Subcellular Targeting of Plant Sucrose Transporters Is Affected by Their Oligomeric State
Abstract
1. Introduction
2. Results
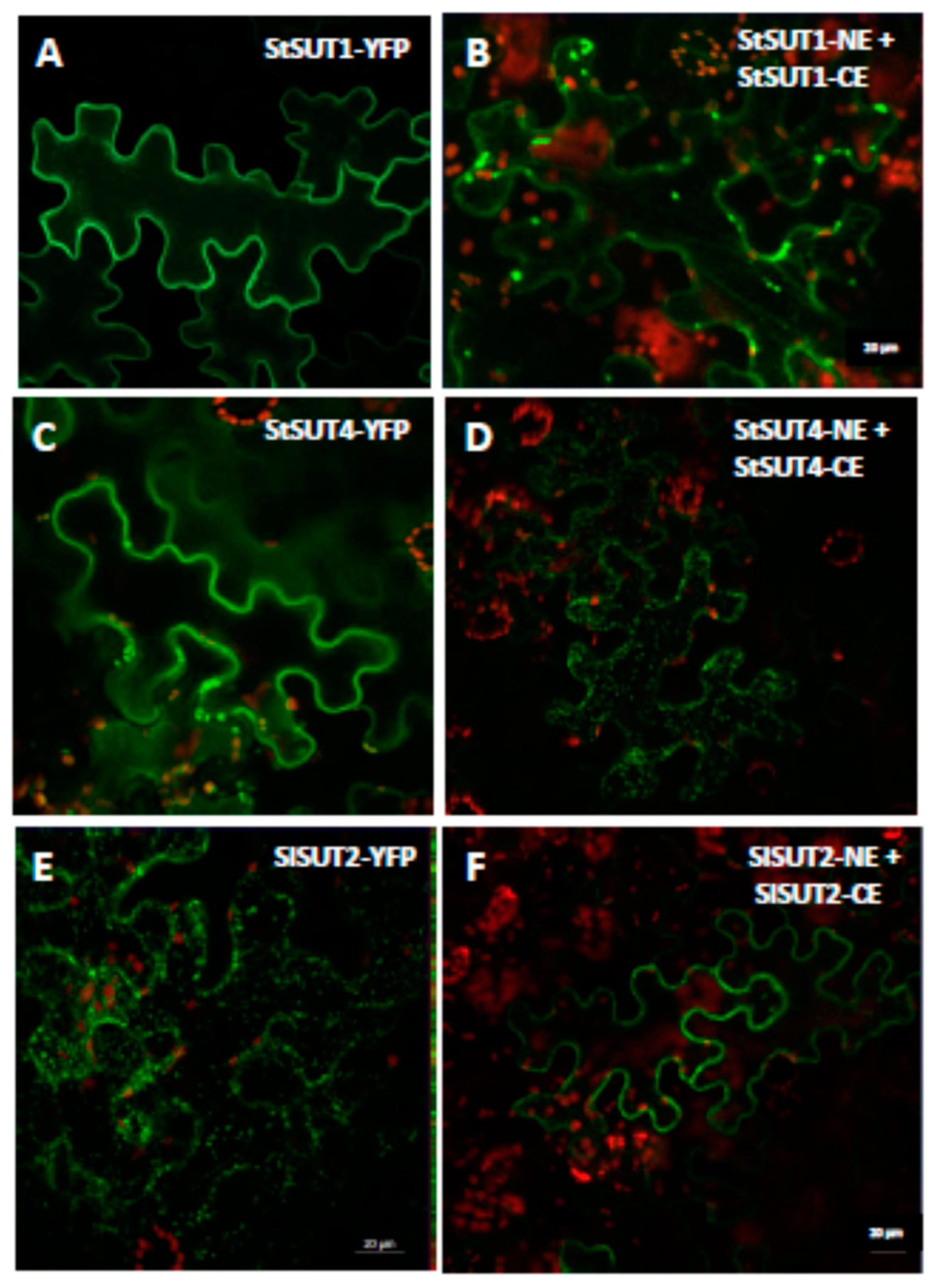
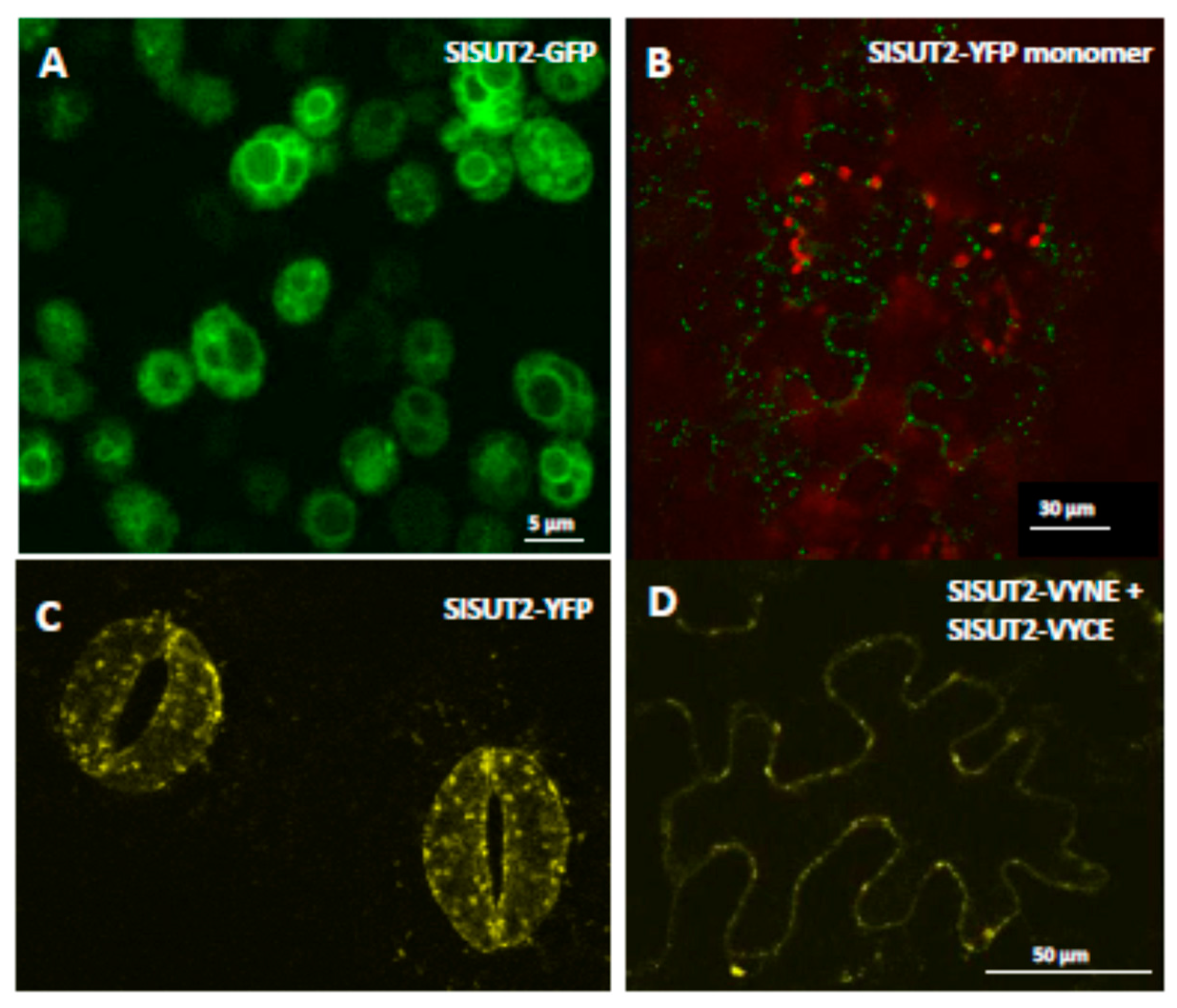
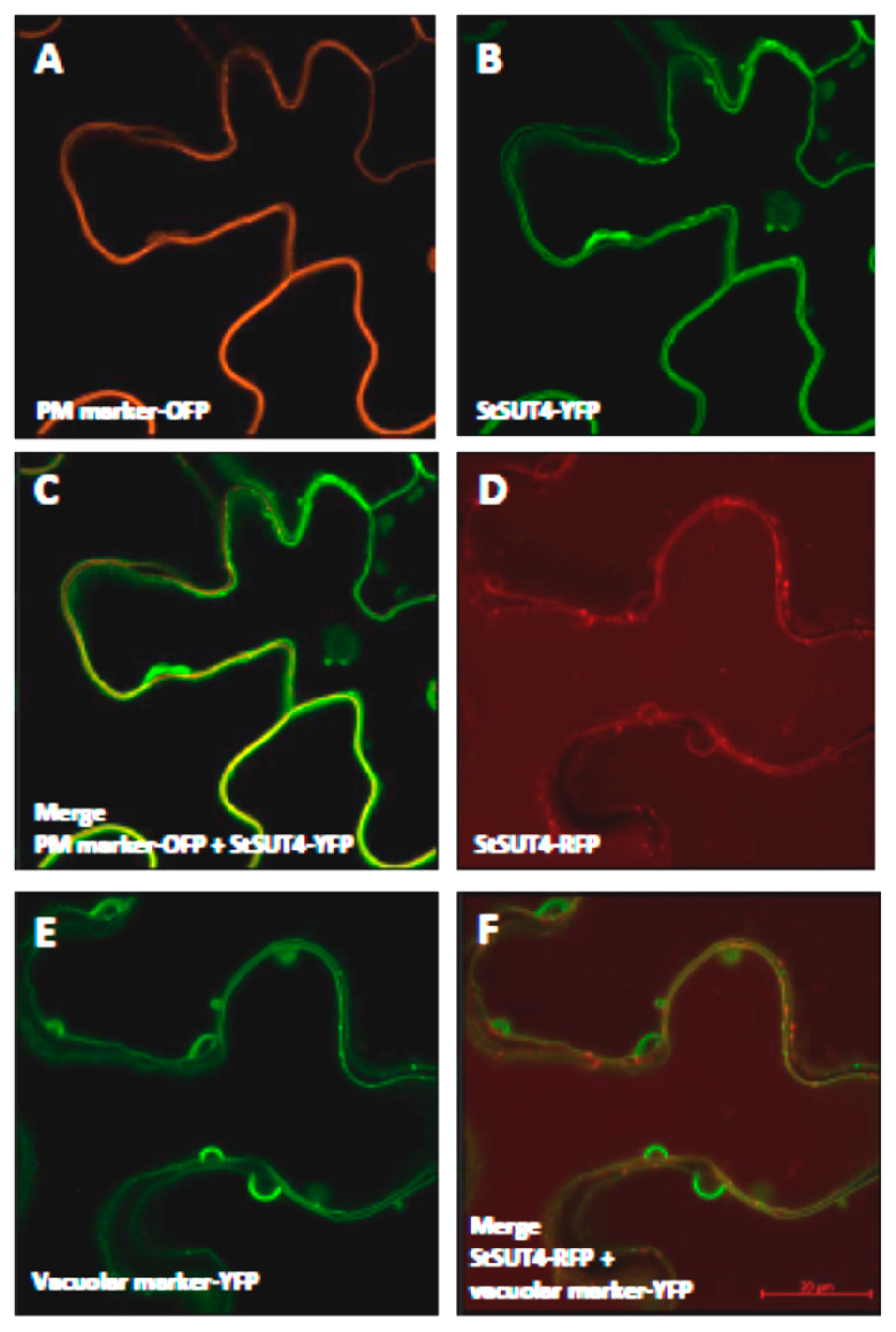
2.1. Homodimerization Affects Subcellular Distribution of Sucrose Transporters in An Opposite Way
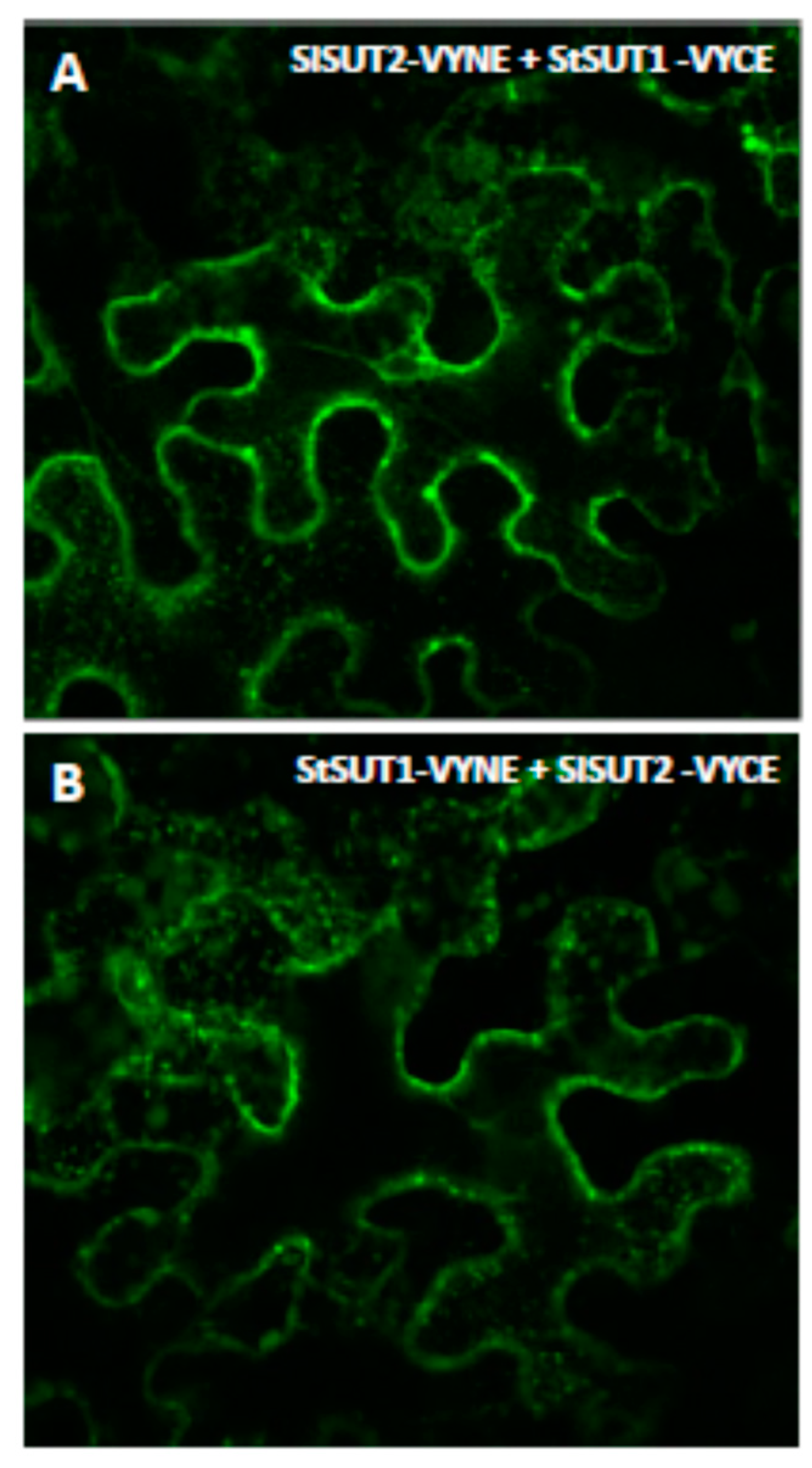
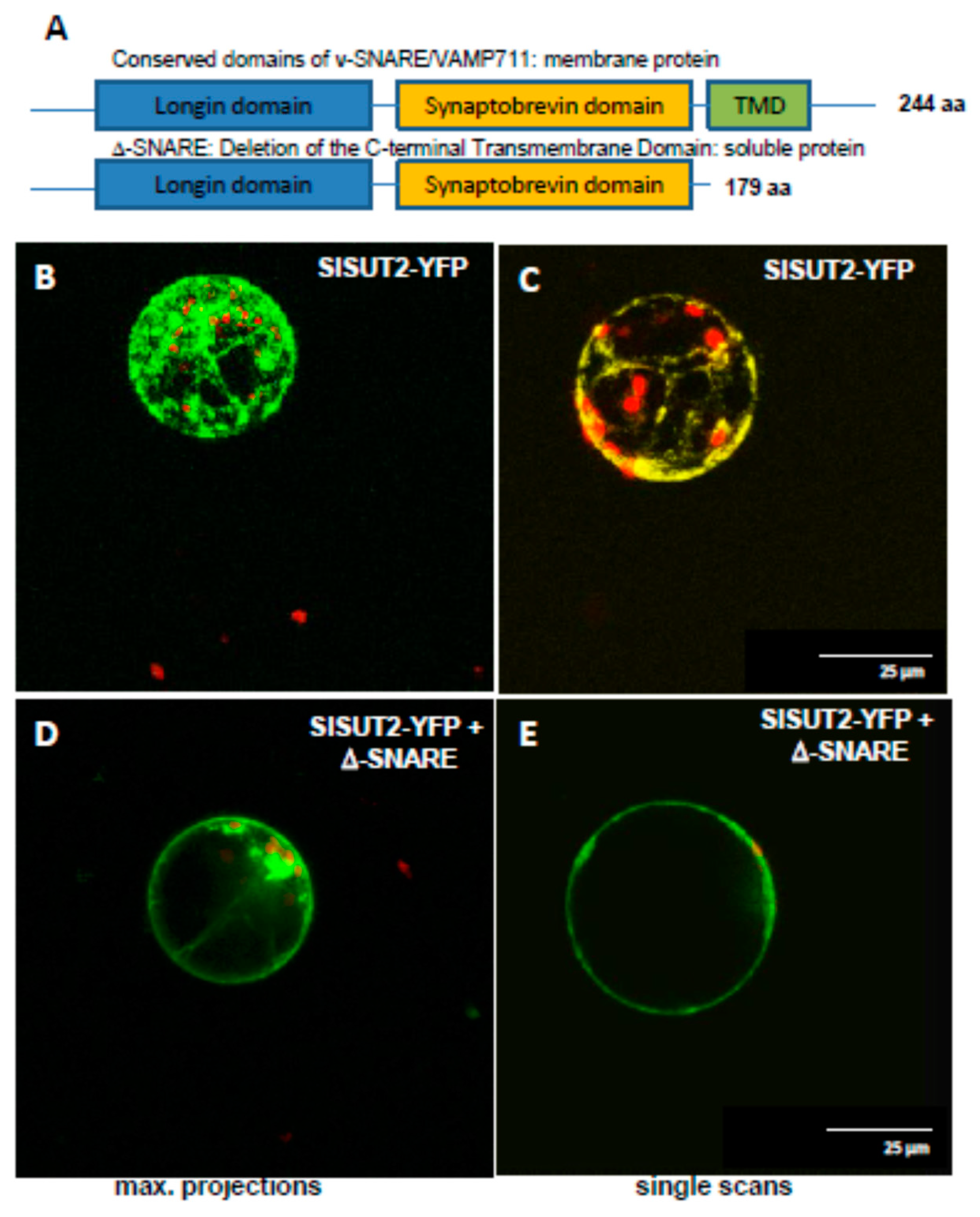
2.2. Heterodimers between SUT1 and SUT2 Are Internalized As Well
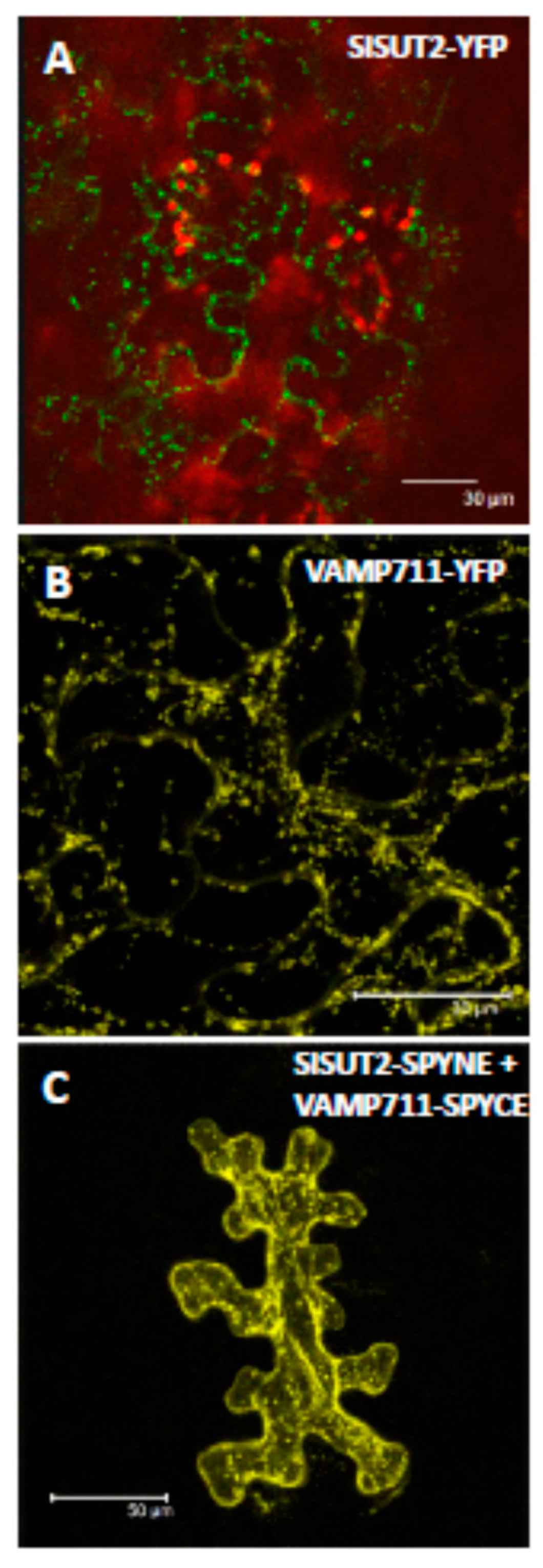
2.3. SlSUT2 Targeting to the Plasma Membrane Is Also Affected by Other Protein–Protein Interaction Partners
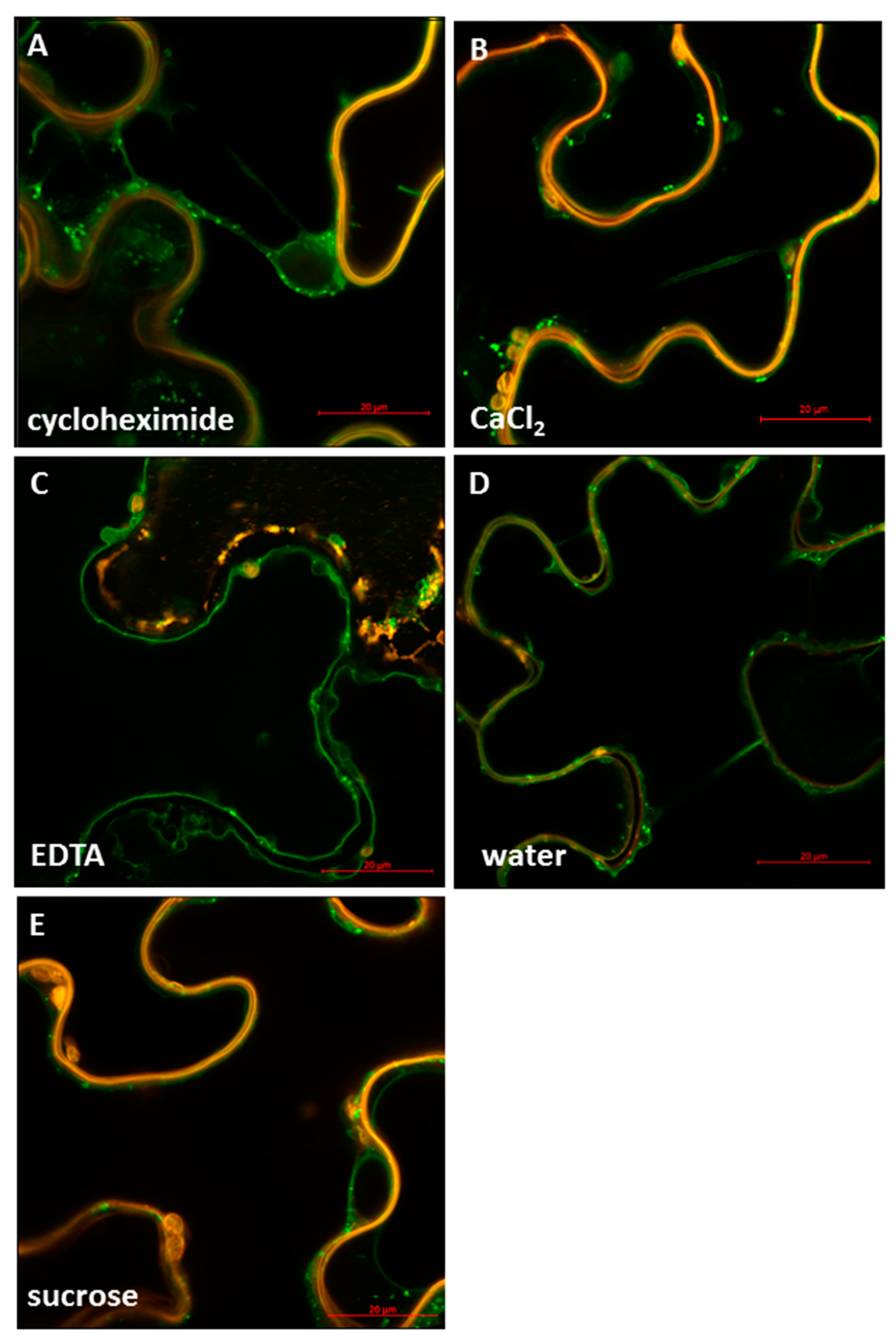
2.4. StSUT4 Targeting Is Affected by Divalent Cations Calcium
3. Discussion
3.1. Dynamic Localization of Sucrose Transporters
3.2. Putative Effect of Sucrose Transporter Phosphorylation
3.3. Effect of Divalent Cations on Sucrose Transporter Activity and Localization
4. Methods
4.1. Recombinant DNA
4.2. Transient Expression of Fluorescent Proteins
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kühn, C.; Quick, W.P.; Schulz, A.; Riesmeier, J.W.; Sonnewald, U.; Frommer, W.B. Companion cell-specific inhibition of the potato sucrose transporter SUT1. Plant Cell Environ. 1996, 19, 1115–1123. [Google Scholar] [CrossRef]
- Bitterlich, M.; Krügel, U.; Boldt-Burisch, K.; Franken, P.; Kühn, C. The sucrose transporter SlSUT2 from tomato interacts with brassinosteroid functioning and affects arbuscular mycorrhiza formation. Plant J. Cell Mol. Biol. 2014, 78, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Chincinska, I.A.; Liesche, J.; Krügel, U.; Michalska, J.; Geigenberger, P.; Grimm, B.; Kühn, C. Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol. 2008, 146, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Hansch, F.; Jaspar, H.; von Sivers, L.; Bitterlich, M.; Franken, P.; Kuhn, C. Brassinosteroids and sucrose transport in mycorrhizal tomato plants. Plant Signal. Behav. 2020, 1714292. [Google Scholar] [CrossRef]
- Ransom-Hodgkins, W.D.; Vaughn, M.W.; Bush, D.R. Protein phosphorylation plays a key role in sucrose-mediated transcriptional regulation of a phloem-specific proton-sucrose symporter. Planta 2003, 217, 483–489. [Google Scholar] [CrossRef]
- Vaughn, M.W.; Harrington, G.N.; Bush, D.R. Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc. Natl. Acad. Sci. USA 2002, 99, 10876–10880. [Google Scholar] [CrossRef]
- Krügel, U.; Kühn, C. Post-translational regulation of sucrose transporters by direct protein-protein interactions. Front. Plant Sci. 2013, 4, 237. [Google Scholar] [CrossRef]
- Krügel, U.; He, H.X.; Gier, K.; Reins, J.; Chincinska, I.; Grimm, B.; Schulze, W.X.; Kühn, C. The potato sucrose transporter StSUT1 interacts with a DRM-associated protein disulfide isomerase. Mol. Plant 2012, 5, 43–62. [Google Scholar] [CrossRef]
- Liesche, J.; He, H.X.; Grimm, B.; Schulz, A.; Kühn, C. Recycling of Solanum sucrose transporters expressed in yeast, tobacco, and in mature phloem sieve elements. Mol. Plant 2010, 3, 1064–1074. [Google Scholar] [CrossRef]
- Eggert, E.; Obata, T.; Gerstenberger, A.; Gier, K.; Brandt, T.; Fernie, A.R.; Schulze, W.; Kühn, C. A sucrose transporter-interacting protein disulphide isomerase affects redox homeostasis and links sucrose partitioning with abiotic stress tolerance. Plant Cell Environ. 2016, 39, 1366–1380. [Google Scholar] [CrossRef]
- Krügel, U.; Veenhoff, L.M.; Langbein, J.; Wiederhold, E.; Liesche, J.; Friedrich, T.; Grimm, B.; Martinoia, E.; Poolman, B.; Kühn, C. Transport and sorting of the Solanum tuberosum sucrose transporter SUT1 is affected by posttranslational modification. Plant Cell 2008, 20, 2497–2513. [Google Scholar] [CrossRef]
- Krügel, U.; Wiederhold, E.; Pustogowa, J.; Hackel, A.; Grimm, B.; Kühn, C. Site directed mutagenesis of StSUT1 reveals target amino acids of regulation and stability. Biochimie 2013, 95, 2132–2144. [Google Scholar] [CrossRef]
- Gehl, C.; Waadt, R.; Kudla, J.; Mendel, R.R.; Hansch, R. New GATEWAY vectors for high throughput analyses of protein-protein interactions by bimolecular fluorescence complementation. Mol. Plant 2009, 2, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.; Kühn, C.; Weise, A.; Schulz, A.; Gebhardt, C.; Hirner, B.; Hellmann, H.; Schulze, W.; Ward, J.M.; Frommer, W.B. SUT2, a putative sucrose sensor in sieve elements. Plant Cell 2000, 12, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Schulze, W.; Weise, A.; Frommer, W.B.; Ward, J.M. Function of the cytosolic N-terminus of sucrose transporter AtSUT2 in substrate affinity. Febs Lett. 2000, 485, 189–194. [Google Scholar] [CrossRef]
- Reinders, A.; Schulze, W.; Kühn, C.; Barker, L.; Schulz, A.; Ward, J.M.; Frommer, W.B. Protein-protein interactions between sucrose transporters of different affinities colocalized in the same enucleate sieve element. Plant Cell 2002, 14, 1567–1577. [Google Scholar] [CrossRef]
- Besserer, A.; Burnotte, E.; Bienert, G.P.; Chevalier, A.S.; Errachid, A.; Grefen, C.; Blatt, M.R.; Chaumont, F. Selective regulation of maize plasma membrane aquaporin trafficking and activity by the SNARE SYP121. Plant Cell 2012, 24, 3463–3481. [Google Scholar] [CrossRef]
- Tyrrell, M.; Campanoni, P.; Sutter, J.U.; Pratelli, R.; Paneque, M.; Sokolovski, S.; Blatt, M.R. Selective targeting of plasma membrane and tonoplast traffic by inhibitory (dominant-negative) SNARE fragments. Plant J. Cell Mol. Biol. 2007, 51, 1099–1115. [Google Scholar] [CrossRef]
- Sutter, J.U.; Campanoni, P.; Tyrrell, M.; Blatt, M.R. Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 2006, 18, 935–954. [Google Scholar] [CrossRef]
- Bozkurt, T.O.; Belhaj, K.; Dagdas, Y.F.; Chaparro-Garcia, A.; Wu, C.H.; Cano, L.M.; Kamoun, S. Rerouting of plant late endocytic trafficking toward a pathogen interface. Traffic 2015, 16, 204–226. [Google Scholar] [CrossRef]
- Chincinska, I.; Gier, K.; Krügel, U.; Liesche, J.; He, H.; Grimm, B.; Harren, F.J.; Cristescu, S.M.; Kühn, C. Photoperiodic regulation of the sucrose transporter StSUT4 affects the expression of circadian-regulated genes and ethylene production. Front. Plant Sci. 2013, 4, 26. [Google Scholar] [CrossRef]
- Jung, B.; Ludewig, F.; Schulz, A.; Meissner, G.; Wostefeld, N.; Flugge, U.I.; Pommerrenig, B.; Wirsching, P.; Sauer, N.; Koch, W.; et al. Identification of the transporter responsible for sucrose accumulation in sugar beet taproots. Nat. Plants 2015, 1, 14001. [Google Scholar] [CrossRef] [PubMed]
- Payyavula, R.S.; Tay, K.H.; Tsai, C.J.; Harding, S.A. The sucrose transporter family in Populus: The importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J. Cell Mol. Biol. 2011, 65, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Nyamdari, B.; Tsai, C.J.; Harding, S.A. The tonoplast-localized sucrose transporter in Populus (PtaSUT4) regulates whole-plant water relations, responses to water stress, and photosynthesis. PLoS ONE 2012, 7, e44467. [Google Scholar] [CrossRef] [PubMed]
- Flemetakis, E.; Dimou, M.; Cotzur, D.; Efrose, R.C.; Aivalakis, G.; Colebatch, G.; Udvardi, M.; Katinakis, P. A sucrose transporter, LjSUT4, is up-regulated during Lotus japonicus nodule development. J. Exp. Bot. 2003, 54, 1789–1791. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.; Hsiao, H.H.; Chen, H.J.; Chang, C.W.; Wang, S.J. Influence of temperature on the expression of the rice sucrose transporter 4 gene, OsSUT4, in germinating embryos and maturing pollen. Acta Physiol. Plant. 2014, 36, 217–229. [Google Scholar] [CrossRef]
- Zheng, W.; Jiang, Y.; Wang, X.; Huang, S.; Yuan, M.; Guo, Y. AP3M harbors actin filament binding activity that is crucial for vacuole morphology and stomatal closure in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 18132–18141. [Google Scholar] [CrossRef]
- Schulze, W.X.; Reinders, A.; Ward, J.; Lalonde, S.; Frommer, W.B. Interactions between co-expressed Arabidopsis sucrose transporters in the split-ubiquitin system. BMC Biochem. 2003, 4, 3. [Google Scholar] [CrossRef]
- Römer, U.; Schrader, H.; Günther, N.; Nettelsroth, N.; Frommer, W.B.; Elling, L. Expression, purification and characterization of recombinant sucrose synthase1 from Solanum tuberosum L. for carbohydrate engineering. J. Biotechnol. 2004, 107, 135–149. [Google Scholar]
- Elling, L. Effect of metal ions on sucrose synthase from rice grains: A study on enzyme inhibition and enzyme topography. Glycobiology 1995, 5, 201–206. [Google Scholar] [CrossRef]
- Huber, S.C.; Huber, J.L.; Liao, P.C.; Gage, D.A.; McMichael, R.W., Jr.; Chourey, P.S.; Hannah, L.C.; Koch, K. Phosphorylation of serine-15 of maize leaf sucrose synthase. Occurrence in vivo and possible regulatory significance. Plant Physiol. 1996, 112, 793–802. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hardin, S.C.; Winter, H.; Huber, S.C. Phosphorylation of the amino terminus of maize sucrose synthase in relation to membrane association and enzyme activity. Plant Physiol. 2004, 134, 1427–1438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liesche, J.; Schulz, A.; Krügel, U.; Grimm, B.; Kühn, C. Dimerization and endocytosis of the sucrose transporter StSUT1 in mature sieve elements. Plant Signal. Behav. 2008, 3, 1136–1137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pumplin, N.; Voinnet, O. RNA silencing suppression by plant pathogens: Defence, counter-defence and counter-counter-defence. Nat. Rev. Microbiol. 2013, 11, 745–760. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, V.; Hackel, A.; Kühn, C. Subcellular Targeting of Plant Sucrose Transporters Is Affected by Their Oligomeric State. Plants 2020, 9, 158. https://doi.org/10.3390/plants9020158
Garg V, Hackel A, Kühn C. Subcellular Targeting of Plant Sucrose Transporters Is Affected by Their Oligomeric State. Plants. 2020; 9(2):158. https://doi.org/10.3390/plants9020158
Chicago/Turabian StyleGarg, Varsha, Aleksandra Hackel, and Christina Kühn. 2020. "Subcellular Targeting of Plant Sucrose Transporters Is Affected by Their Oligomeric State" Plants 9, no. 2: 158. https://doi.org/10.3390/plants9020158
APA StyleGarg, V., Hackel, A., & Kühn, C. (2020). Subcellular Targeting of Plant Sucrose Transporters Is Affected by Their Oligomeric State. Plants, 9(2), 158. https://doi.org/10.3390/plants9020158




