Nitrous Oxide Emissions from Paddies: Understanding the Role of Rice Plants
Abstract
1. Introduction
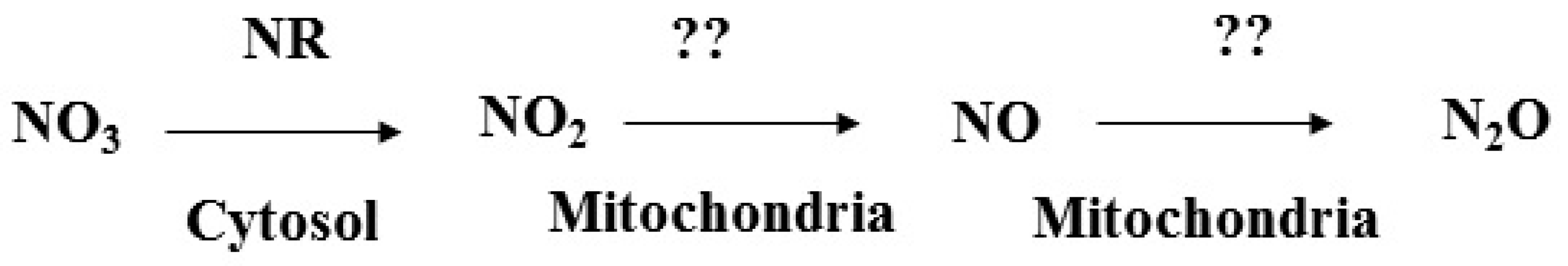
2. Potential Pathway of N2O Formation in Rice Plants
3. Why It Is a Challenge to Understand the Role of Rice Plants with Current Approaches to Methods from Field Studies?
4. Role of NO3, NO2 and NO during Hypoxia and Anoxia Tolerance
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 659–740. [Google Scholar]
- IPCC. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2007: The Physical Science Basis; Solomon, S., Qin, D., Manning, M., Marquis, M., Averyt, K., Marquis, M., Tignor, M.M.B., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410. [Google Scholar] [CrossRef]
- Maclean, J.; Hardy, B.; Hettel, G. Rice Almanac: Source Book for One of the Most Important Economic Activities on Earth; IRRI: Manila, Philippines, 2013. [Google Scholar]
- Linquist, B.; Van Groenigen, K.J.; Adviento-Borbe, M.A.; Pittelkow, C.; Van Kessel, C. An agronomic assessment of greenhouse gas emissions from major cereal crops. Glob. Chang. Biol. 2012, 18, 194–209. [Google Scholar] [CrossRef]
- Heffer, P. Assessment of Fertilizer Use by Crop at the Global Level 2006–2007; International Fertilizer Industry Association: Paris, France, 2009. [Google Scholar]
- Zhao, X.; Pu, C.; Ma, S.T.; Liu, S.L.; Xue, J.F.; Wang, X.; Wang, Y.Q.; Li, S.S.; Lal, R.; Chen, F.; et al. Management-induced greenhouse gases emission mitigation in global rice production. Sci. Total Environ. 2019, 649, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Carrijo, D.; Huang, S.; Chen, J.; Balaine, N.; Zhang, W.; Groenigen, K.J.; Linquist, B. Water management to mitigate the global warming potential of rice systems: A global meta-analysis. Field Crop. Res. 2019, 234, 47–54. [Google Scholar] [CrossRef]
- Ussiri, D.; Lal, R. Soil Emission of Nitrous Oxide and its Mitigation; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Liu, X.; Zhou, T.; Liu, Y.; Zhang, X.; Li, L.; Pan, G. Effect of mid-season drainage on CH4 and N2O emission and grain yield in rice ecosystem: A meta-analysis. Agric. Water Manag. 2019, 213, 1028–1035. [Google Scholar] [CrossRef]
- Yu, K.W.; Wang, Z.P.; Chen, G.X. Nitrous oxide and methane transport through rice plants. Biol. Fert. Soils 1997, 24, 341–343. [Google Scholar] [CrossRef]
- Yan, X.Y.; Shi, S.L.; Du, L.J.; Xing, G. Pathways of N2O emission from rice paddy soil. Soil Biol. Biochem. 2000, 32, 437–440. [Google Scholar] [CrossRef]
- Lenhart, K.; Behrendt, T.; Greiner, S.; Steinkamp, J.; Well, R.; Giesemann, A.; Keppler, F. Nitrous oxide effluxes from plants as a potentially important source to the atmosphere. New Phytol. 2019, 221, 1398–1408. [Google Scholar] [CrossRef]
- Smart, D.R.; Bloom, A.J. Wheat leaves emit nitrous oxide during nitrate assimilation. Proc. Natl. Acad. Sci. USA 2001, 98, 7875–7878. [Google Scholar] [CrossRef]
- Goshima, N.; Mukai, T.; Suemori, M.; Takahashi, M.; Caboche, M.; Morikawa, H. Emission of nitrous oxide (N2O) from transgenic tobacco expressing antisense NiR mRNA. Plant J. 1999, 19, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Lagomarsino, A.; Agnelli, A.E.; Linquist, B.; Adviento-Borbe, M.A.; Agnelli, A.; Gavina, G.; Ravaglia, S. and Ferrara, R.M. Alternate wetting and drying of rice reduced CH4 emissions but triggered N2O peaks in a clayey soil of central Italy. Pedosphere 2016, 26, 533–548. [Google Scholar] [CrossRef]
- Kritee, K.; Nair, D.; Zavala-Araiza, D.; Proville, J.; Rudek, J.; Adhya, T.K.; Loecke, T.; Esteves, T.; Balireddygari, S.; Dava, O.; et al. High nitrous oxide fluxes from rice indicate the need to manage water for both long-and short-term climate impacts. Proc. Natl. Acad. Sci. USA 2018, 115, 9720–9725. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Huang, Y.; Zheng, X.; Wang, Y. Quantifying direct N2O emissions in paddy fields during rice growing season in mainland China: Dependence on water regime. Atmos. Environ. 2007, 41, 8030–8042. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Adviento-Borbe, M.A.; Hill, J.E.; Six, J.; van Kessel, C.; Linquist, B.A. Yield-scaled global warming potential of annual nitrous oxide and methane emissions from continuously flooded rice in response to nitrogen input. Agric. Ecosyst. Environ. 2013, 177, 10–20. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Zhang, Q.; Liu, X.; Xu, J.; Li, Y.; Di, H. Heterotrophic nitrification and denitrification are the main sources of nitrous oxide in two paddy soils. Plant Soil 2018, 445, 39–53. [Google Scholar] [CrossRef]
- Hakata, M.; Takahashi, M.; Zumft, W.; Sakamoto, A.; Morikawa, H. Conversion of the nitrate nitrogen and nitrogen dioxide to nitrous oxides in plants. Acta Biotechnol. 2003, 23, 249–257. [Google Scholar] [CrossRef]
- Dean, J.V.; Harper, J.E. Nitric oxide and nitrous oxide production by soybean and winged bean during the in vivo nitrate reductase assay. Plant. Physiol. 1986, 82, 718–723. [Google Scholar] [CrossRef]
- Lenhart, K.; Weber, B.; Elbert, W.; Steinkamp, J.; Clough, T.; Crutzen, P.; Pöschl, U.; Keppler, F. Nitrous oxide and methane emissions from cryptogamic covers. Glob. Chang. Biol. 2015, 21, 3889–3900. [Google Scholar] [CrossRef]
- Weathers, P.J. N2O evolution by green algae. Appl. Environ. Microbiol. 1984, 48, 1251–1253. [Google Scholar] [CrossRef]
- Brudvig, G.W.; Stevens, T.H.; Chan, S.I. Reactions of nitric oxide with cytochrome c oxidase. Biochemistry 1980, 19, 5275–5285. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. The Biology of Subcellular Nitric Oxide; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant. Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant. Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Igamberdiev, A.U. The anoxic plant mitochondrion as a nitrite: NO reductase. Mitochondrion 2011, 11, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Horchani, F.; Aschi-Smiti, S.; Brouquisse, R. Involvement of nitrate reduction in the tolerance of tomato (Solanum lycopersicum L.) plants to prolonged root hypoxia. Acta Physiol. Plant. 2010, 32, 1113–1123. [Google Scholar] [CrossRef]
- Buwalda, F.; Greenway, H. Nitrogen uptake and growth of wheat during O2 deficiency in root media containing NO3−only, or NO3−plus NH4+. New Phytol. 1989, 111, 161–166. [Google Scholar] [CrossRef]
- Zhang, B.G.; Puard, M.; Couchat, P. Effect of hypoxia, acidity and nitrate on inorganic nutrition in rice plants. Plant Physiol. Biochem. 1990, 28, 655–661. [Google Scholar]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Sodek, L. Effect of oxygen deficiency on nitrogen assimilation and amino acid metabolism of soybean root segments. Amino Acids 2013, 44, 743–755. [Google Scholar] [CrossRef]
- Sugiura, M.; Georgescu, M.N.; Takahashi, M. A nitrite transporter associated with nitrite uptake by higher plant chloroplasts. Plant Cell Physiol. 2007, 48, 1022–1035. [Google Scholar] [CrossRef]
- Stoimenova, M.; Igamberdiev, A.U.; Gupta, K.J.; Hill, R.D. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 2007, 226, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Stoimenova, M.; Kaiser, W.M. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J. Exp. Bot. 2005, 56, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Castello, P.R.; David, P.S.; McClure, T.; Crook, Z.; Poyton, R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006, 3, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, H.H.; Froriep, D.; Havemeyer, A.; Mendel, R.R.; Bittner, F.; Clement, B. The mitochondrial amidoxime reducing component (mARC): Involvement in metabolic reduction of N-oxides, oximes and Nxhydroxyamidinohydrazones. Chem. Med. Chem. 2014, 9, 2381–2387. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.E.; Tejero, J.; Sun, B.; Gauthier, M.C.; Thomas, J.; Ragireddy, V.; Merchant, B.A.; Wang, J.; Azarov, I.; Basu, P.; et al. Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J. Biol. Chem. 2014, 289, 10345–10358. [Google Scholar] [CrossRef]
- Klepper, L.A. Nitric oxide emissions from soybean leaves during in vivo nitrate reductase assays. Plant Physiol. 1987, 85, 96–99. [Google Scholar] [CrossRef]
- Rockel, P.; Strube, F.; Rockel, A.; Wildt, J.; Kaiser, W.M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot. 2002, 53, 103–110. [Google Scholar] [CrossRef]
- Basu, S.; Azarova, N.A.; Font, M.D.; King, S.B.; Hogg, N.; Gladwin, M.T.; Surti, S.; Kim-Shapiro, D.B. Nitrite reductase activity of cytochrome c. J. Biol. Inorg. Chem. 2008, 283, 32590–32597. [Google Scholar] [CrossRef]
- Benamar, A.; Rolletschek, H.; Borisjuk, L.; Avelange-Macherel, M.H.; Curien, G.; Mostefai, H.A.; Macherel, D. Nitrite–nitric oxide control of mitochondrial respiration at the frontier of anoxia. BBA Bioenerg. 2008, 1777, 1268–1275. [Google Scholar] [CrossRef]
- Ascenzi, P.; Marino, M.; Polticelli, F.; Santucci, R.; Coletta, M. Cardiolipin modulates allosterically the nitrite reductase activity of horse heart cytochrome c. J. Biol. Inorg. Chem. 2014, 19, 1195–1201. [Google Scholar] [CrossRef]
- Tischner, R.; Planchet, E.; Kaiser, W.M. Mitochondrial electron transport as a source for nitric oxide in the unicellular green alga Chlorella sorokiniana. FEBS Lett. 2004, 576, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Sampath, V.; Caughey, W.S. Cytochrome c oxidase catalysis of the reduction of nitric oxide to nitrous oxide. Biochem. Biophys. Res. Commun. 1995, 212, 1054–1060. [Google Scholar] [CrossRef]
- Saraste, M.; Castresana, J. Cytochrome oxidase evolved by tinkering with denitrification enzymes. FEBS Lett. 1994, 341, 1–4. [Google Scholar] [CrossRef]
- Blomberg, M.R.; Ädelroth, P. Mechanisms for enzymatic reduction of nitric oxide to nitrous oxide-A comparison between nitric oxide reductase and cytochrome c oxidase. BBA Bioenerg. 2018, 1859, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Alegria, A.E.; Sanchez, S.; Quintana, I. Quinone-enhanced ascorbate reduction of nitric oxide: Role of quinone redox potential. Free Radic. Res. 2004, 38, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Hill, R.D. Plant mitochondrial function during anaerobiosis. Ann. Bot. 2008, 103, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cruz, P.; Alegría, A.E. Quinone-enhanced reduction of nitric oxide by xanthine/xanthine oxidase. Chem. Res. Toxicol. 2009, 22, 818–823. [Google Scholar] [CrossRef]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef]
- Winkel, A.; Colmer, T.D.; Ismail, A.M.; Pedersen, O. Internal aeration of paddy field rice during complete submergence–importance of light and floodwater O2. New Phytol. 2013, 197, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.E. Evolution of nitrogen oxide (s) during in vivo nitrate reductase assay of soybean leaves. Plant Physiol. 1981, 68, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, M.E.; Öhlén, E.; Lind, M.; Larsson, C.M. Nitrate regulation of nitrate uptake and nitrate reductase expression in barley grown at different nitrate: Ammonium ratios at constant relative nitrogen addition rate. Physiol. Plantarum. 1995, 94, 254–260. [Google Scholar] [CrossRef]
- Saravitz, C.H.; Devienne-Barret, F.; Raper, C.D., Jr.; Chaillou, S.; Lamaze, T. Nitrate uptake rate by soybean and wheat plants determined by external nitrate concentration and shoot-mediated demand. Int. J. Plant Sci. 1998, 159, 305–312. [Google Scholar] [CrossRef]
- Kim, G.W.; Lee, J.G.; Gutierrez-Suson, J.; Khan, M.I.; Jung, S.T.; Kim, P.J. Importance of annual monitoring for evaluating the direct nitrous oxide emission factor in temperate mono-rice paddy fields. Appl. Soil. Ecol. 2019, 140, 42–48. [Google Scholar] [CrossRef]
- Chaichana, N.; Bellingrath-Kimura, S.; Komiya, S.; Fujii, Y.; Noborio, K.; Dietrich, O.; Pakoktom, T. Comparison of Closed Chamber and Eddy Covariance Methods to Improve the Understanding of Methane Fluxes from Rice Paddy Fields in Japan. Atmosphere 2018, 9, 356. [Google Scholar] [CrossRef]
- Swain, C.K.; Nayak, A.K.; Bhattacharyya, P.; Chatterjee, D.; Chatterjee, S.; Tripathi, R.; Dhal, B. Greenhouse gas emissions and energy exchange in wet and dry season rice: Eddy covariance-based approach. Environ. Monit. Assess. 2018, 190, 423. [Google Scholar] [CrossRef]
- Machacova, K.; Vainio, E.; Urban, O.; Pihlatie, M. Seasonal dynamics of stem N2O exchange follow the physiological activity of boreal trees. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Decock, C.; Six, J. How reliable is the intramolecular distribution of 15N in N2O to source partition N2O emitted from soil? Soil Biol. Biochem. 2013, 65, 114–127. [Google Scholar] [CrossRef]
- Firestone, M.K.; Firestone, R.B.; Tiedje, J.M. Nitrous oxide from soil denitrification: Factors controlling its biological production. Science 1980, 208, 749–751. [Google Scholar] [CrossRef]
- Limami, A.M.; Diab, H.; Lothier, J. Nitrogen metabolism in plants under low oxygen stress. Planta 2014, 239, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Allègre, A.; Silvestre, J.; Morard, P.; Kallerhoff, J.; Pinelli, E. Nitrate reductase regulation in tomato roots by exogenous nitrate: A possible role in tolerance to long-term root anoxia. J. Exp. Bot. 2004, 55, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Shingaki-Wells, R.A.; Millar, A.H.; Whelan, J.; Narsai, R. What happens to plant mitochondria under low oxygen? An omics review of the responses to low oxygen and reoxygenation. Plant Cell Environ. 2014, 37, 2260–2277. [Google Scholar]
- Gupta, K.J.; Mur, L.A.; Wany, A.; Kumari, A.; Fernie, A.R.; Ratcliffe, R.G. The role of nitrite and nitric oxide under low oxygen conditions in plants. New Phytol. 2019, 225, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Lee, C.P.; Ratcliffe, R.G. Nitrite protects mitochondrial structure and function under hypoxia. Plant Cell Physiol. 2016, 58, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Igamberdiev, A.U. Reactive nitrogen species in mitochondria and their implications in plant energy status and hypoxic stress tolerance. Front. Plant Sci. 2016, 7, 369. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Ratcliffe, R.G.; Gupta, K.J. Plant mitochondria: Source and target for nitric oxide. Mitochondrion 2014, 19, 329–333. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timilsina, A.; Bizimana, F.; Pandey, B.; Yadav, R.K.P.; Dong, W.; Hu, C. Nitrous Oxide Emissions from Paddies: Understanding the Role of Rice Plants. Plants 2020, 9, 180. https://doi.org/10.3390/plants9020180
Timilsina A, Bizimana F, Pandey B, Yadav RKP, Dong W, Hu C. Nitrous Oxide Emissions from Paddies: Understanding the Role of Rice Plants. Plants. 2020; 9(2):180. https://doi.org/10.3390/plants9020180
Chicago/Turabian StyleTimilsina, Arbindra, Fiston Bizimana, Bikram Pandey, Ram Kailash Prasad Yadav, Wenxu Dong, and Chunsheng Hu. 2020. "Nitrous Oxide Emissions from Paddies: Understanding the Role of Rice Plants" Plants 9, no. 2: 180. https://doi.org/10.3390/plants9020180
APA StyleTimilsina, A., Bizimana, F., Pandey, B., Yadav, R. K. P., Dong, W., & Hu, C. (2020). Nitrous Oxide Emissions from Paddies: Understanding the Role of Rice Plants. Plants, 9(2), 180. https://doi.org/10.3390/plants9020180