Functionality of Embryo Sacs in Pear Cultivars ‘Ingeborg’ and ‘Celina’ as Related to Fruit Set under Nordic Climate
Abstract
:1. Introduction
2. Results
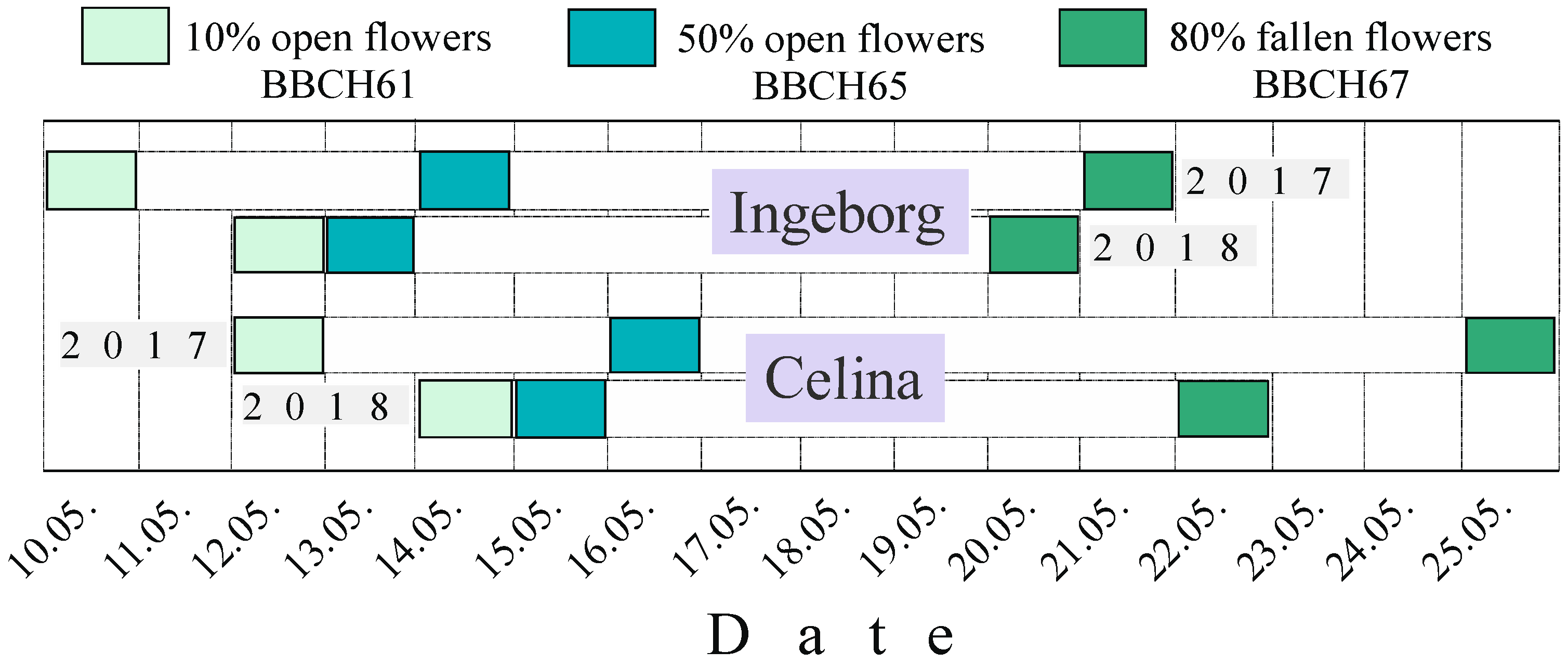
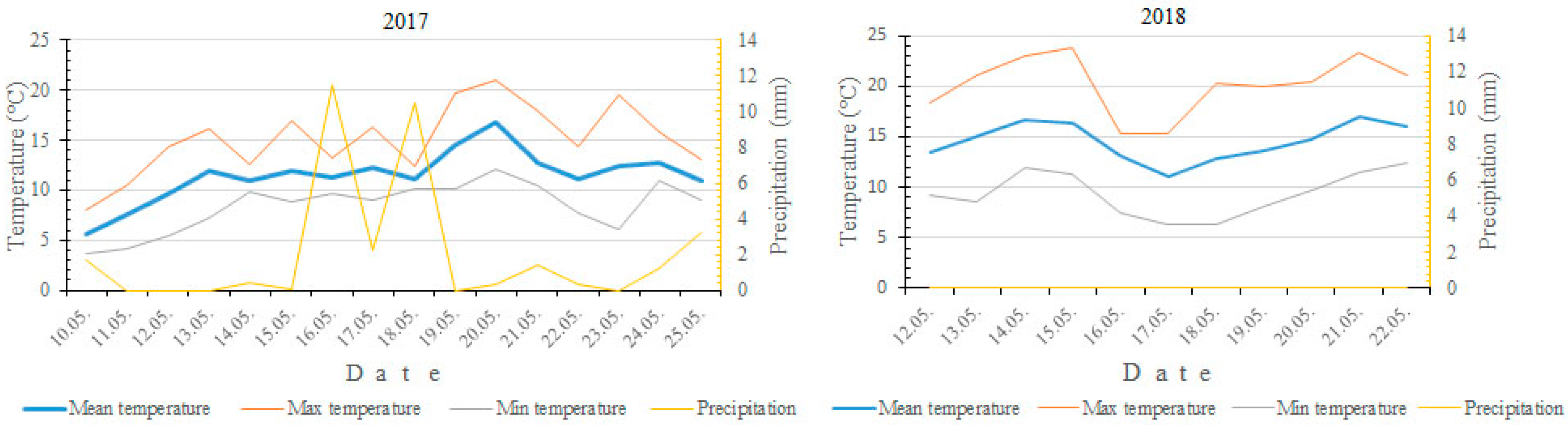
2.1. Flowering Time and Climate Conditions
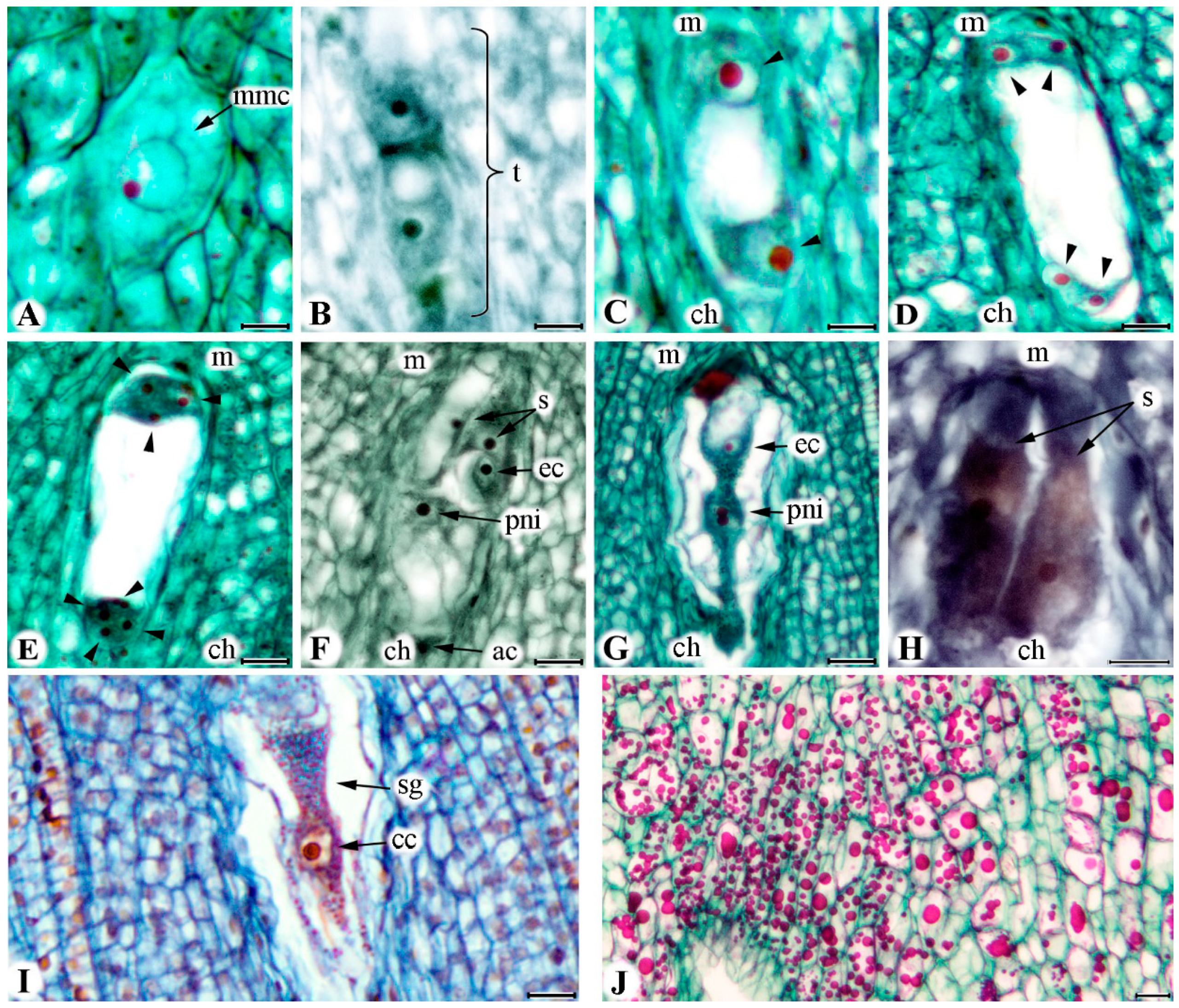
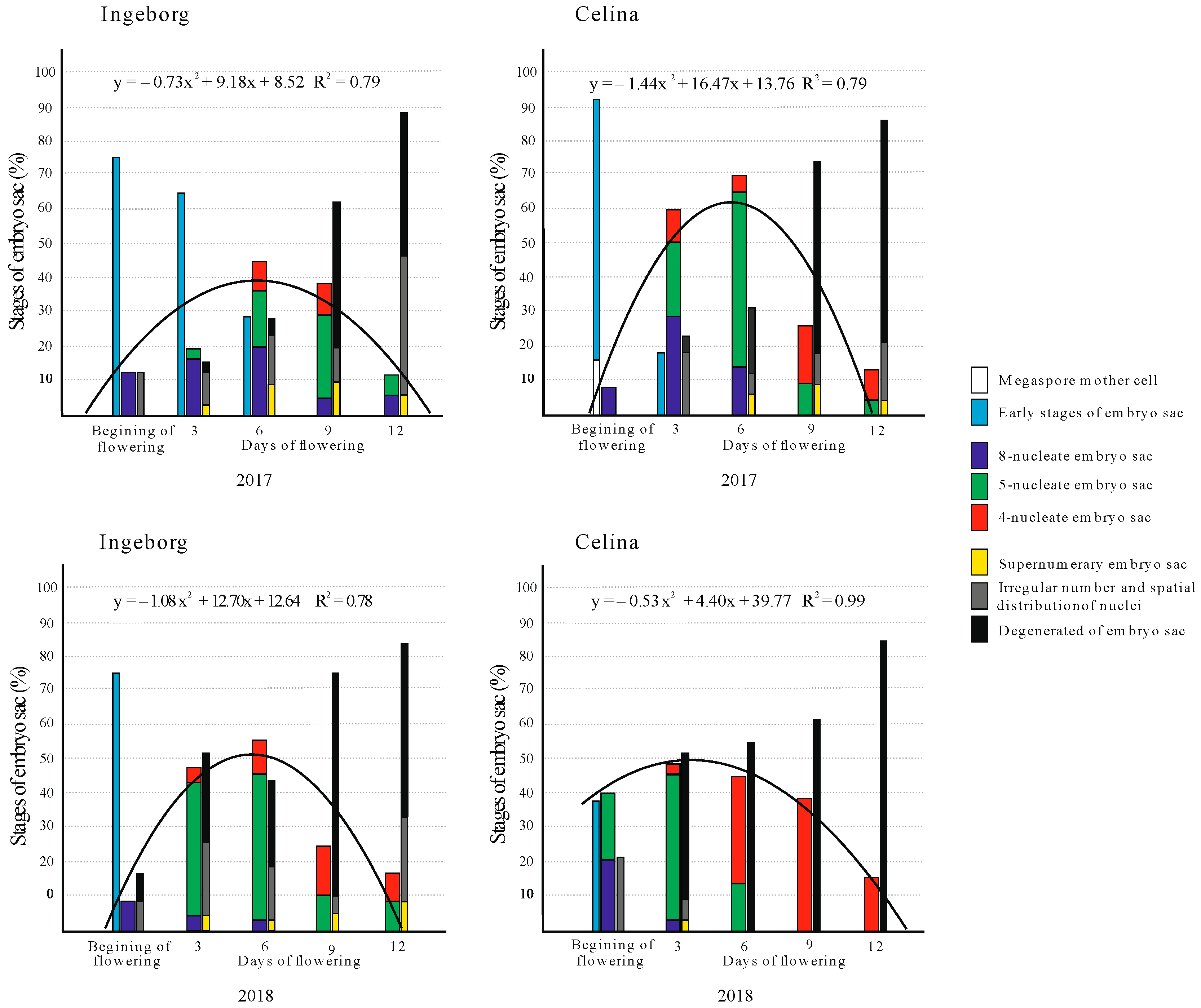
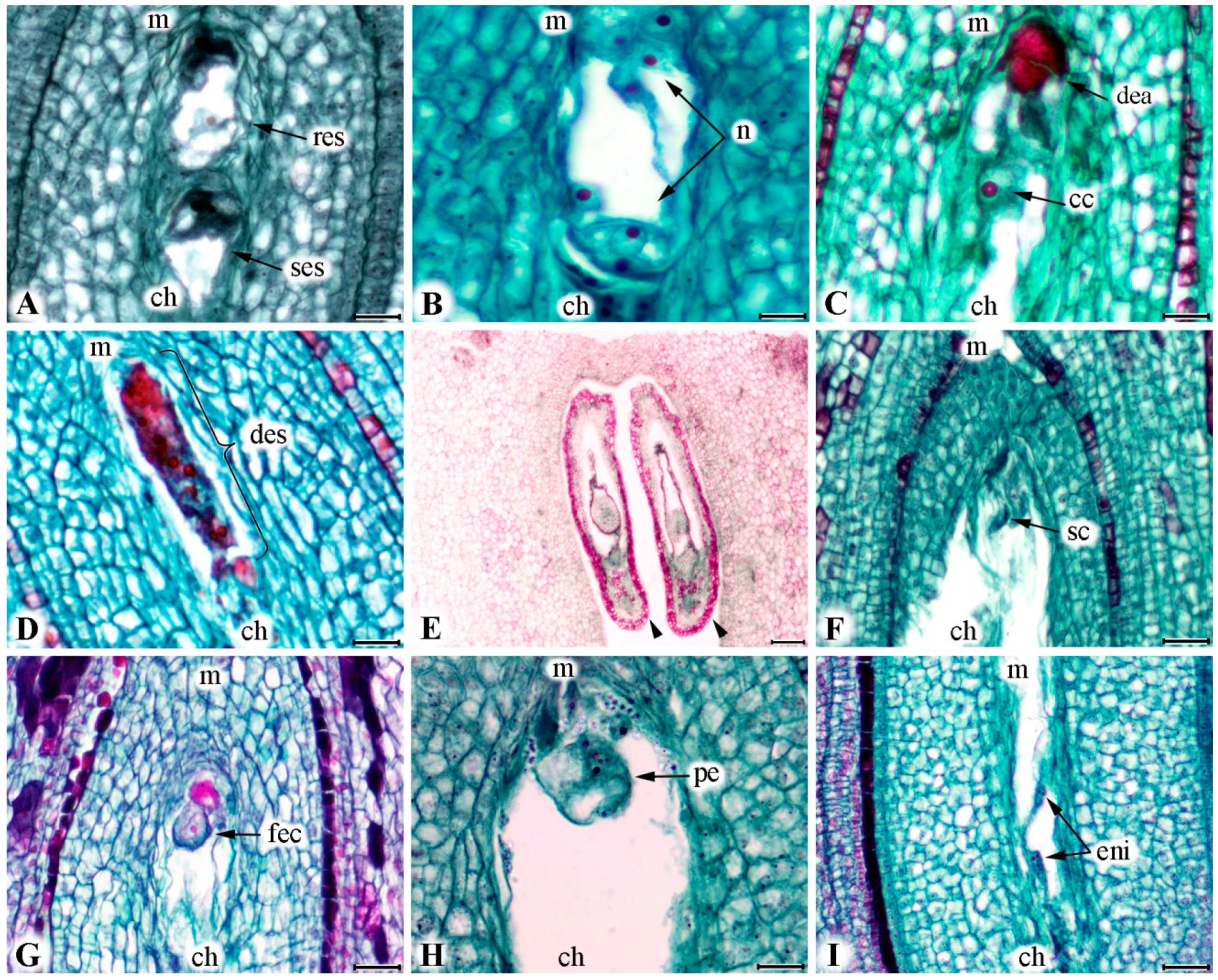
2.2. Development of Embryo Sac
2.3. Viability of Embryo Sac
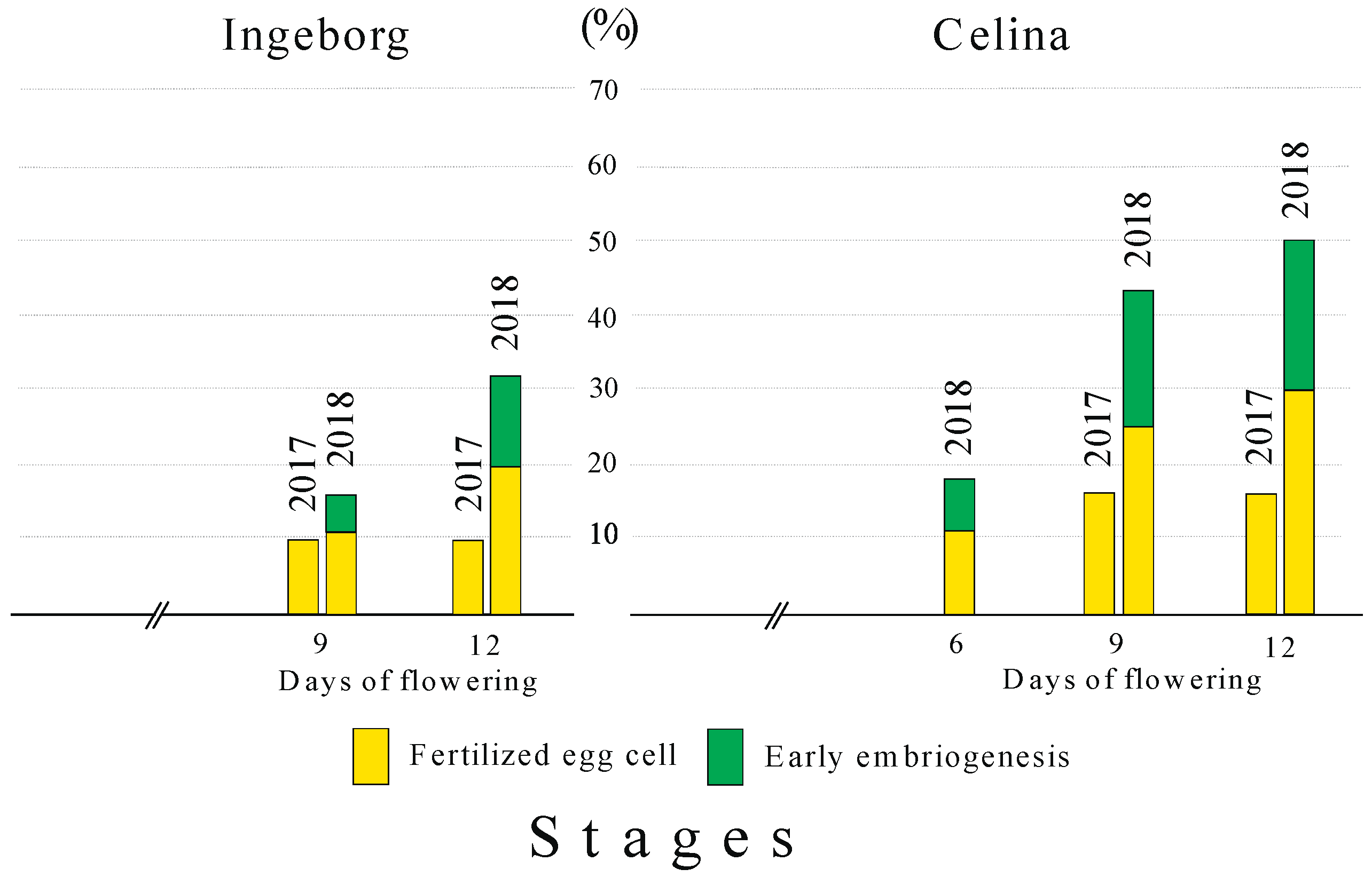
2.4. Fertilization Success
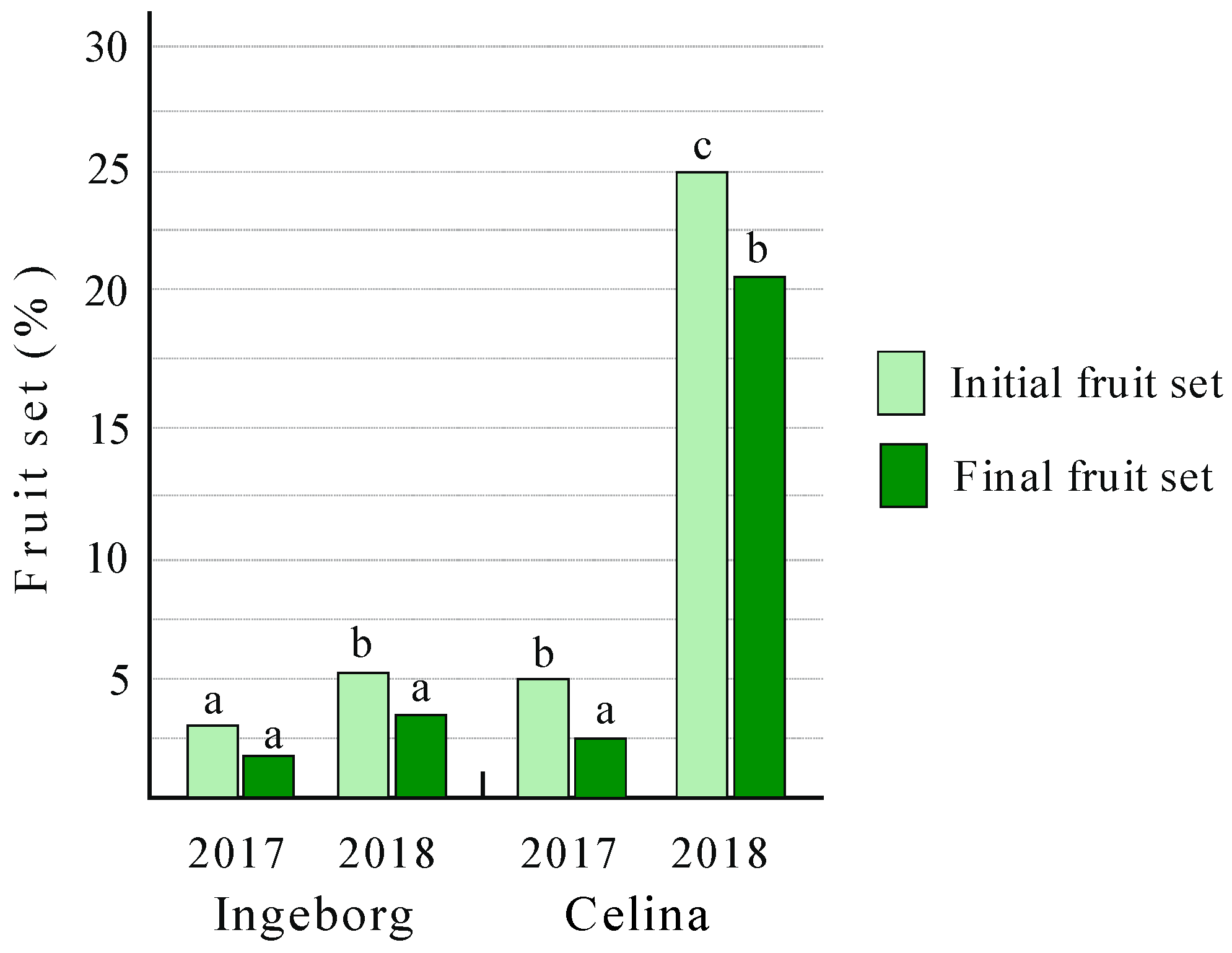
2.5. Fruit Set
3. Discussion
3.1. Developing and Viability of Embryo Sac
3.2. Fertilization Success and Fruit Set
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Flowering Time and Climate Conditions
4.3. Microscopic Preparations
4.4. Fruit Set
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sehic, J.; Garkava-Gustavsson, L.; Fernández-Fernández, F.; Nybom, H. Genetic diversity in a collection of European pear (Pyrus communis) cultivars determined with SSR markers chosen by ECPGR. Sci. Hortic. 2012, 145, 39–45. [Google Scholar] [CrossRef]
- Meland, M.; Frøynes, O.; Fotirić Akšić, M.; Maas, F.M. Performance of ‘Celina’, ‘Ingeborg’ and ‘Kristina’ pear cultivars on Quince rootstocks growing in a Nordic Climate. Acta Hortic. 2020, in press. [Google Scholar]
- Hjeltnes, S.H.; Vercammen, J.; Gomand, A.; Måge, F.; Røen, D. High potential new Norwegian bred pear cultivars. Acta Hortic. 2015, 1094, 111–116. [Google Scholar] [CrossRef]
- Vilegen-Verschure, A. Celina, the new pear variety. Europ. Fruitg. Mag. 2013, 55, 14–17. [Google Scholar]
- Cerović, R.; Fotirić-Akšić, M.; Meland, M. Success Rate of Individual Pollinizers for the Pear Cultivars “Ingeborg” and “Celina” in a Nordic Climate. Agronomy 2020, 10, 970. [Google Scholar] [CrossRef]
- Gasi, F.; Frøynes, O.; Kalamujić Stroil, B.; Lasić, L.; Pojskić, N.; Fotirić Akšić, M.; Meland, M. S-Genotyping and Seed Paternity Testing of the Pear Cultivar ‘Celina’. Agronomy 2020, 10, 1372. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Cerovic, R.; Micic, N.; Duric, G.; Jevtic, S. Modelling pollen tube growth and ovule viability in sour cherry. Acta Hortic. 1998, 468, 621–628. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. The effect of temperature on stigmatic receptivity in sweet cherry (Prunus avium L.). Plant Cell Environ. 2003, 26, 1673–1680. [Google Scholar] [CrossRef] [Green Version]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Effect of temperature on pollen tube kinetics and dynamics in sweet cherry, Prunus avium (Rosaceae). Am. J. Bot. 2004, 91, 558–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radičević, S.; Cerović, R.; Nikolić, D.; Ðorđević, M. The effect of genotype and temperature on pollen tube growth and fertilization in sweet cherry (Prunus avium L.). Euphytica 2016, 209, 121–136. [Google Scholar] [CrossRef]
- Kuroki, K.; Takemura, Y.; Mingfeng, J.; Marumori, H.; Teratani, N.; Matsumoto, K.; Matsumoto, T.; Tamura, F. Pear pollen selection using higher germination properties at low temperatures and the effect on the fruit set and quality of Japanese pear cultivars. Sci. Hortic. 2017, 216, 200–204. [Google Scholar] [CrossRef]
- Cerović, R.; Ružic, Đ.; Mićić, N. Viability of plum ovules at different temperatures. Ann. Appl. Biol. 2000, 137, 53–59. [Google Scholar] [CrossRef]
- Radičević, S.; Cerović, R.; Đorđević, M. Ovule senescence and unusual pollen tube growth in the ovary of sweet cherry as affected by pistilar genotype and temperature. Span. J. Agric. Res. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- Kozai, N.; Beppu, K.; Mochioka, R.; Boonprakob, U.; Subhadrabandhu, S.; Kataoka, L. Adverse effects of high temperature on the development of reproductive organs in ‘Hakuho’ peach trees. J. Hortic. Sci. Biotechnol. 2004, 79, 255–264. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Warm temperatures at bloom reduce fruit set in sweet cherry. J. Appl. Bot. Food Qual. 2007, 81, 158–164. [Google Scholar]
- Bini, G.; Bellini, E. Macrosporogenesi nella cultivar di pero ‘Decana del Comizio’ e ricerca del periodo più opportuno per la sua impollinazione. Riv. Della Ortoflorofruttic. Ital. 1971, 55, 322–337. [Google Scholar]
- Bini, G. Ulteriori osservazioni su alcuni aspetii della biologia fiorale e di fruttificazione della cultivar di pero ‘Decana del Comizio’. Riv. Della Ortoflorofruttic. Ital. 1972, 56, 299–307. [Google Scholar]
- Batygina, B.T.; Vasilyeva, E.V. Peridiozation in the development of flowering plant reproductive structure: Critical periods. Acta Biol. Crac. Ser. Bot. 2003, 451, 27–36. [Google Scholar]
- González-Gutiérrez, G.A.; Antonia Gutiérrez-Mora, A.; Rodríguez-Garay, B. Embryo sac formation and early embryo development in Agave tequilana (Asparagaceae). Springer Plus 2014, 3, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyś, B. Cyto-embryiological studies in self-incompatible and self-fertile cultivars of sour-cherries (Cerasus vulgaris Mill.). II Development of embryo sacs and ovules at some stages of florescence. Genet. Pol. 1984, 25, 171–180. [Google Scholar]
- Furukawa, Y.; Bukovac, M.J. Embryo sac development in sour cherry during the pollination period as related to fruit set. Hortic. Sci. 1989, 24, 1005–1008. [Google Scholar]
- Madlung, A. Polyploidy and its effect on evolutionary success: Old questions revisited with new tools. Heredity 2013, 110, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Kanbe, K. Comparison of the establishment efficiency of well-grown seedlings at the early growth stage from reciprocal crosses between diploid and triploid apple cultivars, and the possibility of these cultivars cross breeding. Hort. Res. 2007, 6, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Park, S.M.; Hiramatsu, M.; Wakana, A. Aneuploid plants derived from crosses with triploid grapes through immature seed culture and subsequent embryo culture. Plant Cell Tiss. Organ Cult. 1999, 59, 125–133. [Google Scholar] [CrossRef]
- Vorsa, N.; Ballington, J.R. Fertility of triploid highbush blueberry. J. Am. Soc. Hort. Sci. 1991, 116, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Phillips, W.D.; Ranney, T.G.; Touchell, D.H.; Eaker, T.A. Fertility and reproductive pathways of triploid flowering pears (Pyrus sp.). HortScience 2016, 51, 968–971. [Google Scholar] [CrossRef] [Green Version]
- Wiliams, R.R. Factors affecting pollination in fruit trees. In Physiology of Tree Crops; Luckwill, L.C., Cutting, C.V., Eds.; Academic Press: London, UK; New York, NY, USA, 1970; pp. 193–207. [Google Scholar]
- Cerović, R.; Mićić, N. Functionality of embryo sacs as related to their viability and fertilization success in sour cherry. Sci. Hortic. 1999, 79, 227–235. [Google Scholar] [CrossRef]
- Đorđević, M.; Cerović, R.; Radičević, S.; Glišić, I.; Milošević, N.; Marić, S.; Lukić, M. Abnormalities in the ovule development of the European plum cultivar ‘Pozna Plava’ in the days following anthesis. Sci. Hortic. 2019, 252, 222–228. [Google Scholar] [CrossRef]
- Gillet, M.E.; Gregorius, H.R. Effects of reproductive resource allocation and pollen density on fertilization success in plants. BMC Ecol. 2020, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Stösser, R.; Anvary, S.F. Pollen tube growth and fruit set as influenced by senescence of stigma, style and ovules. Acta Hortic. 1983, 139, 13–22. [Google Scholar] [CrossRef]
- Webster, A.D. Factors influencing the flowering, fruit set and fruit growth of European pears. Acta Hortic. 2002, 596, 699–709. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Cerović, R.; Slavković, D.; Hjeltnes, S.H.; Meland, M. Selection of the best pollinizer of ‘Celina’ pear. Acta Hortic. 2018, 1229, 365–370. [Google Scholar] [CrossRef]
- Gaši, F.; Pojskić, N.; Kurtović, M.; Kaiser, C.; Hjeltnes, S.H.; Fotirić Akšić, M.; Meland, M. Pollinizer efficacy of several ‘Ingeborg’ pear pollinizers in Hardanger, Norway, examined using microsatellite markers. HortScience 2017, 52, 1722–1727. [Google Scholar] [CrossRef]
- Meland, M.; Kurtovic, M.; Kalamujic, B.; Pojskic, N.; Lasic, L.; Gasi, F. Microsatellites as a tool for identifying successful pollinators of the pear cultivar ‘Ingeborg’ in Ullensvang, Norway. Acta Hortic. 2018, 1229, 57–60. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M.; Rivero, R.; Måge, F.; Remberg, S.F. Phenology, flowering and fruit-set performance of six recent pear cultivars of Nordic origin. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 578–587. [Google Scholar] [CrossRef]
- Stösser, R.; Hartman, W.; Anvari, S.F. General aspects of pollination and fertilization of pome and stone fruit. Acta Hortic. 1996, 423, 15–22. [Google Scholar] [CrossRef]
- Willemse, M.T.M.; van Went, J.L. The female gametophyte. In Embryology of Angisperms; Johri, B.M., Ed.; Springer: Berlin, Germany, 1984; pp. 159–196. [Google Scholar]
- Yadegari, R.; Drews, N.G. Female gametophyte development. Plant Cell 2004, 16, 133–141. [Google Scholar] [CrossRef]
- Costa Tura, J.; Mackenzie, K.A.D. Ovule and embryo sac development in Malus pumila L. cv. Cox’s Orange Pippin, from dormancy to blossom. Ann. Bot. 1990, 66, 443–450. [Google Scholar] [CrossRef]
- Reiser, L.; Fischer, L.R. The Ovule and the Embryo Sac. Plant Cell 1993, 5, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Willemse, M.T.M.; Franssed-Verheijen, M.A.M. Ovular development and pollen tube growth in the ovary of Gasteria verrucosa (Mill.) H. Duval as condition for fertilization. In Sexual Reproduction in Higher Plants; Cresti, M.P., Gori, O., Pacini, E., Eds.; Springer: Berlin, Germany, 1988; pp. 357–362. [Google Scholar]
- Cerović, R.; Vujičić, R.; Mićić, N. Localization of Polysaccharides in the Ovary of Sour Cherry. Gartenbauwissenschaft 1999, 64, 40–46. [Google Scholar]
- Huang, B.Q.; Russell, S.D. Female germ unit: Organization, isolation, and function. Int. Rev. Cytol. 1992, 140, 233–292. [Google Scholar]
- Song, X.; Yuan, L.; Sundaresan, V. Antipodal cells persist through fertilization in the female gametophyte of Arabidopsis. Plant Reprod. 2014, 27, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Stösser, R.; Anvari, S.F. On the senescence of ovules in cherries. Sci. Hortic. 1982, 16, 29–38. [Google Scholar] [CrossRef]
- Eaton, G.W. A study of the megagametophyte in Prunus avium and its relation to fruit setting. Can. J. Plant Sci. 1959, 39, 466–476. [Google Scholar] [CrossRef]
- Fotirić, M. Klonska Selekcija i Biologija Oplodjenja Oblačiske Višnje (Prunus cerasus L.). Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2009; p. 170. [Google Scholar]
- Alburquerque, N.; Burgos, L.; Egea, J. Variability in the developmental stage of apricot ovules at anthesis and its relationship with fruit set. Ann. Appl. Biol. 2002, 141, 147–152. [Google Scholar] [CrossRef]
- Burgos, L.; Alburquerque, N.; Egea, J. Review. Flower biology in apricot and its implications for breeding. Span. J. Agric. Res. 2004, 2, 227–241. [Google Scholar] [CrossRef]
- Piemonta, E.; Polito, V.S. Ovule abortion in ‘Nonpareil’ almond (Prunus dulcis [MILL.] D. A. WEBB). Am. J. Bot. 1982, 69, 913–920. [Google Scholar] [CrossRef]
- Cerović, R. Fertilization Biology of Sour Cherry; Endowment Andrejević: Belgrade, Serbia, 1997; pp. 1–133. [Google Scholar]
- Herrero, M. Male and female synchrony and the regulation of mating in flowering plants. Phil. Trans. R. Soc. Lond. B 2003, 358, 1019–1024. [Google Scholar] [CrossRef]
- Nyéki, J.; Pinter, G.; Szabo, Z. Recent data on fertilization of pear varieties. Acta Hortic. 1984, 367, 87–96. [Google Scholar] [CrossRef]
- Sanzol, J.; Herrero, M. The effective pollination period in fruit trees. Sci. Hortic. 2001, 90, 1–17. [Google Scholar] [CrossRef]
- Meier, U. Growth stages of mono- and dicotyledonous plants. In Federal Biological Research Centre for Agriculture and Forestry, 2nd ed.; BBCH Monograph; Blackwell Wissenschafts-Verlag: Berlin, Germany, 2001; pp. 1–158. [Google Scholar]
- Gerlach, D. A rapid safranin-crystal violet-light green staining sequence for paraffin sections of plant materials. Stain Technol. 1969, 44, 210–211. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerović, R.; Fotirić Akšić, M.; Đorđević, M.; Meland, M. Functionality of Embryo Sacs in Pear Cultivars ‘Ingeborg’ and ‘Celina’ as Related to Fruit Set under Nordic Climate. Plants 2020, 9, 1716. https://doi.org/10.3390/plants9121716
Cerović R, Fotirić Akšić M, Đorđević M, Meland M. Functionality of Embryo Sacs in Pear Cultivars ‘Ingeborg’ and ‘Celina’ as Related to Fruit Set under Nordic Climate. Plants. 2020; 9(12):1716. https://doi.org/10.3390/plants9121716
Chicago/Turabian StyleCerović, Radosav, Milica Fotirić Akšić, Milena Đorđević, and Mekjell Meland. 2020. "Functionality of Embryo Sacs in Pear Cultivars ‘Ingeborg’ and ‘Celina’ as Related to Fruit Set under Nordic Climate" Plants 9, no. 12: 1716. https://doi.org/10.3390/plants9121716
APA StyleCerović, R., Fotirić Akšić, M., Đorđević, M., & Meland, M. (2020). Functionality of Embryo Sacs in Pear Cultivars ‘Ingeborg’ and ‘Celina’ as Related to Fruit Set under Nordic Climate. Plants, 9(12), 1716. https://doi.org/10.3390/plants9121716




