134Cs Uptake and Growth at Various Cs+ and K+ Levels in Arabidopsis AtKUP7 Mutants
Abstract
1. Introduction
2. Results
2.1. Plant Material
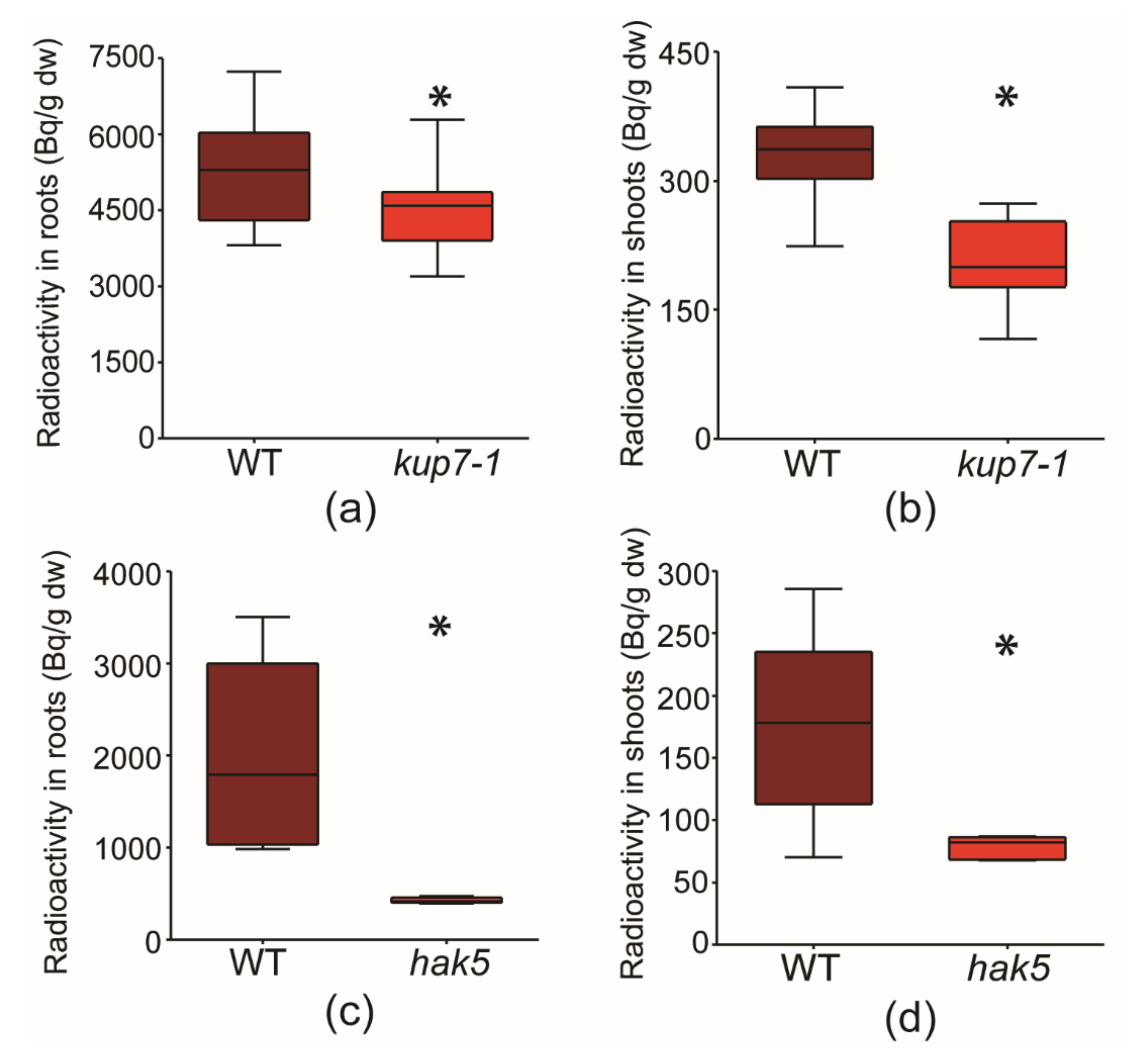
2.2. Short-term Uptake of 134Cs+
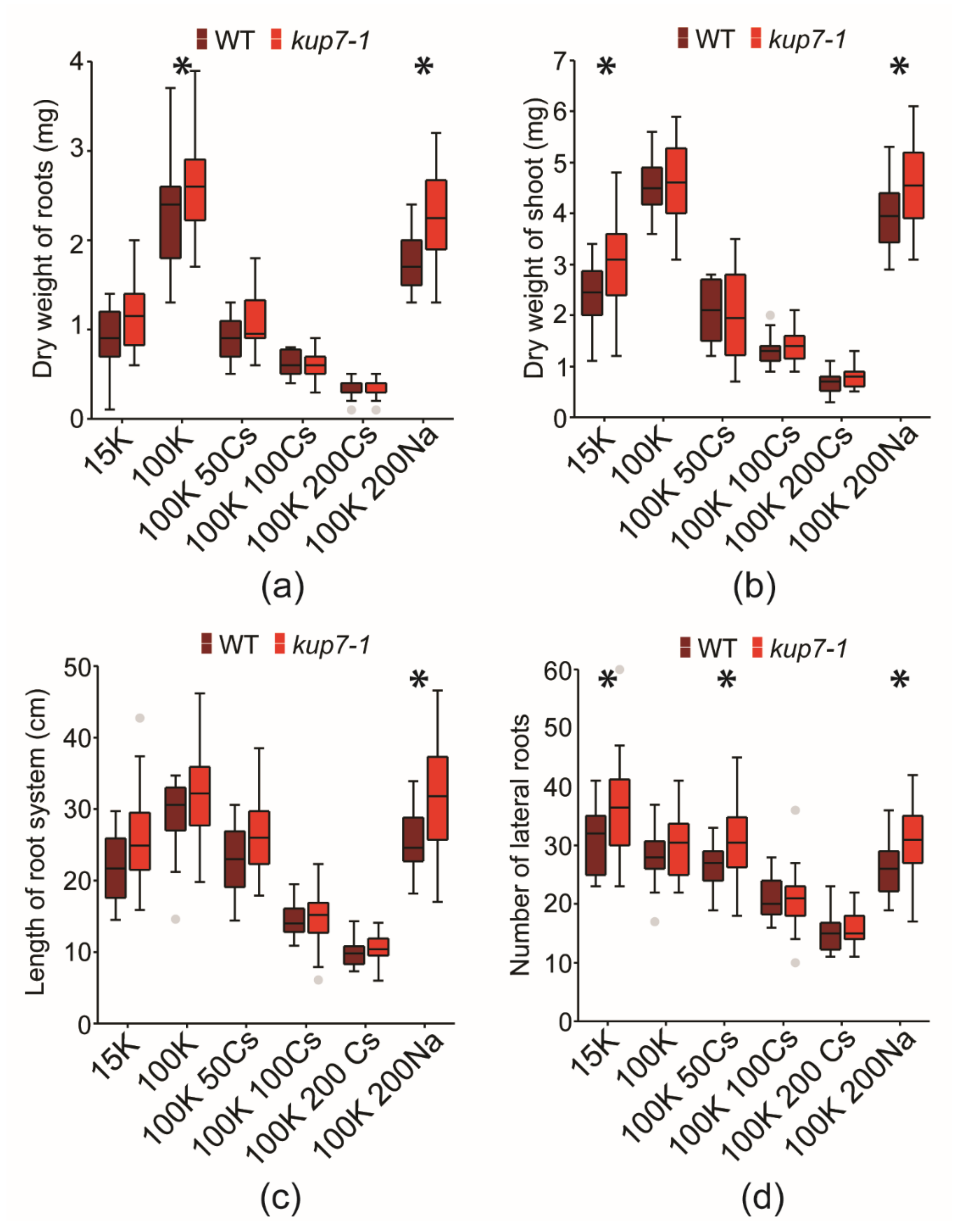
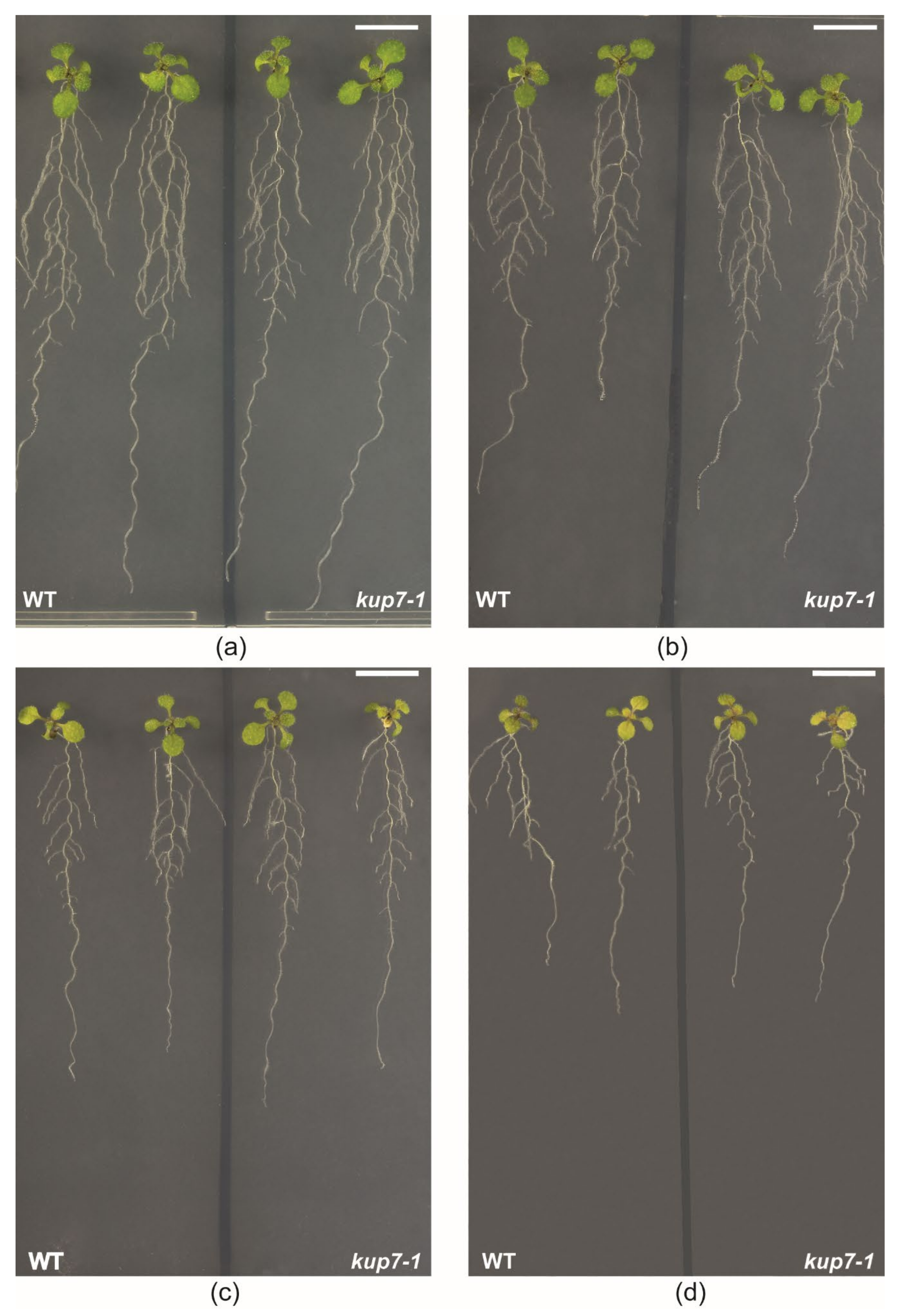
2.3. Plant Susceptibility to 133Cs+ Toxicity
2.4. Response to K+ Shortage
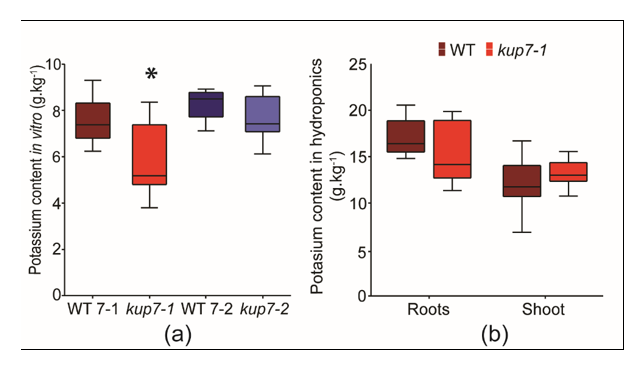
2.5. Potassium Accumulation in Low K+ Conditions
3. Discussion
3.1. Radiocaesium Accumulation and Cs+ Sensitivity of kup7-1 Mutant Plants
3.2. Low K+ Responses and K+ Accumulation in Arabidopsis kup7 Mutants
4. Conclusions
5. Materials and Methods
5.1. Plant Material
5.2. Uptake of 134Cs
5.3. Susceptibility to Cs+ Toxicity and K+ Shortage
5.4. Measurements of Root System Traits and Plant Biomass
5.5. Potassium Accumulation
5.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wai, K.-M.; Krstic, D.; Nikezic, D.; Lin, T.-H.; Yu, P.K.N. External Cesium-137 doses to humans from soil influenced by the Fukushima and Chernobyl nuclear power plants accidents: A comparative study. Sci. Rep. 2020, 10, 7902. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Wulfetange, K.; Porée, F.; Michard, E.; Gajdanowicz, P.; Lacombe, B.; Sentenac, H.; Thibaud, J.-B.; Mueller-Roeber, B.; Blatt, M.R.; et al. External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J. 2006, 46, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Rai, H.; Kawabata, M. The Dynamics of Radio-Cesium in Soils and Mechanism of Cesium Uptake Into Higher Plants: Newly Elucidated Mechanism of Cesium Uptake Into Rice Plants. Front. Plant Sci. 2020, 11, 528. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Tansley review no. 113 Mechanisms of caesium uptake by plants. New Phytol. 2000, 147, 241–256. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Shaw, G.; Nisbet, A.; Wilkins, B. Effect of potassium starvation on the uptake of radiocaesium by spring wheat (Triticum aestivum cv. Tonic). Plant Soil 2000, 220, 27–34. [Google Scholar] [CrossRef]
- Smolders, E.; Sweeck, L.; Merckx, R.; Cremers, A. Cationic interactions in radiocaesium uptake from solution by spinach. J. Environ. Radioact. 1997, 34, 161–170. [Google Scholar] [CrossRef]
- Nishita, H.; Dixon, D.; Larson, K.H. Accumulation of Cs+ and K+ and growth of bean plants in nutrient solution and soils. Plant Soil 1962, 17, 221–242. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Smolders, E. Plant uptake of radiocaesium: A review of mechanisms, regulation and application. J. Exp. Bot. 2000, 51, 1635–1645. [Google Scholar] [CrossRef]
- Chérel, I.; Lefoulon, C.; Boeglin, M.; Sentenac, H. Molecular mechanisms involved in plant adaptation to low K+ availability. J. Exp. Bot. 2014, 65, 833–848. [Google Scholar] [CrossRef]
- Gierth, M.; Mäser, P.; Schroeder, J.I. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant. Physiol. 2005, 137, 1105–1114. [Google Scholar] [CrossRef]
- Qi, Z.; Hampton, C.R.; Shin, R.; Barkla, B.J.; White, P.J.; Schachtman, D.P. The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in Arabidopsis. J. Exp. Bot. 2008, 59, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.; Miyazaki, T.; Shin, R. Contribution of KUPs to potassium and cesium accumulation appears complementary in Arabidopsis. Plant Signal. Behav. 2019, 14, 1554468. [Google Scholar] [CrossRef] [PubMed]
- Ródenas, R.; Nieves-Cordones, M.; Rivero, R.M.; Martinez, V.; Rubio, F. Pharmacological and gene regulation properties point to the SlHAK5 K+ transporter as a system for high-affinity Cs+ uptake in tomato plants. Physiol. Plant. 2018, 162, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Rai, H.; Yokoyama, S.; Satoh-Nagasawa, N.; Furukawa, J.; Nomi, T.; Ito, Y.; Fujimura, S.; Takahashi, H.; Suzuki, R.; Yousra, E.; et al. Cesium uptake by rice roots largely depends upon a single gene, HAK1, which encodes a potassium transporter. Plant Cell Physiol. 2017, 58, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Kanter, U.; Hauser, A.; Michalke, B.; Dräxl, S.; Schäffner, A.R. Caesium and strontium accumulation in shoots of Arabidopsis thaliana: Genetic and physiological aspects. J. Exp. Bot. 2010, 61, 3995–4009. [Google Scholar] [CrossRef]
- Dräxl, S.; Müller, J.; Li, W.B.; Michalke, B.; Scherb, H.; Hense, B.A.; Tschiersch, J.; Kanter, U.; Schäffner, A.R. Caesium accumulation in yeast and plants is selectively repressed by loss of the SNARE Sec22p/SEC22. Nat. Commun. 2013, 4, 2092. [Google Scholar] [CrossRef]
- Plain, C.; Gérant, D.; Epron, D.; Laclau, J.-P.; Bouillet, J.-P.; Nouvellon, Y.; Packer, A.P.; Cabral, O.M.R.; Dannoura, M.; Moreira, M.Z.; et al. In situ13CO2 pulse labelling of field-grown eucalypt trees revealed the effects of potassium nutrition and throughfall exclusion on phloem transport of photosynthetic carbon. Tree Physiol. 2015, 36, 6–21. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, N.; Gao, K.; Chen, F.; Yuan, L.; Mi, G. Ammonium inhibits primary root growth by reducing the length of meristem and elongation yone and decreasing elemental expansion rate in the root apex in Arabidopsis thaliana. PLoS ONE 2013, 8, e61031. [Google Scholar] [CrossRef]
- Broadley, M.R.; Willey, N.J. Differences in root uptake of radiocaesium by 30 plant taxa. Environ. Pollut. 1997, 97, 11–15. [Google Scholar] [CrossRef]
- Djedidi, S.; Kojima, K.; Ohkama-Ohtsu, N.; Bellingrath-Kimura, S.D.; Yokoyama, T. Growth and 137Cs uptake and accumulation among 56 Japanese cultivars of Brassica rapa, Brassica juncea and Brassica napus grown in a contaminated field in Fukushima: Effect of inoculation with a Bacillus pumilus strain. J. Environ. Radioact. 2016, 157, 27–37. [Google Scholar] [CrossRef]
- Kojima, K.; Ookawa, T.; Yamaya-Ito, H.; Salem, D.; Ohkama-Ohtsu, N.; Bellingrath-Kimura, S.D.; Yokoyama, T. Characterization of 140 Japanese and world rice collections cultivated in Nihonmatsu-city in Fukushima in terms of radiocesium activity concentrations in seed grains and straws to explore rice cultivars with low radiocesium accumulation. J. Radianal. Nucl. Chem. 2017, 314, 1009–1021. [Google Scholar] [CrossRef]
- Penrose, B.; Johnson née Payne, K.A.; Arkhipov, A.; Maksimenko, A.; Gaschak, S.; Meacham, M.C.; Crout, N.J.M.; White, P.J.; Beresford, N.A.; Broadley, M.R. Inter-cultivar variation in soil-to-plant transfer of radiocaesium and radiostrontium in Brassica oleracea. J. Environ. Radioact. 2016, 155–156, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.R.; Willey, N.J.; Mead, A. A method to assess taxonomic variation in shoot caesium concentration among flowering plants. Environ. Pollut. 1999, 106, 341–349. [Google Scholar] [CrossRef]
- Gaymard, F.; Pilot, G.; Lacombe, B.; Bouchez, D.; Bruneau, D.; Boucherez, J.; Michaux-Ferriere, N.; Thibaud, J.-B.; Sentenac, H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 1998, 94, 647–655. [Google Scholar] [CrossRef]
- Li, H.; Yu, M.; Du, X.-Q.; Wang, Z.-F.; Wu, W.-H.; Quintero, F.J.; Jin, X.-H.; Li, H.-D.; Wang, Y. NRT1.5/NPF7.3 functions as a proton-coupled H+/K+ antiporter for K+ loading into the xylem in Arabidopsis. Plant Cell 2017, 29, 2016–2026. [Google Scholar] [CrossRef]
- Remy, E.; Cabrito, T.R.; Batista, R.A.; Teixeira, M.C.; Sá-Correia, I.; Duque, P. The major facilitator superfamily transporter ZIFL2 modulates cesium and potassium homeostasis in Arabidopsis. Plant Cell Physiol. 2015, 56, 148–162. [Google Scholar] [CrossRef][Green Version]
- Yang, T.; Zhang, S.; Hu, Y.; Wu, F.; Hu, Q.; Chen, G.; Cai, J.; Wu, T.; Moran, N.; Yu, L. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 2014, 166, 945–959. [Google Scholar] [CrossRef]
- Han, M.; Wu, W.; Wu, W.-H.; Wang, Y. Potassium transporter KUP7 is involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol. Plant 2016, 9, 437–446. [Google Scholar] [CrossRef]
- Hampton, C.R.; Bowen, H.C.; Broadley, M.R.; Hammond, J.P.; Mead, A.; Payne, K.A.; Pritchard, J.; White, P.J. Cesium toxicity in Arabidopsis. Plant Physiol. 2004, 136, 3824–3837. [Google Scholar] [CrossRef]
- Staunton, S.; Hinsinger, P.; Guivarch, A.; Brechignac, F. Root uptake and translocation of radiocaesium from agricultural soils by various plant species. Plant Soil 2003, 254, 443–455. [Google Scholar] [CrossRef]
- Burger, A.; Lichtscheidl, I. Stable and radioactive cesium: A review about distribution in the environment, uptake and translocation in plants, plant reactions and plants’ potential for bioremediation. Sci. Total Environ. 2018, 618, 1459–1485. [Google Scholar] [CrossRef] [PubMed]
- Genies, L.; Orjollet, D.; Carasco, L.; Camilleri, V.; Frelon, S.; Vavasseur, A.; Leonhardt, N.; Henner, P. Uptake and translocation of cesium by Arabidopsis thaliana in hydroponics conditions: Links between kinetics and molecular mechanisms. Environ. Exp. Bot. 2017, 138, 164–172. [Google Scholar] [CrossRef]
- Rubio, F.; Nieves-Cordones, M.; Alemán, F.; Martínez, V. Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol. Plant 2008, 134, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Santa-María, G.E.; Rodríguez-Navarro, A. Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiol. Plant 2000, 109, 34–43. [Google Scholar] [CrossRef]
- Kobayashi, D.; Uozumi, N.; Hisamatsu, S.I.; Yamagami, M. AtKUP/HAK/KT9, a K+ transporter from Arabidopsis thaliana, mediates Cs+ uptake in Escherichia coli. Biosci. Biotechnol. Biochem. 2010, 74, 203–205. [Google Scholar] [CrossRef]
- Al-Younis, I.; Wong, A.; Gehring, C. The Arabidopsis thaliana K+-uptake permease 7 (AtKUP7) contains a functional cytosolic adenylate cyclase catalytic centre. FEBS Lett. 2015, 589, 3848–3852. [Google Scholar] [CrossRef]
- Al-Younis, I.; Wong, A.; Lemtiri-Chlieh, F.; Schmöckel, S.; Tester, M.; Gehring, C.; Donaldson, L. The Arabidopsis thaliana K+-uptake permease 5 (AtKUP5) contains a functional cytosolic adenylate cyclase essential for K+ transport. Front. Plant Sci. 2018, 9, 1645. [Google Scholar] [CrossRef]
- Isaure, M.P.; Fraysse, A.; Devès, G.; Le Lay, P.; Fayard, B.; Susini, J.; Bourguignon, J.; Ortega, R. Micro-chemical imaging of cesium distribution in Arabidopsis thaliana plant and its interaction with potassium and essential trace elements. Biochimie 2006, 88, 1583–1590. [Google Scholar] [CrossRef]
- Hampton, C.R.; White, P.; Broadley, M. Short review: The mechanisms of radiocaesium uptake by Arabidopsis roots. Nukleonika 2005, 50, 3–8. [Google Scholar]
- Tian, H.; Baxter, I.R.; Lahner, B.; Reinders, A.; Salt, D.E.; Ward, J.M. Arabidopsis NPCC6/NaKR1 is a phloem mobile metal binding protein necessary for phloem function and root meristem maintenance. Plant Cell 2010, 22, 3963–3979. [Google Scholar] [CrossRef]
- Rubio, F.; Alemán, F.; Nieves-Cordones, M.; Martínez, V. Studies on Arabidopsis athak5 atakt1 double mutants disclose the range of concentrations at which AtHAK5, AtAKT1 and unknown systems mediate K+ uptake. Physiol. Plant 2010, 139, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Glass, A.D.; Crawford, N.M. Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1, and axr2. Plant Physiol. 1993, 102, 983–989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- T-DNA Express: Arabidopsis Gene Mapping Tool. Available online: http://signal.salk.edu/cgi-bin/tdnaexpress (accessed on 3 July 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šustr, M.; Doksanská, T.; Doležalová, B.; Soukup, A.; Tylová, E. 134Cs Uptake and Growth at Various Cs+ and K+ Levels in Arabidopsis AtKUP7 Mutants. Plants 2020, 9, 1525. https://doi.org/10.3390/plants9111525
Šustr M, Doksanská T, Doležalová B, Soukup A, Tylová E. 134Cs Uptake and Growth at Various Cs+ and K+ Levels in Arabidopsis AtKUP7 Mutants. Plants. 2020; 9(11):1525. https://doi.org/10.3390/plants9111525
Chicago/Turabian StyleŠustr, Marek, Tereza Doksanská, Barbora Doležalová, Aleš Soukup, and Edita Tylová. 2020. "134Cs Uptake and Growth at Various Cs+ and K+ Levels in Arabidopsis AtKUP7 Mutants" Plants 9, no. 11: 1525. https://doi.org/10.3390/plants9111525
APA StyleŠustr, M., Doksanská, T., Doležalová, B., Soukup, A., & Tylová, E. (2020). 134Cs Uptake and Growth at Various Cs+ and K+ Levels in Arabidopsis AtKUP7 Mutants. Plants, 9(11), 1525. https://doi.org/10.3390/plants9111525




