The Effect of Different Extraction Protocols on Brassica oleracea var. acephala Antioxidant Activity, Bioactive Compounds, and Sugar Profile
Abstract
1. Introduction
2. Results
2.1. The Effect of Different Extraction Protocols on Antioxidant Activity, Bioactive Compounds, and Sugar Content in Brassica
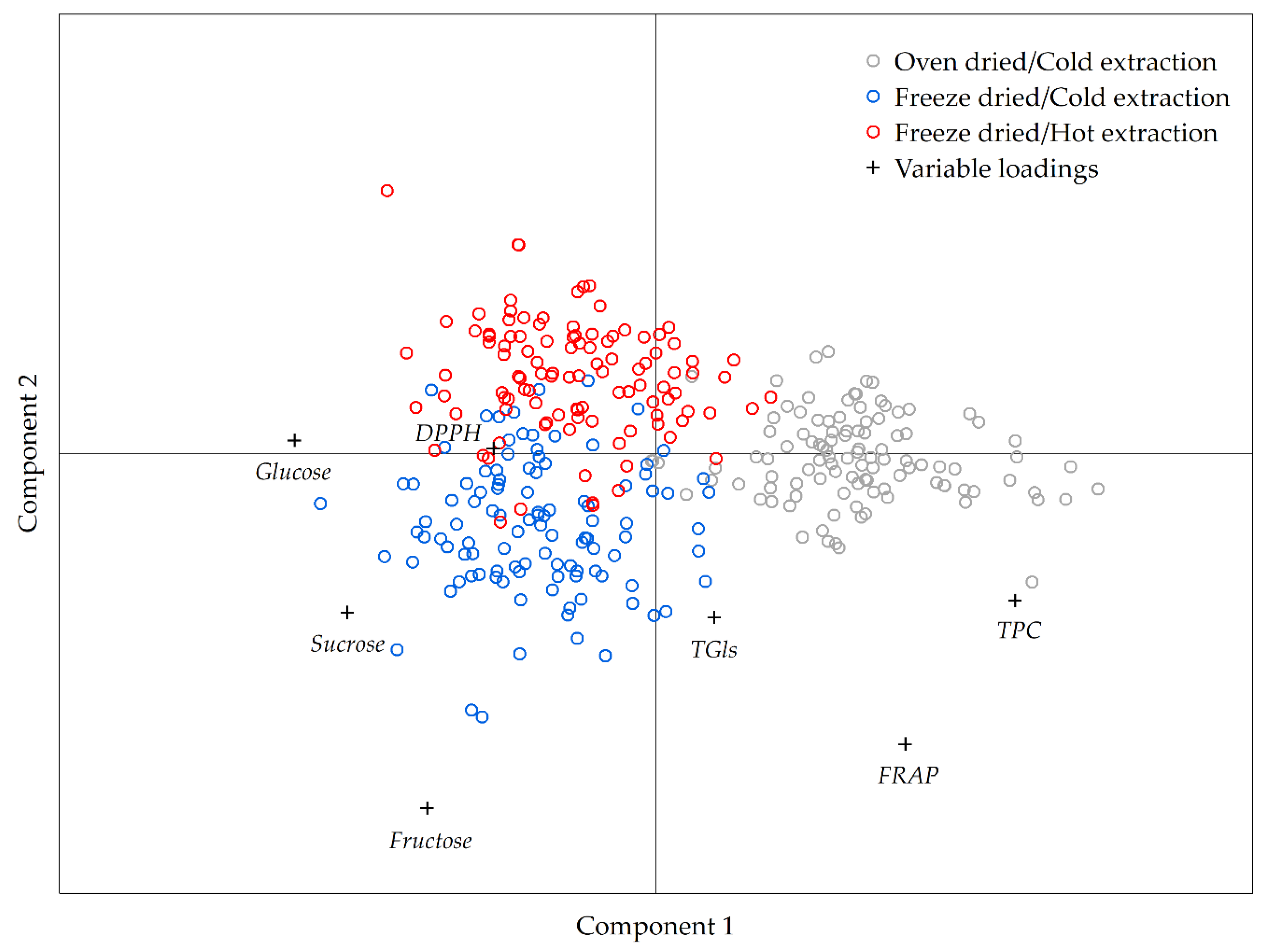
2.2. Multivariate Analysis of the Data Obtained by Different Extraction Protocols
2.3. Correlations between Bioactive Compounds, Antioxidant Activity, and Sugar Content in Brassica Extracts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Hot Methanol Extraction
4.3. Cold Methanol Extraction
4.4. Determination of Total Antioxidant Activity
4.5. Determination of Total Glucosinolates and Total Phenolic Content
4.6. Sugar Analysis by HPLC
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manchali, S.; Chidambara Murthy, K.N.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, A.K.; Prasad, K.; Bahadur, A.; Rai, M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J. Food Compos. Anal. 2007, 20, 106–112. [Google Scholar] [CrossRef]
- Talalay, P.; Fahey, J.W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001, 131, 3027S–3033S. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Hayirlioglu-Ayaz, S.; Alpay-Karaoglu, S.; Grúz, J.; Valentová, K.; Ulrichová, J.; Strnad, M. Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chem. 2008, 107, 19–25. [Google Scholar] [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef]
- Lešić, R.; Borošić, J.; Buturac, I.; Herak Ćustić, M.; Poljak, M.; Romić, D. Povrćarstvo (Vegetable Crops), 3rd ed.; Zrnski d.d.: Čakovec, Croatia, 2016. [Google Scholar]
- Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Chromatographic column evaluation for the untargeted profiling of glucosinolates in cauliflower by means of ultra-high performance liquid chromatography coupled to high resolution mass spectrometry. Talanta 2018, 179, 792–802. [Google Scholar] [CrossRef]
- Novío, S.; Cartea, M.E.; Soengas, P.; Freire-Garabal, M.; Núñez-Iglesias, M.J. Effects of Brassicaceae isothiocyanates on prostate cancer. Molecules 2016, 21, 626. [Google Scholar] [CrossRef]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Herr, I.; Büchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef]
- Mellon, F.A.; Bennett, R.N.; Holst, B.; Williamson, G. Intact glucosinolate analysis in plant extracts by programmed cone voltage electrospray LC/MS: Performance and comparison with LC/MS/MS methods. Anal. Biochem. 2002, 306, 83–91. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Redeker, K.; Kittipol, V.; Bancroft, I.; Hartley, S.E. Development of an efficient glucosinolate extraction method. Plant Methods 2017, 13, 17. [Google Scholar] [CrossRef]
- Ares, A.M.; Nozal, M.J.; Bernal, J.L.; Bernal, J. Effect of Temperature and Light Exposure on the Detection of Total Intact Glucosinolate Content by LC-ESI-MS in Broccoli Leaves. Food Anal. Methods 2014, 7, 1687–1692. [Google Scholar] [CrossRef]
- Grosser, K.; van Dam, N.M. A straightforward method for glucosinolate extraction and analysis with high-pressure liquid chromatography (HPLC). J. Vis. Exp. 2017, 2017, 55425. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Patel, S.M.; Doen, T.; Pikal, M.J. Determination of end point of primary drying in freeze-drying process control. AAPS PharmSciTech 2010, 11, 73–84. [Google Scholar] [CrossRef]
- Song, L.; Thornalley, P.J. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem. Toxicol. 2007, 45, 216–224. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Gratacós-Cubarsí, M.; Ribas-Agustí, A.; García-Regueiro, J.A.; Castellari, M. Simultaneous evaluation of intact glucosinolates and phenolic compounds by UPLC-DAD-MS/MS in Brassica oleracea L. var. botrytis. Food Chem. 2010, 121, 257–263. [Google Scholar] [CrossRef]
- Šamec, D.; Pavlović, I.; Radojčić Redovniković, I.; Salopek-Sondi, B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chem. 2018, 269, 96–102. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Rhee, J.; Choi, C.S.; Jo, J.S.; Shin, Y.K.; Lee, J.G. Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm. Molecules 2020, 25, 1860. [Google Scholar] [CrossRef]
- Tephly, T.R. The toxicity of methanol. Life Sci. 1991, 48, 1031–1041. [Google Scholar] [CrossRef]
- Ashurst, J.V.; Nappe, T.M. Methanol Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kavet, R.; Nauss, K.M. The toxicity of inhaled methanol vapors. Crit. Rev. Toxicol. 1990, 21, 21–50. [Google Scholar] [CrossRef]
- ISO-ISO 9167:2019-Rapeseed and Rapeseed Meals—Determination of Glucosinolates Content—Method Using High-Performance Liquid Chromatography. Available online: https://www.iso.org/standard/72207.html (accessed on 17 November 2020).
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Technol. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef]
- Mao, L.C.; Pan, X.; Que, F.; Fang, X.H. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur. Food Res. Technol. 2006, 222, 236–241. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragón, H.; Ortiz-Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- Michalak, M.; Szwajgier, D.; Paduch, R.; Kukula-Koch, W.; Waśko, A.; Polak-Berecka, M. Fermented curly kale as a new source of gentisic and salicylic acids with antitumor potential. J. Funct. Foods 2020, 67, 103866. [Google Scholar] [CrossRef]
- Olsen, H.; Grimmer, S.; Aaby, K.; Saha, S.; Borge, G.I.A. Antiproliferative effects of fresh and thermal processed green and red cultivars of curly kale (Brassica oleracea L. convar. acephala var. sabellica). J. Agric. Food Chem. 2012, 60, 7375–7383. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoon, S.; Kwon, S.M.; Park, K.S.; Lee-Kim, Y.C. Kale Juice improves coronary artery disease risk factors in hypercholesterolemic men. Biomed. Environ. Sci. 2008, 21, 91–97. [Google Scholar] [CrossRef]
- Sikora, E.; Cieślik, E.; Leszczyńska, T.; Filipiak-Florkiewicz, A.; Pisulewski, P.M. The antioxidant activity of selected cruciferous vegetables subjected to aquathermal processing. Food Chem. 2008, 107, 55–59. [Google Scholar] [CrossRef]
- Sousa, C.; Taveira, M.; Valentão, P.; Fernandes, F.; Pereira, J.A.; Estevinho, L.; Bento, A.; Ferreres, F.; Seabra, R.M.; Andrade, P.B. Inflorescences of Brassicacea species as source of bioactive compounds: A comparative study. Food Chem. 2008, 110, 953–961. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Camacho, M.d.M.; Martínez-Navarrete, N. The Impact of Freeze-Drying Conditions on the Physico-Chemical Properties and Bioactive Compounds of a Freeze-Dried Orange Puree. Foods 2019, 9, 32. [Google Scholar] [CrossRef]
- Krokida, M.K.; Karathanos, V.T.; Maroulis, Z.B. Effect of freeze-drying conditions on shrinkage and porosity of dehydrated agricultural products. J. Food Eng. 1998, 35, 369–380. [Google Scholar] [CrossRef]
- Rutnakornpituk, B.; Boonthip, C.; Sanguankul, W.; Sawangsup, P.; Rutnakornpituk, M. Study in Total Phenolic Contents, Antioxidant Activity and Analysis of Glucosinolate Compounds in Cruciferous Vegetables. Naresuan Univ. J. Sci. Technol. 2018, 26, 27–37. [Google Scholar]
- Tetteh, O.N.A.; Ulrichs, C.; Huyskens-Keil, S.; Mewis, I.; Amaglo, N.K.; Oduro, I.N.; Adarkwah, C.; Obeng-Ofori, D.; Förster, N. Effects of harvest techniques and drying methods on the stability of glucosinolates in Moringa oleifera leaves during post-harvest. Sci. Hortic. 2019, 246, 998–1004. [Google Scholar] [CrossRef]
- Managa, M.G.; Sultanbawa, Y.; Sivakumar, D. Effects of Different Drying Methods on Untargeted Phenolic Metabolites, and Antioxidant Activity in Chinese Cabbage (Brassica rapa L. subsp. chinensis) and Nightshade (Solanum retroflexum Dun.). Molecules 2020, 25, 1326. [Google Scholar] [CrossRef]
- Korus, A. Effect of preliminary processing, method of drying and storage temperature on the level of antioxidants in kale (Brassica oleracea L. var. acephala) leaves. LWT-Food Sci. Technol. 2011, 44, 1711–1716. [Google Scholar] [CrossRef]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Effect of freeze drying and oven drying on antioxidant properties of fresh wheatgrass. Int. J. Food Sci. Nutr. 2012, 63, 718–721. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of freezing and dehydration of olive leaves (var. Serrana) on extract composition and antioxidant potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Clarke, G.; Ting, K.; Wiart, C.; Fry, J. High Correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest. Antioxidants 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Yusoff, N.A.M.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Supriatno; Ooi, K.L. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). J. Food Compos. Anal. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Michalska, A.; Honke, J.; Łysiak, G.; Andlauer, W. Effect of drying parameters on the formation of early and intermediate stage products of the Maillard reaction in different plum (Prunus domestica L.) cultivars. LWT-Food Sci. Technol. 2016, 65, 932–938. [Google Scholar] [CrossRef]
- Fante, L.; Noreña, C.P.Z. Quality of hot air dried and freeze-dried of garlic (Allium sativum L.). J. Food Sci. Technol. 2015, 52, 211–220. [Google Scholar] [CrossRef]
- Iombor, T.T.; Olaitan, I.N.; Ede, R.A. Proximate composition, antinutrient content and functional properties of soursop flour as influenced by oven and freeze drying methods. Curr. Res. Nutr. Food Sci. 2014, 2, 106–110. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Zhang, Y. Physicochemical, thermal, and pasting properties of Chinese chestnut (Castanea mollissima Bl.) starches as affected by different drying methods. Starch-Stärke 2011, 63, 260–267. [Google Scholar] [CrossRef]
- Karaman, S.; Toker, O.S.; Çam, M.; Hayta, M.; Doğan, M.; Kayacier, A. Bioactive and Physicochemical Properties of Persimmon as Affected by Drying Methods. Dry. Technol. 2014, 32, 258–267. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, C.S.; Wang, M.; Xu, B.N.; Du, L.J. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J. Agric. Food Chem. 2012, 60, 9642–9648. [Google Scholar] [CrossRef]
- Hu, Q.-g.; Zhang, M.; Mujumdar, A.S.; Du, W.-h.; Sun, J.-c. Effects of Different Drying Methods on the Quality Changes of Granular Edamame. Dry. Technol. 2006, 24, 1025–1032. [Google Scholar] [CrossRef]
- Woo, K.S.; Kim, H.Y.; Hwang, I.G.; Lee, S.H.; Jeong, H.S. Characteristics of the thermal degradation of glucose and maltose solutions. Prev. Nutr. Food Sci. 2015, 20, 102–109. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Lai, P.; Tang, B.; Wu, L. Influence of drying methods on the physicochemical properties and nutritional composition of instant Tremella fuciformis. Food Sci. Technol. 2020, 40, 741–748. [Google Scholar] [CrossRef]
- Johansen, H.N.; Glitsø, V.; Bach Knudsen, K.E. Influence of extraction solvent and temperature on the quantitative determination of oligosaccharides from plant materials by high-performance liquid chromatography. J. Agric. Food Chem. 1996, 44, 1470–1474. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ishida, M.; Nagata, M.; Ohara, T.; Kakizaki, T.; Hatakeyama, K.; Nishio, T. Small variation of glucosinolate composition in Japanese cultivars of radish (Raphanus sativus L.) requires simple quantitative analysis for breeding of glucosinolate component. Breed. Sci. 2012, 62, 63–70. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]



| TGls | TPC | DPPH | FRAP | Sucrose | Glucose | Fructose | |
|---|---|---|---|---|---|---|---|
| TGls | 1.00 | ||||||
| TPC | 0.14 1 | 1.00 | |||||
| DPPH | 0.06 | −0.09 | 1.00 | ||||
| FRAP | 0.31 | 0.78 | 0.09 | 1.00 | |||
| Sucrose | −0.08 | −0.44 | 0.27 | −0.23 | 1.00 | ||
| Glucose | −0.13 | −0.65 | 0.26 | −0.41 | 0.47 | 1.00 | |
| Fructose | −0.01 | −0.23 | 0.12 | 0.09 | 0.55 | 0.43 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major, N.; Prekalj, B.; Perković, J.; Ban, D.; Užila, Z.; Ban, S.G. The Effect of Different Extraction Protocols on Brassica oleracea var. acephala Antioxidant Activity, Bioactive Compounds, and Sugar Profile. Plants 2020, 9, 1792. https://doi.org/10.3390/plants9121792
Major N, Prekalj B, Perković J, Ban D, Užila Z, Ban SG. The Effect of Different Extraction Protocols on Brassica oleracea var. acephala Antioxidant Activity, Bioactive Compounds, and Sugar Profile. Plants. 2020; 9(12):1792. https://doi.org/10.3390/plants9121792
Chicago/Turabian StyleMajor, Nikola, Bernard Prekalj, Josipa Perković, Dean Ban, Zoran Užila, and Smiljana Goreta Ban. 2020. "The Effect of Different Extraction Protocols on Brassica oleracea var. acephala Antioxidant Activity, Bioactive Compounds, and Sugar Profile" Plants 9, no. 12: 1792. https://doi.org/10.3390/plants9121792
APA StyleMajor, N., Prekalj, B., Perković, J., Ban, D., Užila, Z., & Ban, S. G. (2020). The Effect of Different Extraction Protocols on Brassica oleracea var. acephala Antioxidant Activity, Bioactive Compounds, and Sugar Profile. Plants, 9(12), 1792. https://doi.org/10.3390/plants9121792






