Direct and Bicarbonate-Induced Iron Deficiency Differently Affect Iron Translocation in Kiwifruit Roots
Abstract
1. Introduction
2. Results
2.1. The Traits of 57Fe Uptake and Translocation in -Fe and Bicarbonate-Treated Kiwifruit Plants
2.2. Fe Forms in Roots of -Fe and Bicarbonate-Treated Kiwifruit Plants
2.3. Physiological Responses in Roots of -Fe and Bicarbonate-Treated Kiwifruit Plants
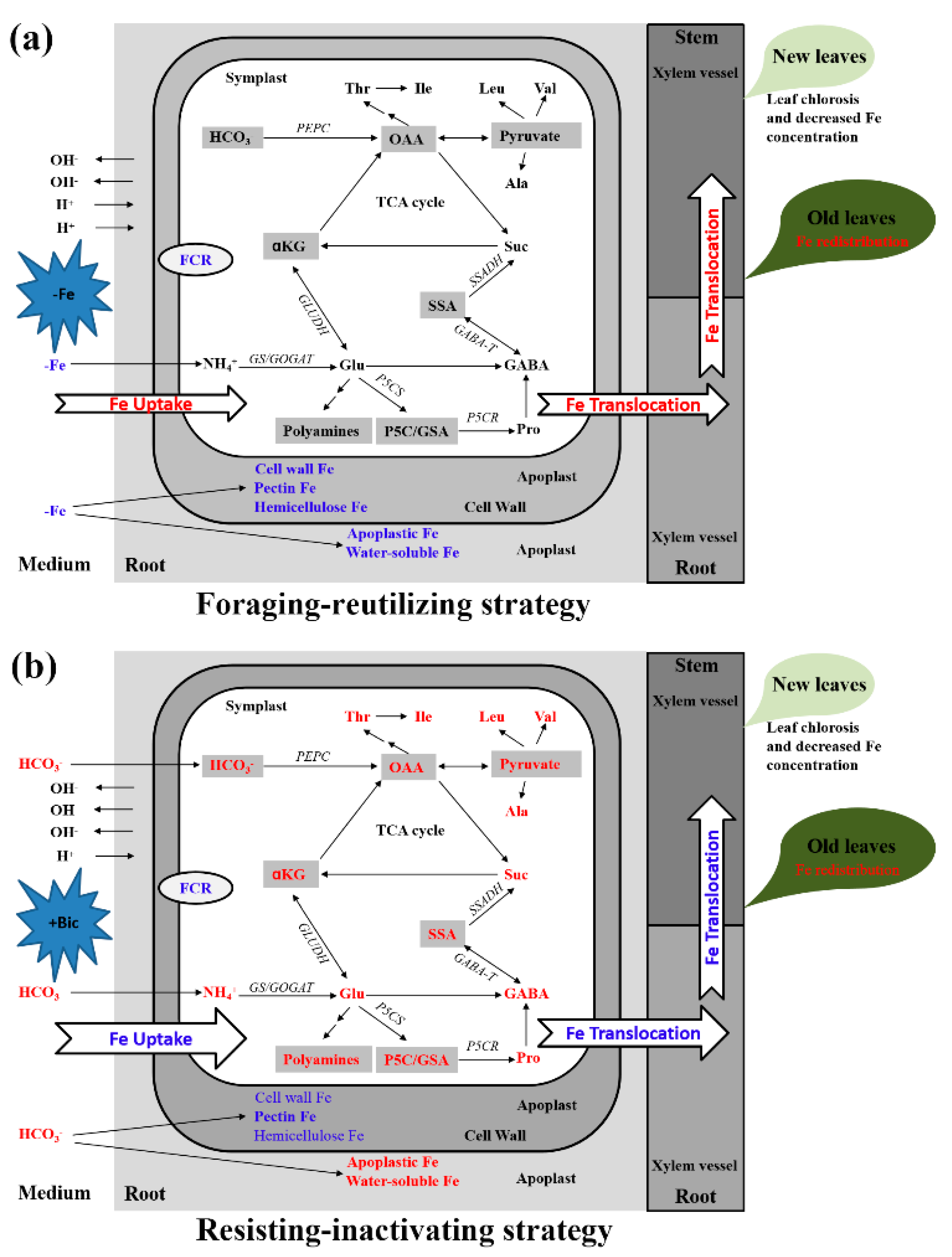
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Determination of Total Fe and 57Fe Concentration
4.3. Measurement of Various Fe Forms
4.4. Assay of Ferric Chelate Reductase (FCR) Activity, Proton (H+) Extrusion in Roots and Fe Deposition on Root Surface
4.5. Detection of NH4+ and NO3- Concentrations
4.6. Analysis of Succinic Acid and Various Amino Acids
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tagliavini, M.; Rombolà, A.D. Iron deficiency and chlorosis in orchard and vineyard ecosystems. Eur. J. Agron. 2001, 15, 71–92. [Google Scholar] [CrossRef]
- Chen, Y.; Barak, P. Iron nutrition of plants in calcareous soils. Adv. Agron. 1982, 35, 217–240. [Google Scholar]
- Alhendawi, R.A.; Römheld, V.; Kirkby, E.; Marschner, H. Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum, and maize. J. Plant. Nutr. 1997, 20, 1731–1753. [Google Scholar] [CrossRef]
- Ding, W.; Clode, P.L.; Lambers, H. Effects of pH and bicarbonate on the nutrient status and growth of three Lupinus species. Plant. Soil 2020, 447, 9–28. [Google Scholar] [CrossRef]
- Yao, C.C.; Long, Z.X.; Liu, X.F. Effect of iron deficiency chlorosis on kiwifruit. J. Northwest. For. Univ. 2005, 20, 148–149. [Google Scholar]
- Xiong, Y.; Li, Q.K. China Soil, 2nd ed.; Science Press: Beijing, China, 1987; p. 85. [Google Scholar]
- Zhai, J.L. Problems and development countermeasures of kiwifruit industry in China. Sci. Technol. Dev. 2015, 4, 521–529. [Google Scholar]
- Cesco, S.; Rombolà, A.D.; Tagliavini, M.; Varanini, Z.; Pinton, R. Phytosiderophores released by graminaceous species promote 59Fe-uptake in citrus. Plant. Soil 2006, 287, 223–233. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.R.; Forner-Giner, M.A.; Iglesias, D.J.; Primo-Millo, E.; Legaz, F. Strategy I responses to Fe-deficiency of two citrus rootstocks differing in their tolerance to iron chlorosis. Sci. Hortic. 2013, 153, 56–63. [Google Scholar] [CrossRef]
- Nikolic, M.; Römheld, V.; Merkt, N. Effect of bicarbonate on uptake and translocation of 59Fe in two grapevine rootstocks differing in their resistance to Fe deficiency chlorosis. Vitis 2000, 39, 145–150. [Google Scholar]
- Martínez-Cuenca, M.R.; Iglesias, D.J.; Forner-Giner, M.A.; Primo-Millo, E.; Legaz, F. The effect of sodium bicarbonate on plant performance and iron acquisition system of FA-5 (Forner-Alcaide 5) citrus seedlings. Acta Physiol. Plant. 2013, 35, 2833–2845. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.R.; Legaz, F.; Forner-Giner, M.A.; Primo-Millo, E.; Iglesias, D.J. Bicarbonate blocks iron translocation from cotyledons inducing iron stress responses in citrus roots. J. Plant. Physiol. 2013, 170, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Pavlovic, J. Plant responses to iron deficiency and toxicity and iron use efficiency in plants. Chapter 3. In Plant Micronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Tran, L.P., Fujiwara, T., Eds.; Academic Press: London, UK, 2018; pp. 55–69. [Google Scholar]
- Zhang, X.X.; Zhang, D.; Sun, W.; Wang, T.Z. The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Weber, G.; Köster, J.; Hajirezaei, M.; Zou, C.; Zhang, F.; Wirén, N. Senescence-induced iron mobilization in source leaves of barley (Hordeum vulgare) plants. New Phytol. 2012, 195, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Yan, T.S.; Fu, L.N.; Zhou, G.F.; Liu, Y.Z.; Peng, S.A. Differences in boron distribution and forms in four citrus scion-rootstock combinations with contrasting boron efficiency under boron-deficient conditions. Trees 2014, 28, 1589–1598. [Google Scholar] [CrossRef]
- Shi, R.; Melzer, M.; Zheng, S.; Benke, A.; Stich, B.; Wirén, N. Iron retention in root hemicelluloses causes genotypic variability in the tolerance to iron deficiency-induced chlorosis in maize. Front. Plant. Sci. 2018, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.J.; Zhu, X.F.; Wang, Z.W.; Dong, F.; Dong, N.Y.; Zheng, S.J. Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant. Cell Environ. 2014, 37, 852–863. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wu, Q.; Zheng, L.; Shen, R.F. NaCl alleviates iron deficiency through facilitating root cell wall iron reutilization and its translocation to the shoot in Arabidopsis thaliana. Plant. Soil 2017, 417, 155–167. [Google Scholar] [CrossRef]
- Zhu, X.F.; Dong, X.Y.; Wu, W.; Shen, R.F. Ammonium regulates Fe deficiency responses by enhancing nitric oxide signaling in Arabidopsis thaliana. Planta 2019, 250, 1089–1102. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Zhang, J.H.; Zhu, L.F.; Abliz, B.; Zhong, C.; Bai, Z.G.; Hu, W.J.; Hussain, S.; James, A.B.; Cao, X.C.; et al. NH4+ facilitates iron reutilization in the cell walls of rice (Oryza sativa) roots under iron-deficiency conditions. Environ. Exp. Bot. 2018, 151, 21–31. [Google Scholar] [CrossRef]
- Donnini, S.; De Nisi, P.; Gabotti, D.; Tato, L.; Zocchi, G. Adaptive strategies of Parietaria diffusa (M.&K.) to calcareous habitat with limited iron availability. Plant. Cell Environ. 2012, 35, 1171–1184. [Google Scholar]
- Chen, H.F.; Zhang, Q.; Cai, H.M.; Zhou, W.; Xu, F.S. H2O2 mediates nitrate-induced iron chlorosis by regulating iron homeostasis in rice: N forms, rhizosphere pH and iron homeostasis. Plant. Cell Environ. 2018, 41, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Vizzotto, G.; Matosevic, I.; Pinton, R.; Varanini, Z.; Costa, G. Iron deficiency responses in roots of kiwi. J. Plant. Nutr. 1997, 20, 327–334. [Google Scholar] [CrossRef]
- Vizzotto, G.; Pinton, R.; Bomben, C.; Cesco, S.; Varanini, Z.; Costa, G. Iron reduction in iron-stressed plants of Actinidia deliciosa genotypes: Involvement of PM Fe(III)-chelate reductase and H+-ATPase activity. J. Plant. Nutr. 1999, 22, 479–488. [Google Scholar] [CrossRef]
- Rombolà, A.D.; Brüggemann, W.; López-Millán, A.F.; Tagliavini, M.; Abadía, J.; Marangoni, B.; Moog, P.R. Biochemical responses to iron deficiency in kiwifruit (Actinidia deliciosa). Tree Physiol. 2002, 22, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Jiao, X.Y.; Guo, T.L.; Li, C.Y.; Liu, Z.D.; Ma, F.W. Time course of physiological responses in kiwifruit induced by bicarbonate. Trees 2019, 33, 1711–1722. [Google Scholar] [CrossRef]
- Wang, N.N.; Yao, C.C.; Li, M.J.; Li, C.Y.; Liu, Z.D.; Ma, F.W. Anatomical and physiological responses of two kiwifruit cultivars to bicarbonate. Sci. Hortic. 2019, 243, 528–536. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant. Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant. Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Liu, X.F.; Fan, X.F.; Zhang, L.S.; Yao, C.C.; Long, Z.X.; Wang, X.L. Inducing factors of iron deficiency chlorosis of kiwis in Guanzhong area of Shaanxi. Acta Agric. Boreali-occidentalis Sin. 2002, 11, 57–59. [Google Scholar]
- Wang, N.N.; He, H.H.; Lacroix, C.; Morris, C.; Liu, Z.D.; Ma, F.W. Soil fertility, leaf nutrients and their relationship in kiwifruit orchards of China’s central Shaanxi province. Soil Sci. Plant. Nutr. 2019, 65, 369–376. [Google Scholar] [CrossRef]
- Jelali, N.; Dell’Orto, M.; Rabhi, M.; Zocchi, G.; Abdelly, C.; Gharsalli, M. Physiological and biochemical responses for two cultivars of Pisum sativum (“Merveille de Kelvedon” and “Lincoln”) to iron deficiency conditions. Sci. Hortic. 2010, 124, 116–121. [Google Scholar] [CrossRef]
- Ksouri, R.; Debez, A.; Mahmoudi, H.; Ouerghi, Z.; Gharsalli, M.; Lachaâl, M. Genotypic variability within Tunisian grapevine varieties (Vitis vinifera L.) facing bicarbonate-induced iron deficiency. Plant. Physiol. Biochem. 2007, 45, 315–322. [Google Scholar] [CrossRef] [PubMed]
- M’Sehli, W.; Youssfi, S.; Donnini, S.; Dell’Orto, M.; De Nisi, P.; Zocchi, G.; Abdelly, C.; Gharsalli, M. Root exudation and rhizosphere acidification by two lines of Medicago ciliaris in response to lime-induced iron deficiency. Plant. Soil 2008, 312, 151–162. [Google Scholar] [CrossRef]
- Shen, J.B.; Zeng, Y.L.; Zhuang, X.H.; Sun, L.; Yao, X.Q.; Pimpl, P.; Jiang, L.W. Organelle pH in the Arabidopsis endomembrane system. Mol. Plant. 2013, 6, 1419–1437. [Google Scholar] [CrossRef]
- Fodor, F.; Kovács, K.; Czech, V.; Solti, Á.; Tóth, B.; Lévai, L.; Bóka, K.; Vértes, A. Effects of short term iron citrate treatments at different pH values on roots of iron-deficient cucumber: A Mössbauer analysis. J. Plant. Physiol. 2012, 169, 1615–1622. [Google Scholar] [CrossRef]
- Mengel, K. Iron availability in plant tissues-iron chlorosis on calcareous soils. Plant. Soil 1994, 165, 275–283. [Google Scholar] [CrossRef]
- Chaney, R.L.; Brown, J.C.; Tiffin, L.O. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant. Physiol. 1972, 50, 208–213. [Google Scholar] [CrossRef]
- Van Beusichem, M.L.; Kirkby, E.A.; Baas, R. Influence of nitrate and ammonium nutrition on the uptake assimilation, and distribution of nutrients in Ricinus communis. Plant. Physiol. 1988, 86, 914–921. [Google Scholar] [CrossRef]
- Molina, J.; Covarrubias, J.I. Influence of nitrogen on physiological responses to bicarbonate in a grapevine rootstock. J. Soil Sci. Plant. Nutr. 2019, 19, 305–312. [Google Scholar] [CrossRef]
- Feng, H.M.; Fan, X.R.; Miller, A.J.; Xu, G.H. Plant nitrogen uptake and assimilation: Regulation of cellular pH homeostasis. J. Exp. Bot. 2020, 71, 4380–4392. [Google Scholar] [CrossRef] [PubMed]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Wang, B.; Song, W.F.; Zheng, S.J.; Shen, R.F. Putrescine alleviates iron deficiency via NO-dependent reutilization of root cell-wall Fe in Arabidopsis. Plant. Physiol. 2016, 170, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Li, X.; VandenLangenberg, K.M.; Wen, D.; Sun, S.S.; Wei, M.; Li, Y.; Yang, F.J.; Shi, Q.H.; Wang, X.F. Overexpression of S-adenosyl-L-methionine synthetase increased tomato tolerance to alkali stress through polyamine metabolism. Plant. Biotechnol. J. 2014, 12, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Gilliham, M.; Tyerman, S.D. Linking metabolism to membrane signaling: The GABA–malate connection. Trends Plant. Sci. 2016, 21, 295–301. [Google Scholar] [CrossRef]
- Li, Y.X.; Liu, B.Y.; Peng, Y.X.; Liu, C.L.; Zhang, X.Z.; Zhang, Z.J.; Liang, W.; Ma, F.W.; Li, C.Y. Exogenous GABA alleviates alkaline stress in Malus hupehensis by regulating the accumulation of organic acids. Sci. Hortic. 2020, 261, 108982. [Google Scholar] [CrossRef]
- Yu, J.Y.; Liu, Z.D.; Yao, C.C.; Chen, Y.A. A new kiwifruit cultivar ‘Qihong’. Acta Hortic. Sin. 2015, 42, 1409–1410. [Google Scholar]
- Pavlovic, J.; Samardzic, J.; Maksimović, V.; Timotijevic, G.; Stevic, N.; Laursen, K.H.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Liang, Y. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol. 2013, 198, 1096–1107. [Google Scholar] [CrossRef]
- Jin, C.W.; You, G.Y.; He, Y.F.; Tang, C.; Wu, P.; Zheng, S.J. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant. Physiol. 2007, 144, 278–285. [Google Scholar] [CrossRef]
- Ma, B.Q.; Yuan, Y.Y.; Gao, M.; Li, C.Y.; Ogutu, C.; Li, M.J.; Ma, F.W. Determination of predominant organic acid components in Malus species: Correlation with apple domestication. Metabolism 2018, 8, 74. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Li, H.X.; Fan, Y.L.; Song, Y.; Wu, L.J.; Tian, H.G.; Liu, X.F.; Liu, T.X. Simultaneous analysis of 23 amino acids in different plant tissues by liquid chromatography ion trap tandem mass spectrometry. Acta Agric. Boreali Occidentalis Sin. 2016, 25, 1229–1236. [Google Scholar]
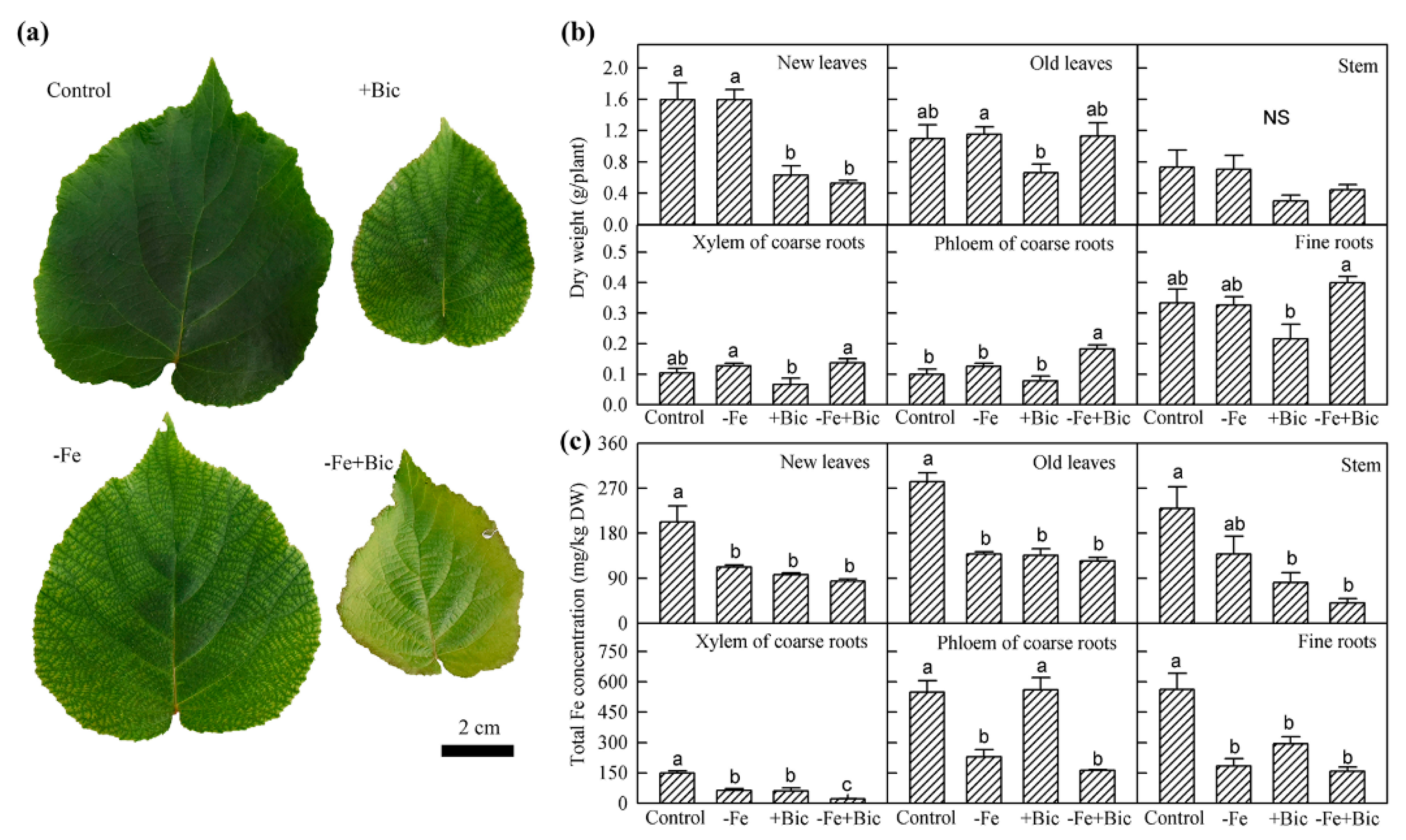
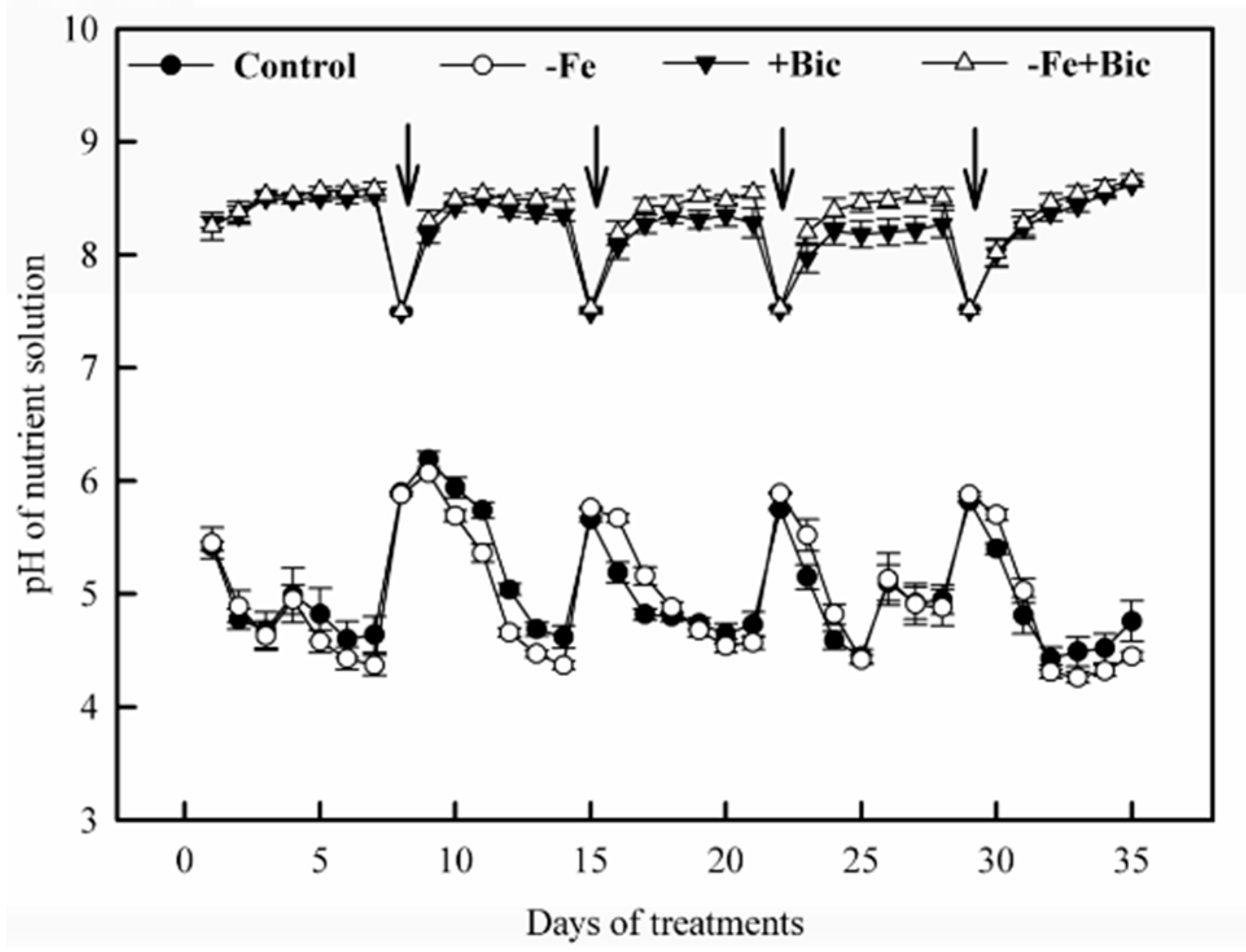
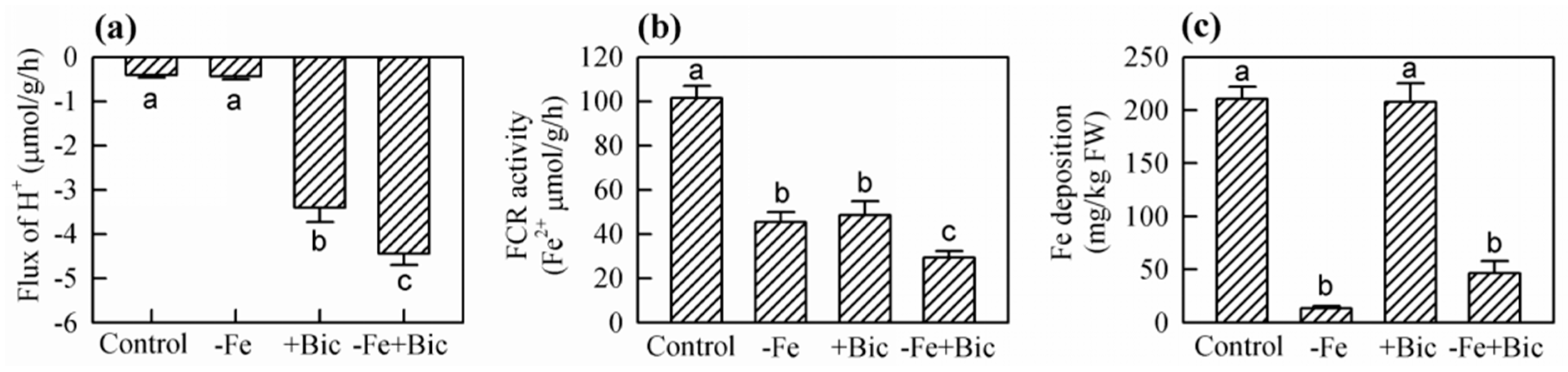
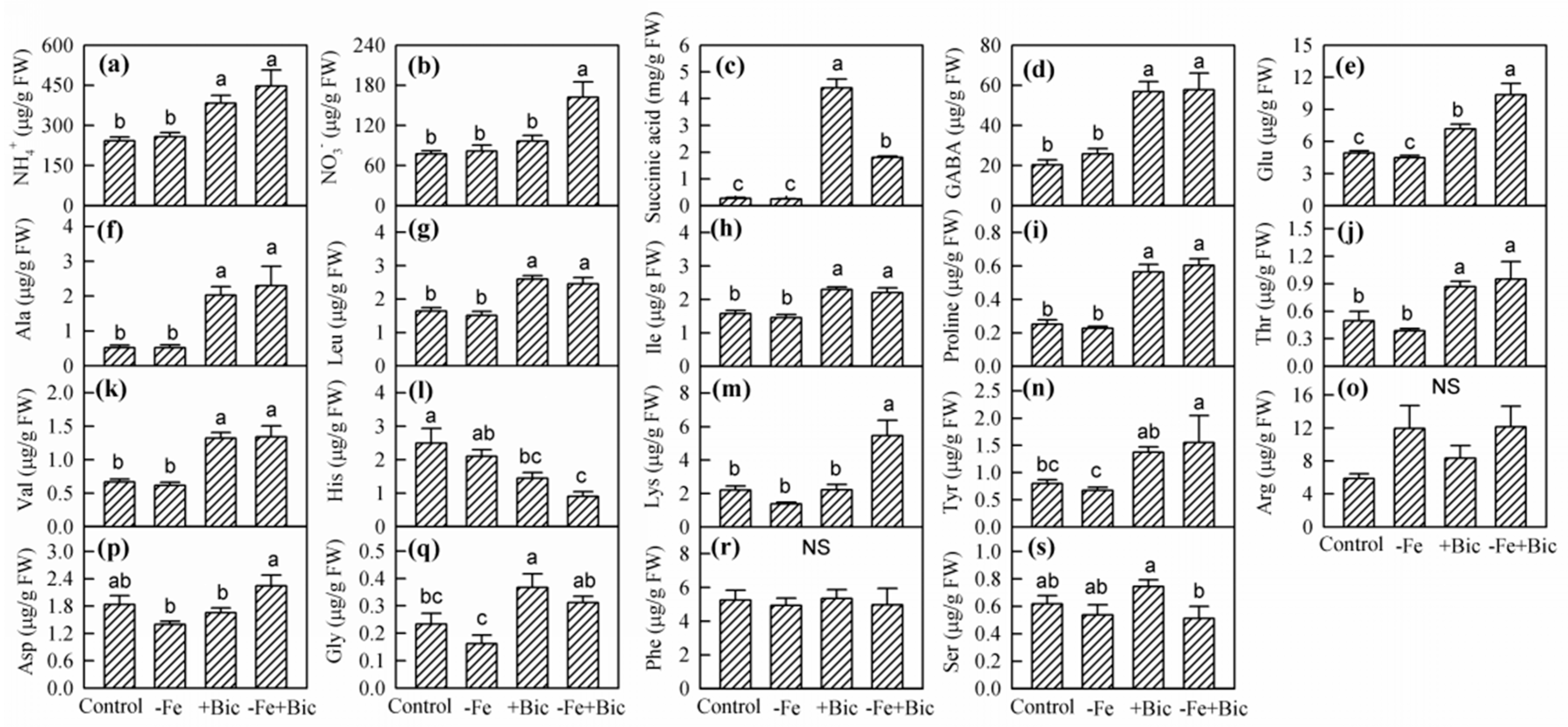
| Treatment | New Leaves | Old Leaves | Stem | Xylem of Coarse Roots | Phloem of Coarse Roots | Fine Roots |
|---|---|---|---|---|---|---|
| 57Fedfs Concentration (mg/kg DW) | ||||||
| Control | 141.2 ± 29.4a | 132.5 ± 23.3a | 154.6 ± 24.2a | 95.5 ± 12.5a | 216.9 ± 24.6a | 398.8 ± 66.3a |
| -Fe | 36.9 ± 2.9b | 14.7 ± 5.3b | 52.3 ± 15.3b | 19.0 ± 2.8bc | 24.4 ± 3.3b | 26.9 ± 6.9c |
| +Bic | 44.6 ± 6.3b | 16.8 ± 2.7b | 39.5 ± 9.1b | 24.8 ± 2.4b | 245.4 ± 29.8a | 189.7 ± 28.8b |
| -Fe+Bic | 0.9 ± 0.5b | 0.7 ± 0.2b | 1.3 ± 0.2b | 2.0 ± 0.1c | 12.8 ± 0.3b | 31.8 ± 2.0c |
| 57Fedfs Distribution (%) | ||||||
| Control | 34.8 ± 3.1b | 22.5 ± 0.9a | 16.9 ± 2.2b | 1.6 ± 0.2ab | 3.4 ± 0.2b | 20.9 ± 1.2c |
| -Fe | 49.1 ± 4.9a | 13.4 ± 3.6b | 26.3 ± 1.1a | 2.0 ± 0.1a | 2.5 ± 0.1b | 6.8 ± 0.7d |
| +Bic | 25.0 ± 0.4c | 10.1 ± 1.7bc | 9.9 ± 1.2c | 1.4 ± 0.1b | 17.3 ± 2.5a | 36.4 ± 3.3b |
| -Fe+Bic | 2.6 ± 1.1d | 4.5 ± 1.6c | 3.3 ± 0.5d | 1.6 ± 0.1ab | 13.8 ± 1.0a | 74.3 ± 2.2a |
| Treatment | 57Fedfs Translocation Rate | 57Fedfs Relative Translocation Rate | ||||
|---|---|---|---|---|---|---|
| NL/OL | S/R | XCR/FR | NL/OL | S/R | XCR/FR | |
| Control | 1.05 ± 0.06b | 0.46 ± 0.05b | 0.25 ± 0.04b | 1.56 ± 0.16b | 2.88 ± 0.20b | 0.08 ± 0.01b |
| -Fe | 3.10 ± 0.93a | 1.34 ± 0.07a | 0.75 ± 0.10a | 4.48 ± 1.54a | 7.97 ± 0.67a | 0.29 ± 0.02a |
| +Bic | 2.71 ± 0.31ab | 0.19 ± 0.03c | 0.14 ± 0.03b | 2.60 ± 0.41ab | 0.82 ± 0.07c | 0.04 ± 0.01bc |
| -Fe+Bic | 1.20 ± 0.25b | 0.04 ± 0.01d | 0.06 ± 0.00b | 0.56 ± 0.10b | 0.12 ± 0.04c | 0.02 ± 0.00c |
| Water-Soluble Fe (mg/kg FW) | Apoplastic Fe (Fe2+ nmol/g/h) | Cell Wall Fe (mg/kg Cell Wall) | Pectin Fe (mg/kg Cell Wall) | Hemicellulose Fe (mg/kg Cell Wall) | |
|---|---|---|---|---|---|
| Control | 1.7 ± 0.3a | 28.3 ± 1.4b | 3907.5 ± 300.4a | 490.2 ± 103.7a | 358.9 ± 45.1a |
| -Fe | 0.5 ± 0.1b | 20.3 ± 2.7b | 572.5 ± 42.1c | 5.9 ± 0.6b | 44.7 ± 5.2b |
| (−72.7) | (−28.4) | (−85.3) | (−98.8) | (−87.6) | |
| +Bic | 2.0 ± 0.2a | 46.0 ± 4.5a | 1186.2 ± 115.4b | 2.1 ± 0.1b | 71.3 ± 8.5b |
| (+12.6) | (+62.1) | (−69.6) | (−99.6) | (−80.1) | |
| -Fe+Bic | 0.3 ± 0.1b | 29.6 ± 6.6b | 527.6 ± 49.5c | 1.6 ± 0.7b | 45.5 ± 5.9b |
| (−80.9) | (+4.6) | (−86.5) | (−99.7) | (−87.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Dong, X.; Chen, Y.; Ma, B.; Yao, C.; Ma, F.; Liu, Z. Direct and Bicarbonate-Induced Iron Deficiency Differently Affect Iron Translocation in Kiwifruit Roots. Plants 2020, 9, 1578. https://doi.org/10.3390/plants9111578
Wang N, Dong X, Chen Y, Ma B, Yao C, Ma F, Liu Z. Direct and Bicarbonate-Induced Iron Deficiency Differently Affect Iron Translocation in Kiwifruit Roots. Plants. 2020; 9(11):1578. https://doi.org/10.3390/plants9111578
Chicago/Turabian StyleWang, Nannan, Xiaoke Dong, Yuanlei Chen, Baiquan Ma, Chunchao Yao, Fengwang Ma, and Zhande Liu. 2020. "Direct and Bicarbonate-Induced Iron Deficiency Differently Affect Iron Translocation in Kiwifruit Roots" Plants 9, no. 11: 1578. https://doi.org/10.3390/plants9111578
APA StyleWang, N., Dong, X., Chen, Y., Ma, B., Yao, C., Ma, F., & Liu, Z. (2020). Direct and Bicarbonate-Induced Iron Deficiency Differently Affect Iron Translocation in Kiwifruit Roots. Plants, 9(11), 1578. https://doi.org/10.3390/plants9111578






