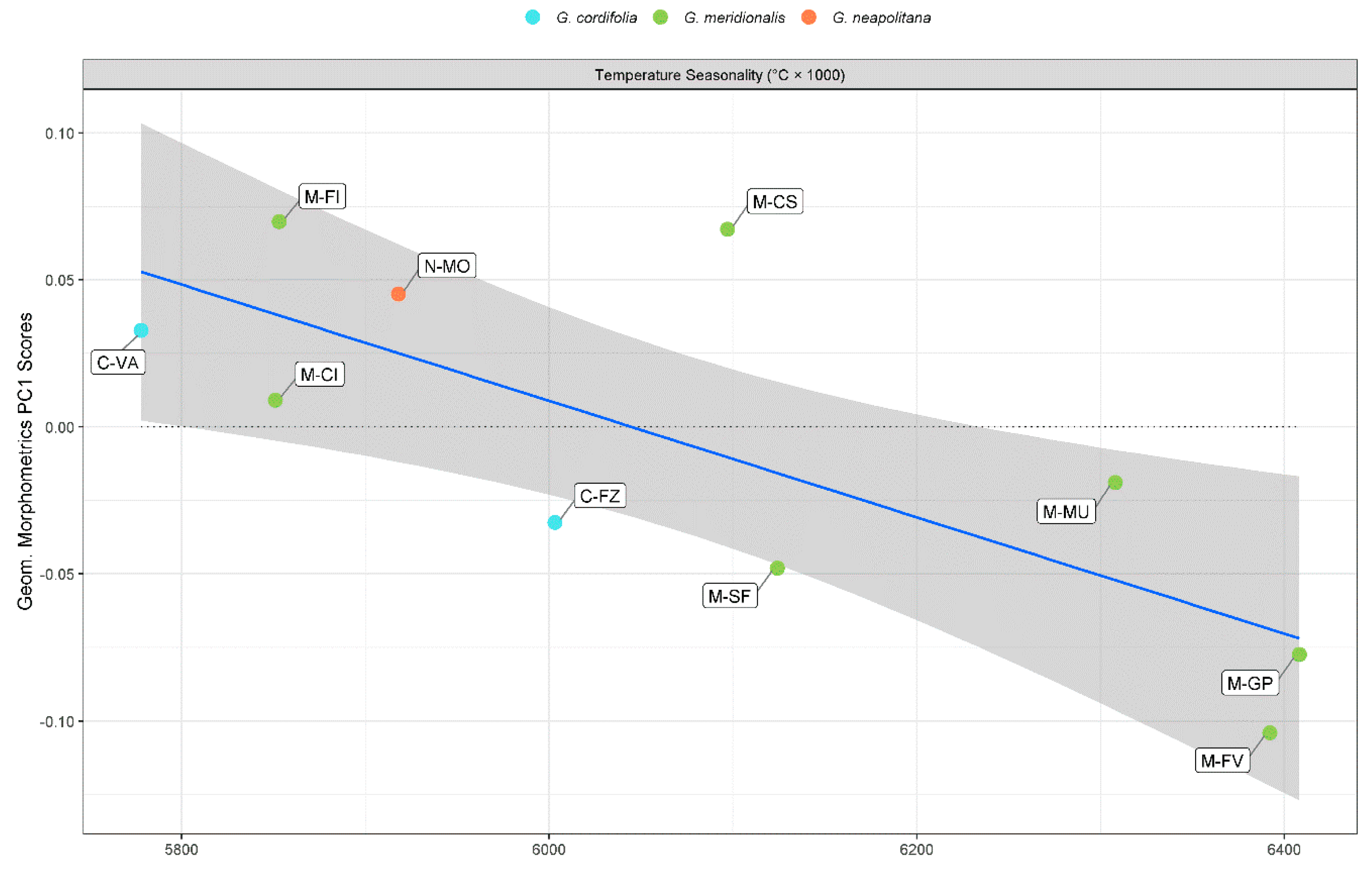
2.2. Geometric Morphometrics
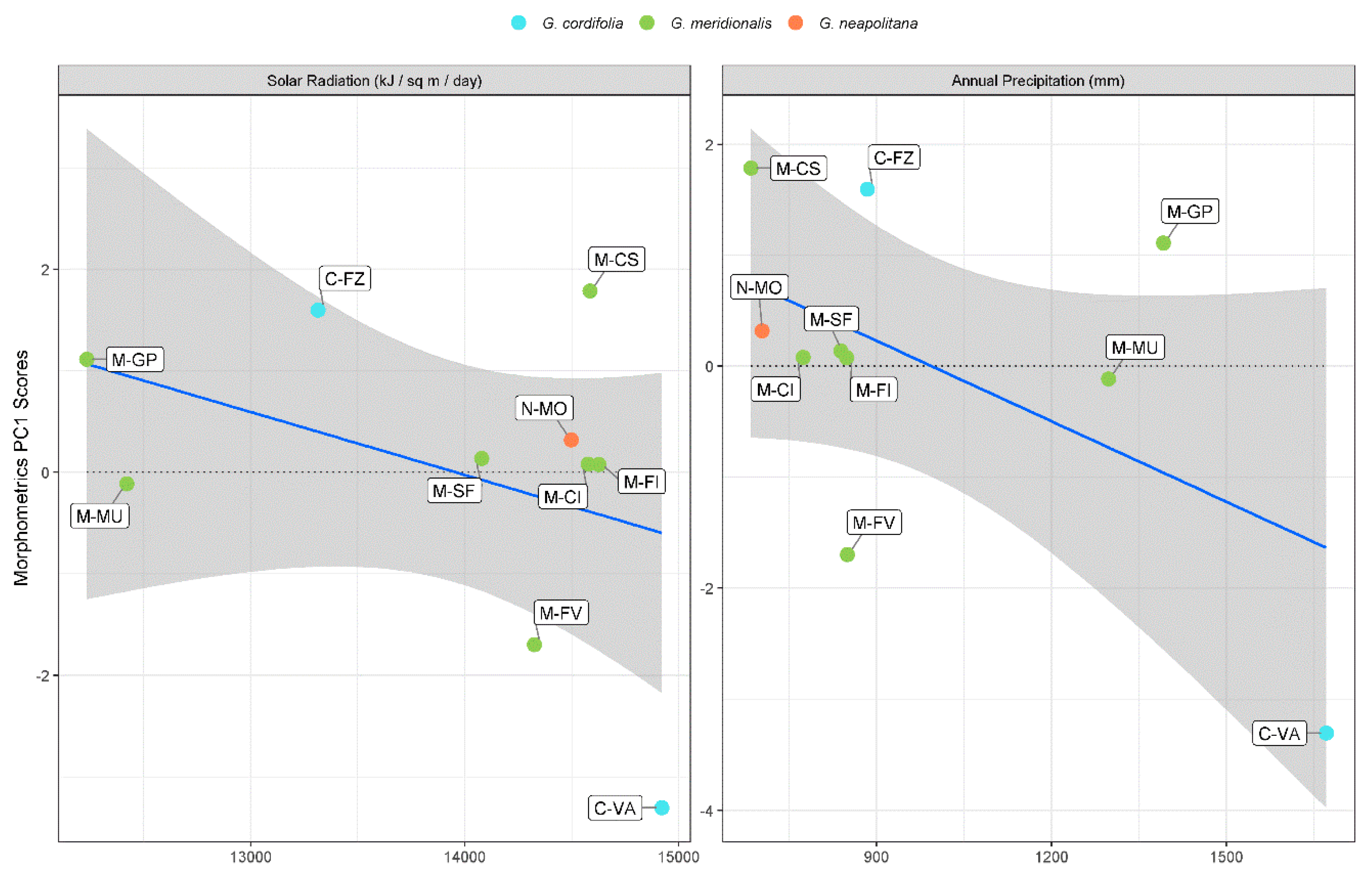
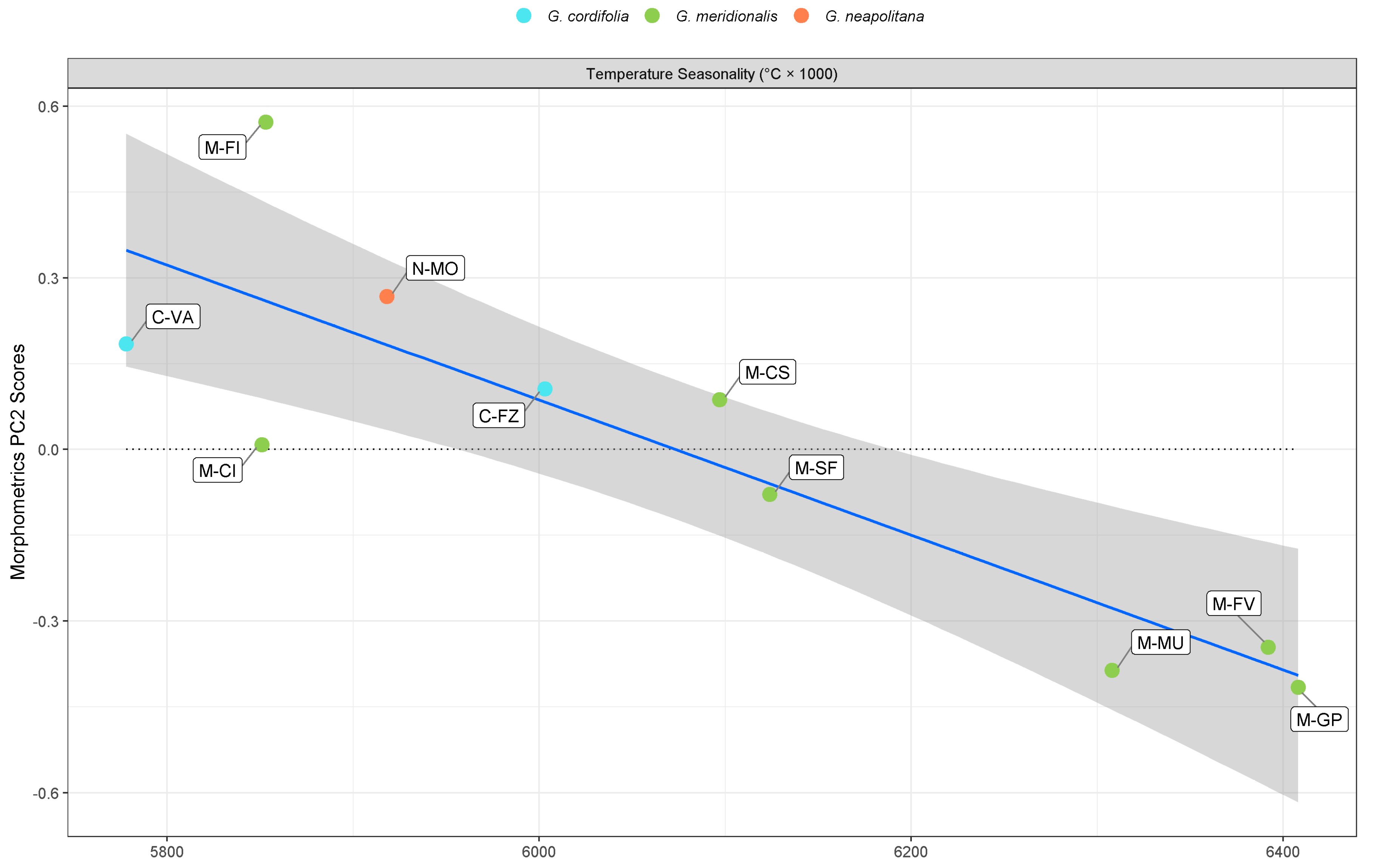
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample Collection
5.2. Classical Morphometrics
5.3. Geometric Morphometrics
5.4. Statistics and Modeling
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hazler Pilepić, K.; Friščić, M.; Duran, A.; Maslo, S.; Garić, R.; Čuljak, S.; Šutalo, K. Contribution to Globularia phylogeny based on nuclear ribosomal spacer and two chloroplast DNA regions. Period. Biol. 2016, 118, 417–424. [Google Scholar] [CrossRef][Green Version]
- Pignatti, S. Flora d’Italia; Edagricole: Firenze, Italy, 2019; Volume 3, ISBN 8850652429. [Google Scholar]
- Schwarz, O. Die Gattung Globularia. Bot. Jahrb. Syst. 1938, 69, 318–373. [Google Scholar]
- Holländer, K.; Jäger, E.J. Morphologie, Biologie und ökogeographische Differenzierung von Globularia: I. Progessionen in der Wuchsform, Infloreszenz, Blattnervatur und Verbreitung. Flora 1994, 189, 223–254. [Google Scholar] [CrossRef]
- Affenzeller, M.; Kadereit, J.W.; Comes, H.P. Parallel bursts of recent and rapid radiation in the Mediterranean and Eritreo-Arabian biodiversity hotspots as revealed by Globularia and Campylanthus (Plantaginaceae). J. Biogeogr. 2018, 45, 552–566. [Google Scholar] [CrossRef]
- Milletti, N. Revisione sistematica del genere Globularia L. (Globulariaceae) in Italia. Ph.D. Thesis, University of Florence, Florence, Italy, 1987. [Google Scholar]
- Duman, H. A new species of Globularia L. (Globulariaceae) from South Anatolia. Bot. J. Linn. Soc. 2001, 137, 425–428. [Google Scholar] [CrossRef]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Schwarz, O. Chromosomenzahlen, Lebensformen und Evolution der Gattung Globularia L. Drudea 1963, 3, 5–16. [Google Scholar]
- Contandriopoulos, C. Contribution a l’etude cytobiogeographique du genre Globularia L. Biol. Ecol. Medit 1978, 5, 3–14. [Google Scholar]
- Kadereit, J.W.; Griebeler, E.M.; Comes, H.P. Quaternary diversification in European alpine plants: Pattern and process. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 265–274. [Google Scholar] [CrossRef]
- Comes, H.P.; Kadereit, J.W. Tests of Pleistocene speciation among alpine and montane species of Globularia (Globulariaceae) from the European high mountains. Bauhinia 2001, 15, 76. [Google Scholar]
- Kadereit, J.W.; Comes, H.P. The temporal course of alpine plant diversification in the Quaternary. Regnum Veg. 2005, 143, 117–130. [Google Scholar]
- Comes, H.P.; Kadereit, J.W. Spatial and temporal patterns in the evolution of the flora of the European Alpine System. Taxon 2003, 52, 451–462. [Google Scholar] [CrossRef]
- Hegi, G. Scrophulariaceae, Orobanchaceae, Lentibulariaceae, Globulariaceae, Plantaginaceae. In Illustrierte Flora von Mitteleuropa; Carl Hanser Verlag: München, Germany, 1974; Volume VI/1, pp. 551–558. [Google Scholar]
- De Natale, A.; Pollio, A. A forgotten collection: The Libyan ethnobotanical exhibits (1912–1914) by A. Trotter at the Museum O. Comes at the University Federico II in Naples, Italy. J. Ethnobiol. Ethnomed. 2012, 8, 4. [Google Scholar] [CrossRef]
- Johnson, T. CRC Ethnobotany Desk Reference; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781315891842. [Google Scholar]
- Friščić, M.; Maslo, S.; Garić, R.; Maleš, Ž.; Hazler Pilepić, K. Comparative analysis of specialized metabolites and antioxidant capacity in vitro of different natural populations of Globularia spp. Acta Bot. Croat. 2018, 77, 1–9. [Google Scholar] [CrossRef]
- Mateos, M.A.; Valdés, B. A new species of Globularia (Globulariaceae) from the Talassemtane National Park, N Morocco. Willdenowia 2006, 36, 409–412. [Google Scholar] [CrossRef]
- Duran, A.; Cetin, Ö.; Öztürk, M. Globularia anatolica sp. nov. (Globulariaceae) from the Honaz Mountain National Park, southwest Turkey. Nord. J. Bot. 2009, 27, 232–237. [Google Scholar] [CrossRef]
- Ravnik, V. Zur morphologischen und taxonomischen Problematik von Globularia cordifolia L. im Bereiche der sudostlichen Kalkalpen und des illyrischen Übergangsgebietes. lahrb. Ver. Schutze Alpenpfl. Tiere 1962, 27, 119–121. [Google Scholar]
- Ravnik, V. Zur morphologisch-systematischen und chorologischen Problematik der Art Globularia cordifolia L. s. lat. Razpr. Slov. Akad. Znan. Umet. Razred Prir. Med. Vede 1965, 8, 5–41. [Google Scholar]
- Milletti, N.; Mori, B. Números Cromosomáticos de Plantas Occidentales, 466-471. An. Jardín Botánico Madrid 1988, 45, 267–272. [Google Scholar]
- Milletti, N.; Strid, A. Globulariaceae. In Mountain Flora of Greece; Strid, A., Tan, K., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 257–259. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Innangi, M.; Izzo, A.; La Valva, V. Revisione dello status IUCN per alcuni taxa inclusi nella Lista Rossa della Regione Campania. Delpinoa 2007, 49, 77–88. [Google Scholar]
- Caputo, G.; La Valva, V.; Nazzaro, R.; Ricciardi, M. La flora della Penisola Sorrentina (Campania). Delpinoa 1990, 31–32, 3–97. [Google Scholar]
- Caneva, G.; Cancellieri, L. il Paesaggio Vegetale della Costa d’Amalfi; Gangemi: Roma, Italy, 2007. [Google Scholar]
- Guarino, C.; Napolitano, F. Community habitats and biodiversity in the Taburno-Camposauro Regional Park. Woodland, rare species, endangered species and their conservation. Forest 2006, 3, 527–541. [Google Scholar] [CrossRef]
- Corazzi, G. Contributo alla conoscenza della flora del Sannio: Il complesso montuoso del Camposauro (Benevento, Campania). Webbia 2008, 63, 215–250. [Google Scholar] [CrossRef]
- Larsen, K. Cytological observations on some species of Globularia. Bot. Not. 1957, 110, 265–270. [Google Scholar]
- Willkomm, M. Récherches sur l’organographie et la Classification des Globulariées; Gustav Mayer: Leipsick, Germany, 1850. [Google Scholar]
- Perrone, R.; Salmeri, C.; Brullo, S.; Colombo, P.; De Castro, O. What do leaf anatomy and micro-morphology tell us about the psammophilous Pancratium maritimum L. (Amaryllidaceae) in response to sand dune conditions? Flora Morphol. Distrib. Funct. Ecol. Plants 2015, 213, 20–31. [Google Scholar] [CrossRef]
- Schoettle, A.W.; Rochelle, S.G. Morphological variation of Pinus flexilis (Pinaceae), a bird-dispersed pine, across a range of elevations. Am. J. Bot. 2000, 87, 1797–1806. [Google Scholar] [CrossRef]
- Campbell, D.R.; Powers, J.M. Natural selection on floral morphology can be influenced by climate. Proc. R. Soc. B Biol. Sci. 2015, 282, 1–7. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Warton, D.I.; McGeoch, M.A. Technical advances at the interface between ecology and statistics: Improving the biodiversity knowledge generation workflow. Methods Ecol. Evol. 2017, 8, 396–397. [Google Scholar] [CrossRef]
- Henderson, A. Traditional morphometrics in plant systematics and its role in palm systematics. Bot. J. Linn. Soc. 2006, 151, 103–111. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kull, K. Leaf weight per area and leaf size of 85 Estonian woody species in relation to shade tolerance and light availability. For. Ecol. Manage. 1994, 70, 1–10. [Google Scholar] [CrossRef]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf Form and Photosynthesis. Bioscience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Cardini, A.; Loy, A. Virtual Morphology and Evolutionary Morphometrics in the new millenium. Hystrix 2013, 24, 1–5. [Google Scholar]
- Musarella, C.M.; Cano-Ortiz, A.; Piñar Fuentes, J.C.; Navas-Ureña, J.; Pinto Gomes, C.J.; Quinto-Canas, R.; Cano, E.; Spampinato, G. Similarity analysis between species of the genus Quercus L. (Fagaceae) in southern Italy based on the fractal dimension. PhytoKeys 2018, 113, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.; Luo, H.; Chen, L.; Peng, J.; Fan, J. Plant recognition via leaf shape and margin features. Multimed. Tools Appl. 2019, 78, 27463–27489. [Google Scholar] [CrossRef]
- Kimball, S. Links between floral morphology and floral visitors along an elevational gradient in a Penstemon hybrid zone. Oikos 2008, 117, 1064–1074. [Google Scholar] [CrossRef]
- Kuzmanović, N.; Šinžar-Sekulić, J.; Lakušić, D. Ecologically Determined Variation in Leaf Anatomical Traits of Sesleria rigida (Poaceae) in Serbia—Multivariate Morphometric Evidence. Folia Geobot. 2012, 47, 41–57. [Google Scholar] [CrossRef]
- Koti, S.; Reddy, K.R.; Kakani, V.G.; Zhao, D.; Reddy, V.R. Soybean (Glycine max) pollen germination characteristics, flower and pollen morphology in response to enhanced ultraviolet-B radiation. Ann. Bot. 2004, 94, 855–864. [Google Scholar] [CrossRef]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A.K. Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef]
- Rodrigues Pereira, T.A.; da Silva, L.C.; Azevedo, A.A.; Francino, D.M.T.; dos Santos Coser, T.; Pereira, J.D. Leaf morpho-anatomical variations in Billbergia elegans and Neoregelia mucugensis (Bromeliaceae) exposed to low and high solar radiation. Botany 2013, 91, 327–334. [Google Scholar] [CrossRef]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which is a better predictor of plant traits: Temperature or precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Pierce, S.; Brusa, G.; Vagge, I.; Cerabolini, B.E.L.L. Allocating CSR plant functional types: The use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 2013, 27, 1002–1010. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic Predictors for Supporting Ecological Applications in the Conterminous United States. U.S Geol. Surv. Data Ser. 2012, 691, 10. [Google Scholar]
- Lomba, A.; Pellissier, L.; Randin, C.; Vicente, J.; Moreira, F.; Honrado, J.; Guisan, A. Overcoming the rare species modelling paradox: A novel hierarchical framework applied to an Iberian endemic plant. Biol. Conserv. 2010, 143, 2647–2657. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Innangi, M.; D’Alessandro, F.; Fioretto, A.; Di Febbraro, M. Modeling distribution of Mediterranean beech forests and soil carbon stock under climate change scenarios. Clim. Res. 2015, 66, 25–36. [Google Scholar] [CrossRef]
- De Castro, O.; Di Maio, A.; Di Febbraro, M.; Imparato, G.; Innangi, M.; Vela, E.; Menale, B. A multi-faceted approach to analyse the effects of environmental variables on geographic range and genetic structure of a perennial psammophilous geophyte: The case of the sea daffodil Pancratium maritimum L. in the mediterranean basin. PLoS ONE 2016, 11, e0164816. [Google Scholar] [CrossRef]
- Brus, D.J.; Hengeveld, G.M.; Walvoort, D.J.J.; Goedhart, P.W.; Heidema, A.H.; Nabuurs, G.J.; Gunia, K. Statistical mapping of tree species over Europe. Eur. J. For. Res. 2011, 131, 145–157. [Google Scholar] [CrossRef]
- Abrams, M.D.; Kloeppel, B.D.; Kubiske, M.E. Ecophysiological and morphological responses to shade and drought in two contrasting ecotypes of Prunus serotina. Tree Physiol. 1992, 10, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Xu, G.; Zou, T. Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant Cell Environ. 2007, 30, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, L.; Alvarez, N.; Guisan, A. Pollinators as drivers of plant distribution and assemblage into communities. In Evolution Plant-Pollinator Relationships; Cambridge University Press: Cambridge, UK, 2012; pp. 392–413. [Google Scholar]
- Espíndola, A.; Pellissier, L.; Alvarez, N. Variation in the proportion of flower visitors of Arum maculatum along its distributional range in relation with community-based climatic niche analyses. Oikos 2011, 120, 728–734. [Google Scholar] [CrossRef]
- De Manincor, N.; Hautekeete, N.; Piquot, Y.; Schatz, B.; Vanappelghem, C.; Massol, F. Does phenology explain plant–pollinator interactions at different latitudes? An assessment of its explanatory power in plant–hoverfly networks in French calcareous grasslands. Peer Community Ecol. 2019, 1–55. [Google Scholar] [CrossRef]
- Fantinato, E.; Del Vecchio, S.; Buffa, G. The co-occurrence of different grassland communities increases the stability of pollination networks. Flora 2019, 255, 11–17. [Google Scholar] [CrossRef]
- Fossel, A.M. Les globulariacees visitees par les abeilles. Ann. L’abeille 1967, 10, 17–27. [Google Scholar] [CrossRef][Green Version]
- Outomuro, D.; Johansson, F. A potential pitfall in studies of biological shape: Does size matter? J. Anim. Ecol. 2017, 86, 1447–1457. [Google Scholar] [CrossRef]
- Viscosi, V. Geometric morphometrics and leaf phenotypic plasticity: Assessing fluctuating asymmetry and allometry in European white oaks (Quercus). Bot. J. Linn. Soc. 2015, 179, 335–348. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Size, shape, and form: Concepts of allometry in geometric morphometrics. Dev. Genes Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D. Factors affecting leaf morphology: A case study of Ranunculus natans C. A. Mey. (Ranunculaceae) in the arid zone of northwest China. Ecol. Res. 2009, 24, 1323–1333. [Google Scholar] [CrossRef]
- Zhao, L.L.; Zhang, Y.; Wang, P.C.; Luo, T.Q.; Zhang, W.; Chen, J. Morphological and genetic variations of Sophora davidii populations originating from different altitudes in the mountains of southwestern China. Flora Morphol. Distrib. Funct. Ecol. Plants 2016, 224, 1–6. [Google Scholar] [CrossRef]
- Tutin, T.G. Globulariaceae. In Flora Europaea; Tutin, T., Heywood, V., Burges, N., Moore, D., Valentine, D., Walters, S., Webb, D., Eds.; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 282–283. [Google Scholar]
- Friščić, M. Botanical Characteristics, Phytochemical Profile and Biological Effects of Globularia L. Species. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2017. [Google Scholar]
- Crkvenčić, M.; Dudaš, S.; Jerković, I.; Marijanović, Z.; Poljuha, D.; Hazler Pilepić, K. Essential Oil Composition of Three Globularia Species. Chem. Biodivers. 2016, 13, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Friščić, M.; Bucar, F.; Hazler Pilepić, K. LC-PDA-ESI-MSn analysis of phenolic and iridoid compounds from Globularia spp. J. Mass Spectrom. 2016, 51, 1211–1236. [Google Scholar] [CrossRef] [PubMed]
- Siljak-Yakovlev, S.; Peruzzi, L. Cytogenetic characterization of endemics: Past and future. Plant Biosyst. 2012, 146, 694–702. [Google Scholar]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- De Castro, O.; Innangi, M.; Di Maio, A.; Menale, B.; Bacchetta, G.; Pires, M.; Noble, V.; Gestri, G.; Conti, F.; Peruzzi, L. Disentangling phylogenetic relationships in a hotspot of diversity: The butterworts (Pinguicula L., Lentibulariaceae) endemic to Italy. PLoS ONE 2016, 11, e0167610. [Google Scholar] [CrossRef]
- Bellino, A.; Bellino, L.; Baldantoni, D.; Saracino, A. Evolution, ecology and systematics of Soldanella (Primulaceae) in the southern Apennines (Italy). BMC Evol. Biol. 2015, 15, 158. [Google Scholar] [CrossRef]
- Peruzzi, L.; Innangi, M.; Tatino, F.; Santangelo, A. Fritillaria messanensis subsp. gracilis (Liliaceae), a new record for the Italian flora (S Italy). Phytologia 2017, 307, 167–170. [Google Scholar]
- Cordell, S.; Goldstein, G.; Mueller-Dombois, D.; Webb, D.; Vitousek, P.M. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: The role of phenotypic plasticity. Oecologia 1998, 113, 188–196. [Google Scholar] [CrossRef]
- Rohlf, F.J. The tps series of software. Hystrix 2015, 26, 1–4. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Viscosi, V.; Cardini, A. Leaf morphology, taxonomy and geometric morphometrics: A simplified protocol for beginners. PLoS ONE 2011, 6, e25630. [Google Scholar] [CrossRef] [PubMed]
- Viscosi, V.; Lepais, O.; Gerber, S.; Fortini, P. Leaf morphological analyses in four European oak species (Quercus) and their hybrids: A comparison of traditional and geometric morphometric methods. Plant Biosyst. 2009, 143, 564–574. [Google Scholar] [CrossRef]
- Shipunov, A.B.; Bateman, R.M. Geometric morphometrics as a tool for understanding Dactylorhiza (Orchidaceae) diversity in European Russia. Biol. J. Linn. Soc. 2005, 85, 1–12. [Google Scholar] [CrossRef]
- Van der Niet, T.; Zollikofer, C.P.E.; Ponce De León, M.S.; Johnson, S.D.; Linder, H.P. Three-dimensional geometric morphometrics for studying floral shape variation. Trends Plant Sci. 2010, 15, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Innangi, M.; Izzo, A. Pinguicula lavalvae (Lentibulariaceae), a new endemic butterwort from southern Italy diagnosed with the aid of geometric morphometrics. Plant Biosyst. 2015, 149, 990–999. [Google Scholar] [CrossRef]
- Astuti, G.; Peruzzi, L. Are shoots of diagnostic value in Central European bladderworts (Utricularia L., Lentibulariaceae)? Plant Biosyst. 2018, 152, 1214–1226. [Google Scholar] [CrossRef]
- Püschel, T.A.; Espejo, J.; Sanzana, M.J.; Benítez, H.A. Analysing the floral elements of the lost tree of Easter Island: A morphometric comparison between the remaining ex-situ lines of the endemic extinct species Sophora toromiro. PLoS ONE 2014, 9, e115548. [Google Scholar] [CrossRef][Green Version]
- Chuanromanee, T.S.; Cohen, J.I.; Ryan, G.L. Morphological Analysis of Size and Shape (MASS): An integrative software program for morphometric analyses of leaves. Appl. Plant Sci. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Bardua, C.; Felice, R.N.; Watanabe, A.; Fabre, A.-C.; Goswami, A. A Practical Guide to Sliding and Surface Semilandmarks in Morphometric Analyses. Integr. Org. Biol. 2019, 1, obz016. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Innangi, M.; Balestrieri, R.; Danise, T.; d’Alessandro, F.; Fioretto, A. From soil to bird community: A Partial Least Squares approach to investigate a natural wooded area surrounded by urban patchwork (Astroni crater, southern Italy). Ecol. Modell. 2019, 394, 1–10. [Google Scholar] [CrossRef]
- Gómez, R.; Asencio, A.D.; Picón, J.M.; Del Campo, R.; Arce, M.I.; del Mar Sánchez-Montoya, M.; Suárez, M.L.; Vidal-Abarca, M.R. The effect of water salinity on wood breakdown in semiarid Mediterranean streams. Sci. Total Environ. 2016, 541, 491–501. [Google Scholar] [CrossRef]
- Jinggut, T.; Yule, C.M. Leaf-litter breakdown in streams of East Malaysia (Borneo) along an altitudinal gradient: Initial nitrogen content of litter limits shredder feeding. Freshw. Sci. 2015, 34, 691–701. [Google Scholar] [CrossRef]
- Lin, M.; Chevalier, M.; Lek, S.; Zhang, L.; Gozlan, R.E.; Liu, J.; Zhang, T.; Ye, S.; Li, W.; Li, Z. Eutrophication as a driver of r-selection traits in a freshwater fish. J. Fish Biol. 2014, 85, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, L.; Pottier, J.; Vittoz, P.; Dubuis, A.; Guisan, A. Spatial pattern of floral morphology: Possible insight into the effects of pollinators on plant distributions. Oikos 2010, 119, 1805–1813. [Google Scholar] [CrossRef]
- Cardini, A.; Alexandre, J.; Diniz, F.; Polly, P.D. Biogeographic Analysis Using Geometric Morphometrics: Clines in Skull Size and Shape in a Widespread African Arboreal Monkey. In Morphometrics for Nonmorphometricians; Elewa, A.M.T., Ed.; Lecture Notes in Earth Sciences; Springer: Berlin/Heidelberg, Germany, 2010; Volume 124, pp. 191–217. ISBN 978-3-540-95852-9. [Google Scholar]
- Botes, A.; McGeoch, M.A.; Robertson, H.G.; van Niekerk, A.; Davids, H.P.; Chown, S.L. Ants, altitude and change in the northern Cape Floristic Region. J. Biogeogr. 2006, 33, 71–90. [Google Scholar] [CrossRef]
- Danise, T.; Fioretto, A.; Innangi, M. Spectrophotometric methods for lignin and cellulose in forest soils as predictors for humic substances. Eur. J. Soil Sci. 2018, 69, 856–867. [Google Scholar] [CrossRef]
- Zhang, L.; Wylie, B.K.; Ji, L.; Gilmanov, T.G.; Tieszen, L.L. Climate-driven interannual variability in net ecosystem exchange in the Northern Great Plains grasslands. Rangel. Ecol. Manag. 2010, 63, 40–50. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Gustafson, W.I.; Yu, S. Generalized approach for using unbiased symmetric metrics with negative values: Normalized mean bias factor and normalized mean absolute error factor. Atmos. Sci. Lett. 2012, 13, 262–267. [Google Scholar] [CrossRef]
- King, T.S.; Chinchilli, V.M. A generalized concordance correlation coefficient for continuous and categorical data. Stat. Med. 2001, 20, 2131–2147. [Google Scholar] [CrossRef] [PubMed]
- Green, P.J.; Silverman, B.W. Chapman & Hall/CRC Monographs on Statistics & Applied Probability. In Nonparametric Regression and Generalized Linear Models: A Roughness Penalty Approach; CRC Press: Boca Raton, FL, USA, 1993; ISBN 9780412300400. [Google Scholar]
- R Core Team. Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org/ (accessed on 12 January 2019).


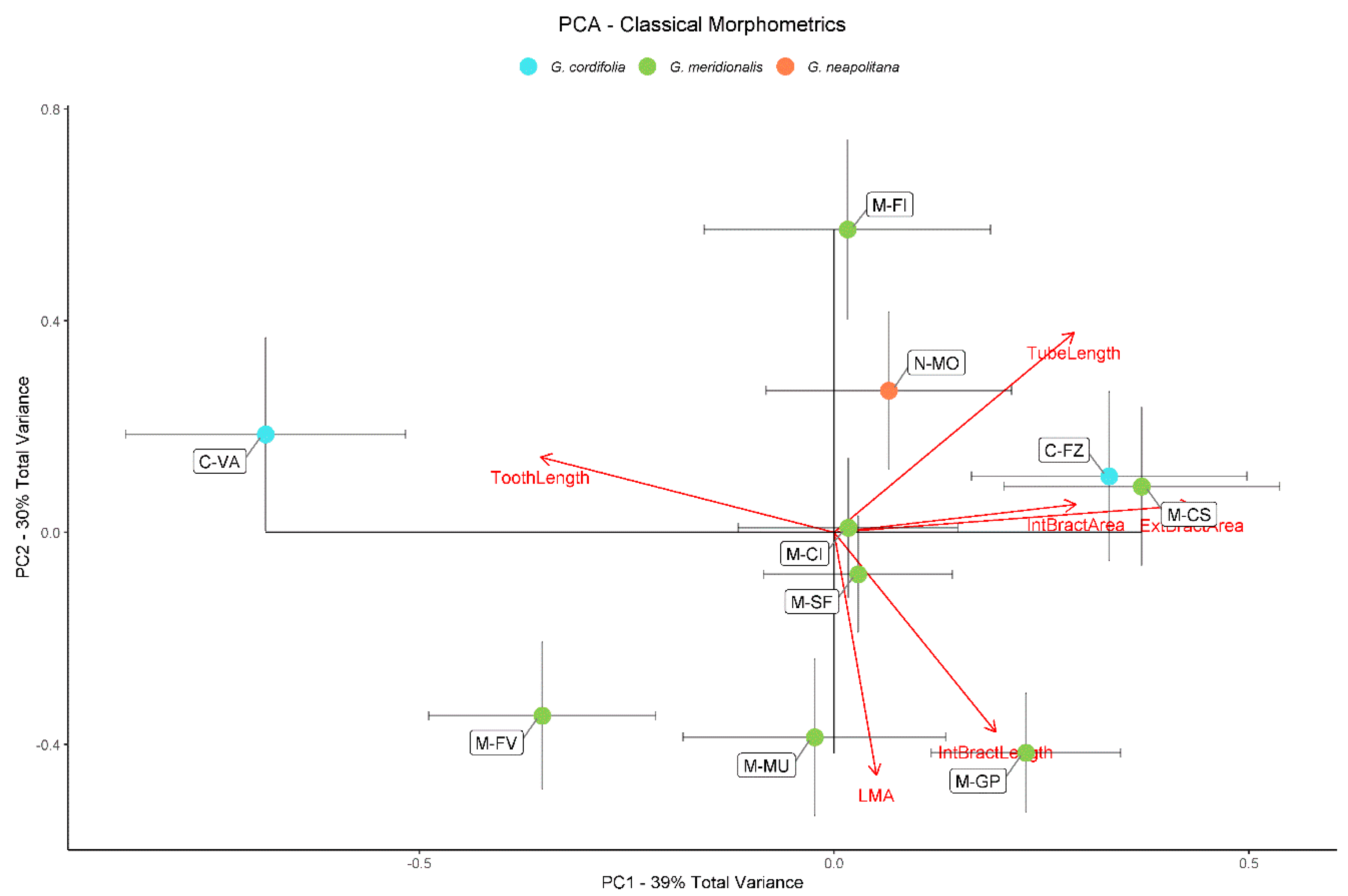
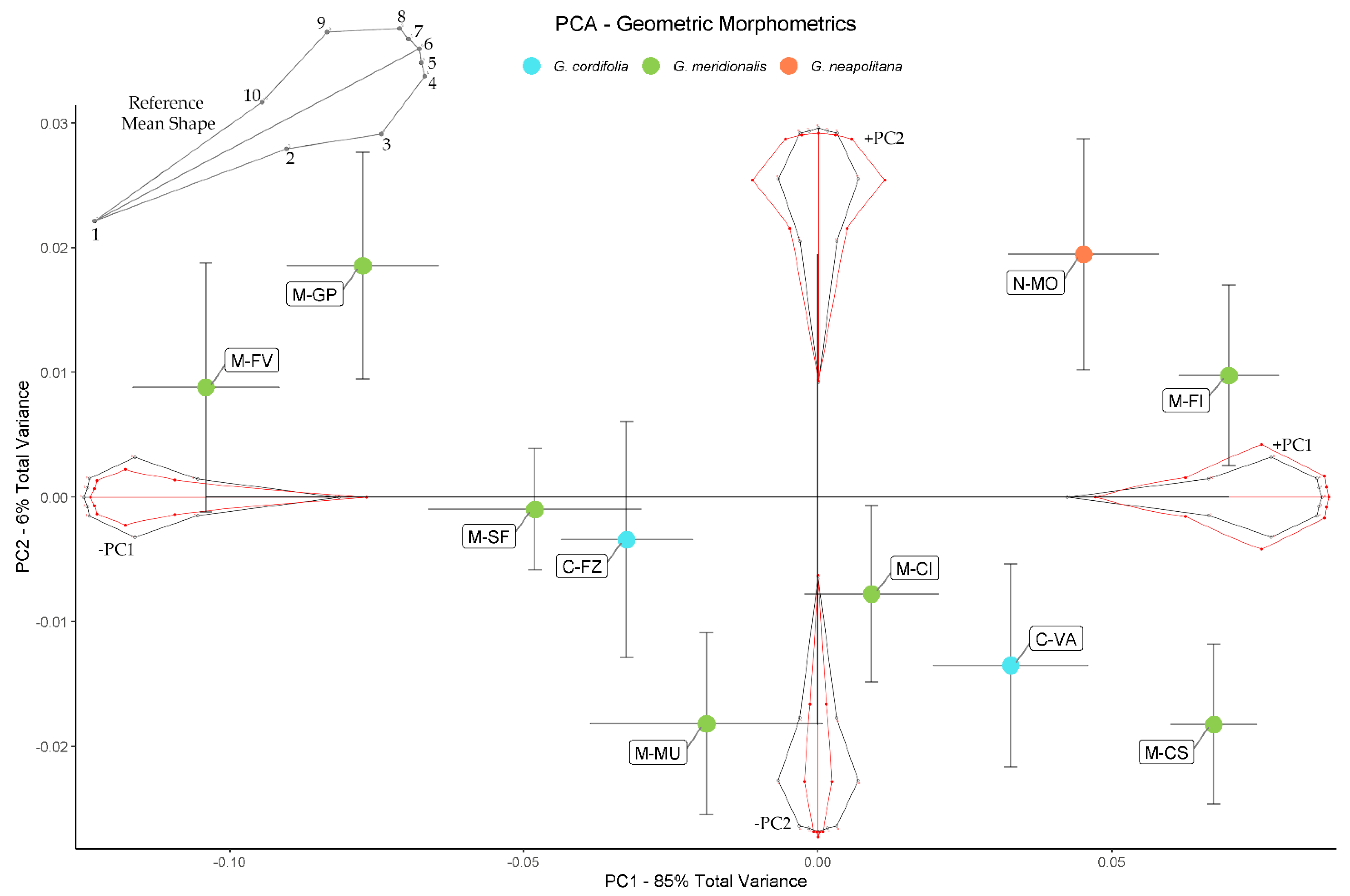
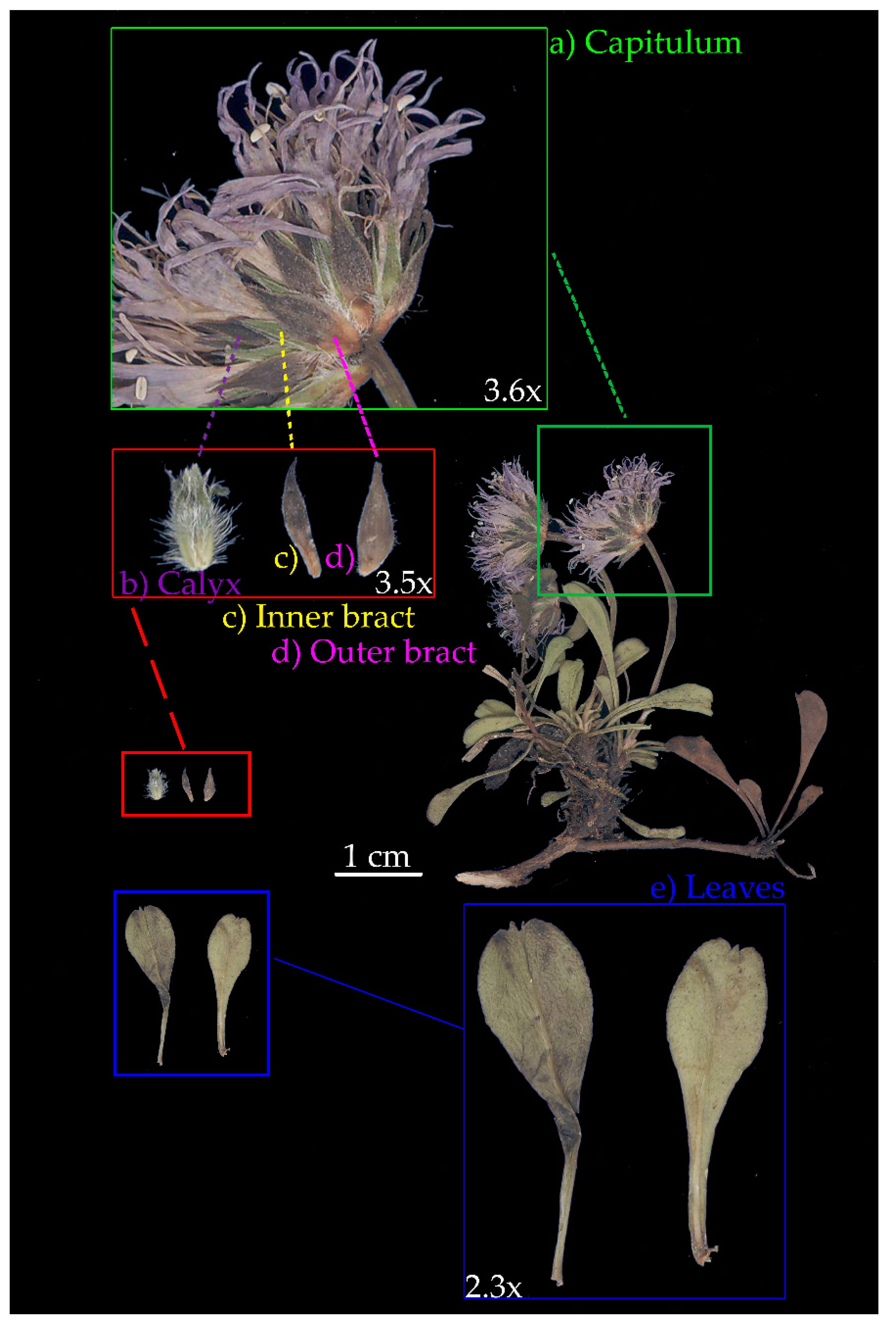

| Population | Leaves | Calyces | Outer Bracts | Inner Bracts | |||
|---|---|---|---|---|---|---|---|
| ID | Species | LMA (g/m2) | Tube Length (mm) | Teeth Length (mm) | Area (mm2) | Length (mm) | Area (mm2) |
| C-VA | G. cordifolia | 182.24 ± 11.25 (9) bc | 1.56 ± 0.04 (9) bc | 2.33 ± 0.11 (9) a | 2.73 ± 0.53 (5) a | 3.62 ± 0.18 (7) ab | 2.49 ± 0.32 (7) ab |
| C-FZ | G. cordifolia | 187.82 ± 12.93 (11) bc | 1.85 ± 0.04 (11) a | 1.92 ± 0.07 (11) bc | 4.67 ± 0.50 (5) a | 4.08 ± 0.17 (9) ab | 2.95 ± 0.17 (9) ab |
| M-GP | G. meridionalis | 257.86 ± 9.70 (15) a | 1.74 ± 0.04 (15) ab | 2.00 ± 0.03 (15) abc | 4.44 ± 0.14 (7) a | 4.2 ± 0.09 (15) a | 2.92 ± 0.14 (15) ab |
| M-MU | G. meridionalis | 216.41 ± 9.59 (11) ab | 1.47 ± 0.07 (6) c | 1.97 ± 0.11 (6) abc | 4.09 ± 0.24 (6) a | 4.13 ± 0.18 (5) ab | 2.80 ± 0.13 (5) ab |
| M-SF | G. meridionalis | 217.07 ± 6.95 (9) ab | 1.68 ± 0.08 (6) abc | 1.85 ± 0.04 (6) bc | 3.69 ± 0.77 (4) a | 3.73 ± 0.17 (7) ab | 2.88 ± 0.22 (7) ab |
| M-FV | G. meridionalis | 214.26 ± 11.76 (9) ab | 1.57 ± 0.04 (9) bc | 2.00 ± 0.07 (9) abc | 2.97 ± 0.28 (3) a | 4.13 ± 0.18 (8) ab | 2.18 ± 0.16 (8) b |
| M-CI | G. meridionalis | 188.65 ± 8.67 (11) bc | 1.74 ± 0.06 (11) ab | 2.06 ± 0.08 (11) abc | 3.61 ± 0.25 (5) a | 4.14 ± 0.15 (10) ab | 3.07 ± 0.21 (10) ab |
| M-CS | G. meridionalis | 184.09 ± 8.29 (15) bc | 1.85 ± 0.06 (15) a | 1.82 ± 0.06 (15) c | 4.46 ± 0.31 (7) a | 4.09 ± 0.18 (13) ab | 2.99 ± 0.22 (13) ab |
| M-FI | G. meridionalis | 176.49 ± 5.64 (11) bc | 1.98 ± 0.12 (11) a | 2.02 ± 0.1 (11) abc | 4.38 ± 0.55 (6) a | 3.45 ± 0.23 (10) b | 2.45 ± 0.32 (10) ab |
| N-MO | G. neapolitana | 169.90 ± 5.88 (15) c | 1.81 ± 0.04 (15) a | 2.14 ± 0.06 (15) ab | 3.72 ± 0.32 (9) a | 4.03 ± 0.26 (9) ab | 3.41 ± 0.31 (9) a |
| All | G. cordifolia | 185.32 ± 8.53 (20) b | 1.72 ± 0.04 (20) a | 2.10 ± 0.07 (20) a | 3.70 ± 0.47 (10) a | 3.88 ± 0.13 (16) a | 2.75 ± 0.17 (16) a |
| All | G. meridionalis | 208.74 ± 4.51 (81) a | 1.75 ± 0.03 (73) a | 1.96 ± 0.03 (73) a | 4.07 ± 0.15 (38) a | 4.00 ± 0.07 (68) a | 2.79 ± 0.08 (68) a |
| All | G. neapolitana | 169.90 ± 5.88 (15) b | 1.81 ± 0.04 (15) a | 2.14 ± 0.06 (15) a | 3.72 ± 0.32 (9) a | 4.03 ± 0.26 (9) a | 3.41 ± 0.31 (9) a |
| Predictors | Dependent Variables | |||
|---|---|---|---|---|
| PC1 Classical Morphometrics | PC2 Classical Morphometrics | PC1 Geometric Morphometrics | PC2 Geometric Morphometrics | |
| Estimate (Confidence Interval) | Estimate (Confidence Interval | Estimate (Confidence Interval | Estimate (Confidence Interval) | |
| (Intercept) | 18.70 (7.91–29.50) * | 7.16 (4.31–10.02) ** | 1.20 (0.49–1.91) * | −0.01 (−0.03–0.01) |
| Solar radiation | −1.10 × 10−3 (−1.80 × 10−3 to −4.00 × 10−4) * | |||
| Annual precipitation | −3.60 × 10−3 (−5.60 × 10−3 to −1.30 × 10−3) * | |||
| Temperature seasonality | −1.18 × 10−3 (−1.65 × 10−3 to −7.10 × 10−4) ** | −2.0 × 10−4 (−3.10 × 10−4 to −8.00 × 10−5) * | ||
| Mean Temperature driest quarter | 8.00 × 10−5 (−8.00 × 10−5 to 1.90 × 10−4) | |||
| R2e/R2p | 0.697/0.545 | 0.760/0.617 | 0.580/0.417 | 0.179/0.000 |
| CCC/nMAE | 0.683/0.212 | 0.782/0.144 | 0.624/0.229 | 0.000/0.377 |
| ID | Description | Species | Date of Collection | Elevation | Latitude | Longitude |
|---|---|---|---|---|---|---|
| C-VA | Val de Rhêmes, Vallée d’Aoste, Italy | G. cordifolia | 13 July 2017 | 2428 | 45.51 | 7.08 |
| C-FZ | Campocecina-Fivizzano, Toscana, Italy | G. cordifolia | 24 July 2017 | 1293 | 44.11 | 10.15 |
| M-GP | Grobničko polje, Primorje-Gorski kotar, Croatia | G. meridionalis | 8 October 2017 | 308 | 45.38 | 14.51 |
| M-MU | Mala Učka, Primorje-Gorski kotar, Croatia | G. meridionalis | 8 October 2017 | 926 | 45.30 | 14.19 |
| M-SF | Monte S. Franco, Abruzzo, Italy | G. meridionalis | 1 August 2017 | 1647 | 42.46 | 13.41 |
| M-FV | Fonte Vedice, Abruzzo, Italy | G. meridionalis | 24 October 2017 | 1080 | 42.34 | 13.58 |
| M-CI | Monte Cigno, Campania, Italy | G. meridionalis | 8 October 2017 | 360 | 41.30 | 14.54 |
| M-CS | Camposauro, Campania, Italy | G. meridionalis | 25 June 2017 | 1182 | 41.18 | 14.60 |
| M-FI | Timpa del Principe-Frascineto, Calabria, Italy | G. meridionalis | 8 August 2017 | 1668 | 39.87 | 16.27 |
| N-MO | Monte S. Angelo a Tre Pizzi, Campania, Italy | G. neapolitana | 16 July 2017 | 1385 | 40.65 | 14.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innangi, M.; Friščić, M.; Hazler Pilepić, K.; Danise, T.; Conti, F.; Bartolucci, F.; Fioretto, A.; Peruzzi, L. Explaining Intricate Morphometric Variability with Environmental Predictors: The Case of Globularia cordifolia Species Complex. Plants 2020, 9, 314. https://doi.org/10.3390/plants9030314
Innangi M, Friščić M, Hazler Pilepić K, Danise T, Conti F, Bartolucci F, Fioretto A, Peruzzi L. Explaining Intricate Morphometric Variability with Environmental Predictors: The Case of Globularia cordifolia Species Complex. Plants. 2020; 9(3):314. https://doi.org/10.3390/plants9030314
Chicago/Turabian StyleInnangi, Michele, Maja Friščić, Kroata Hazler Pilepić, Tiziana Danise, Fabio Conti, Fabrizio Bartolucci, Antonietta Fioretto, and Lorenzo Peruzzi. 2020. "Explaining Intricate Morphometric Variability with Environmental Predictors: The Case of Globularia cordifolia Species Complex" Plants 9, no. 3: 314. https://doi.org/10.3390/plants9030314
APA StyleInnangi, M., Friščić, M., Hazler Pilepić, K., Danise, T., Conti, F., Bartolucci, F., Fioretto, A., & Peruzzi, L. (2020). Explaining Intricate Morphometric Variability with Environmental Predictors: The Case of Globularia cordifolia Species Complex. Plants, 9(3), 314. https://doi.org/10.3390/plants9030314








