Enhancing Conservation of a Globally Imperiled Rockland Herb (Linum arenicola) through Assessments of Seed Functional Traits and Multi-Dimensional Germination Niche Breadths
Abstract
1. Introduction
2. Results
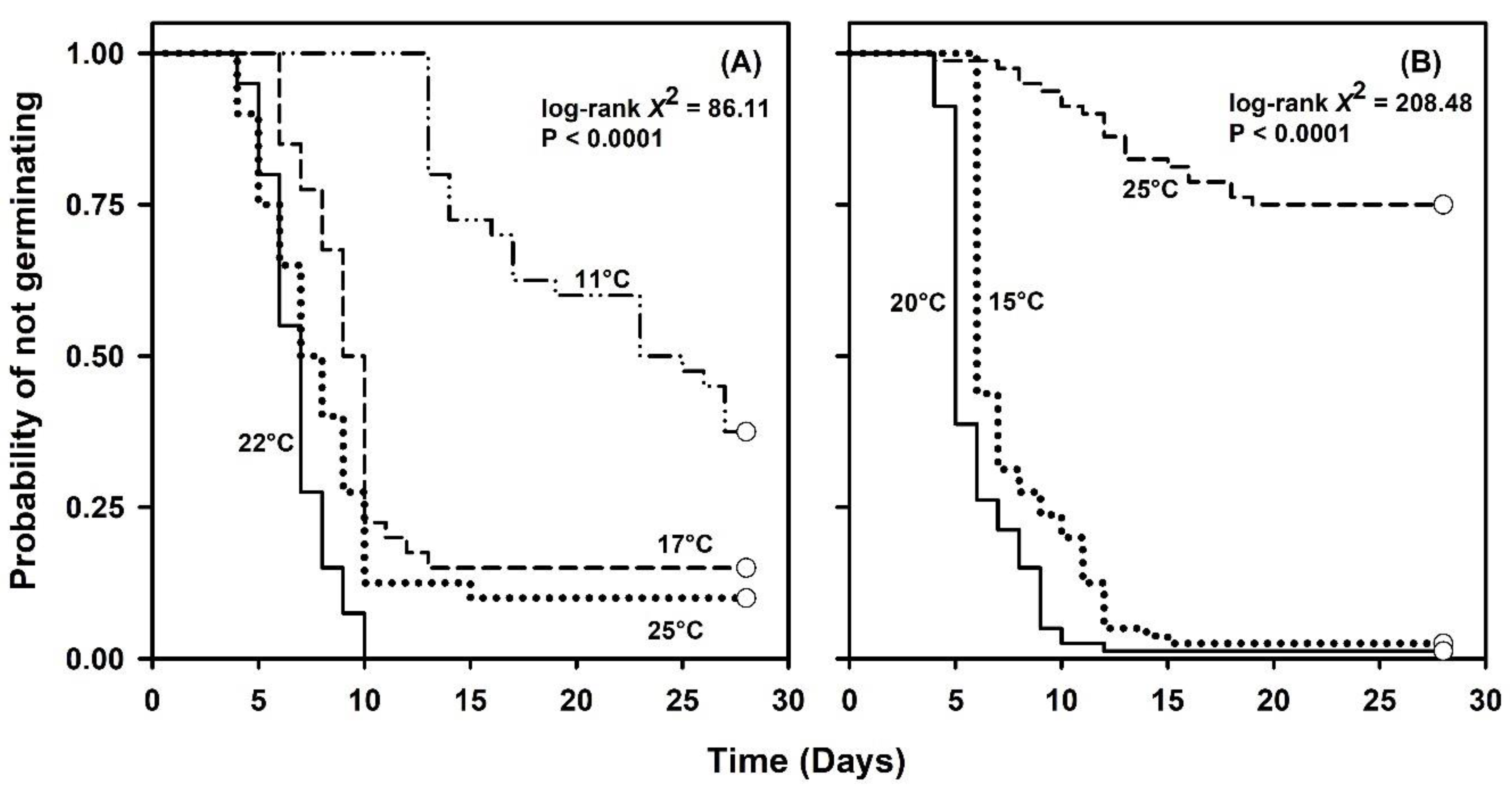
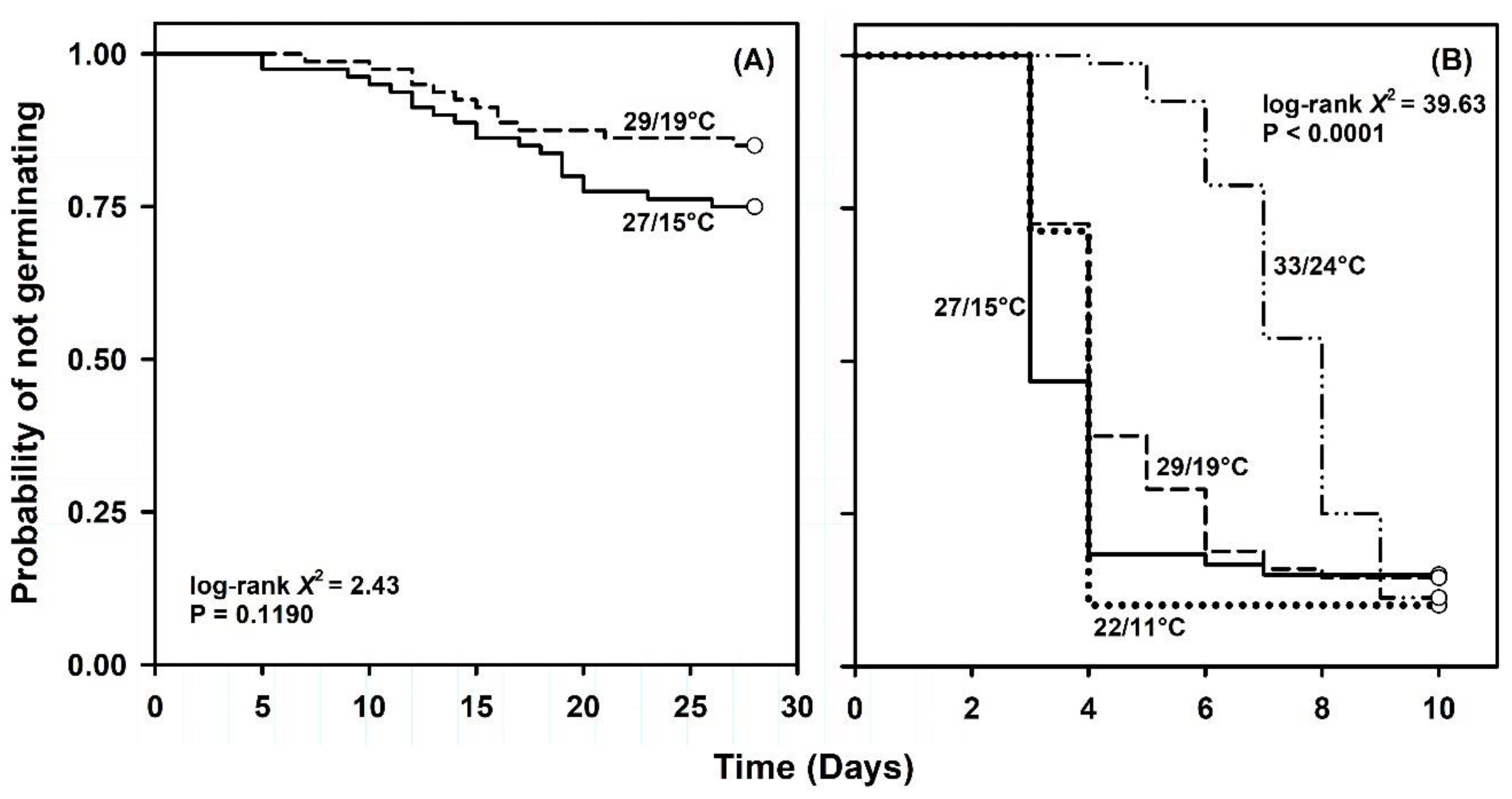
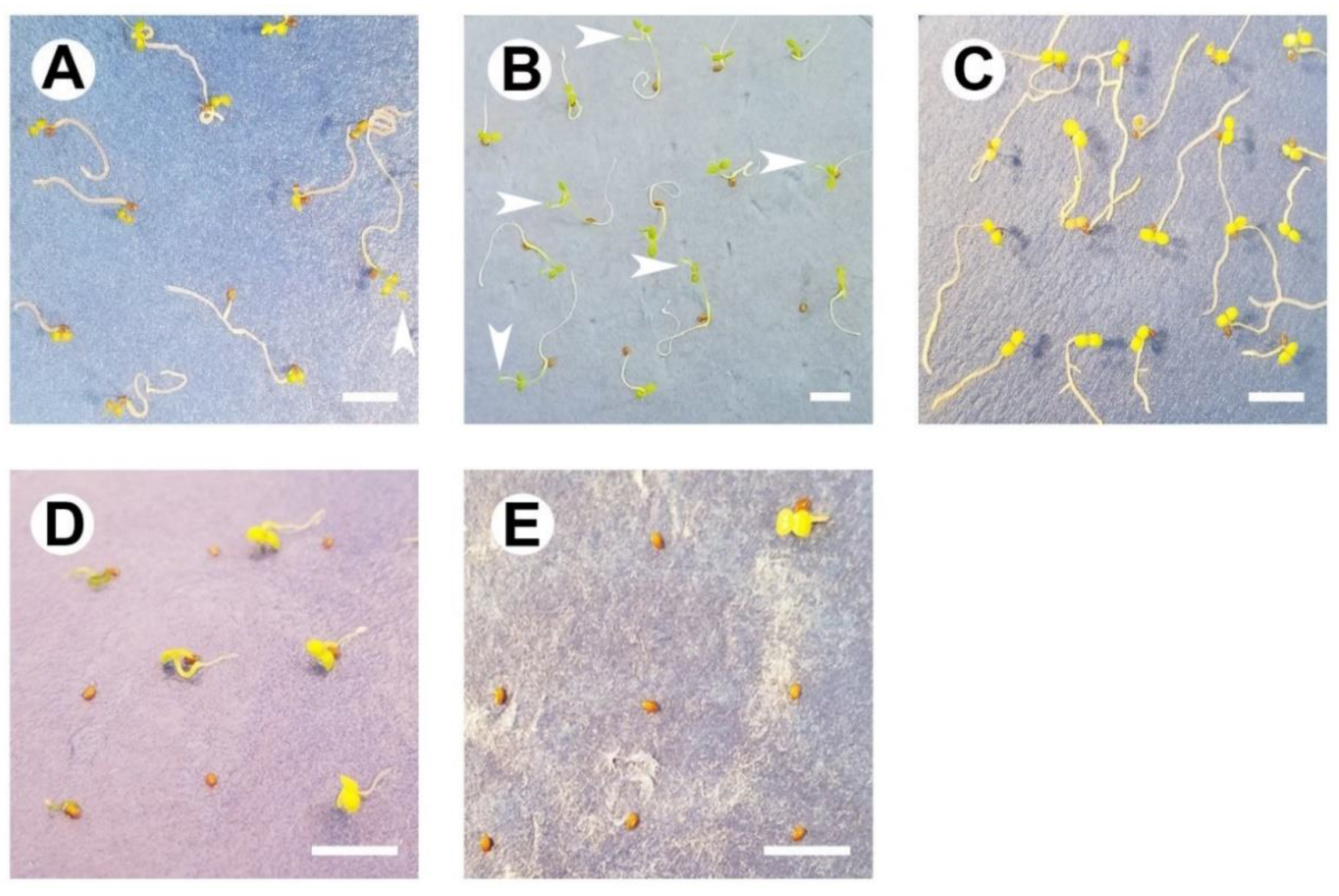
2.1. Germination Capacity under Constant or Simulated Seasonal Temperatures
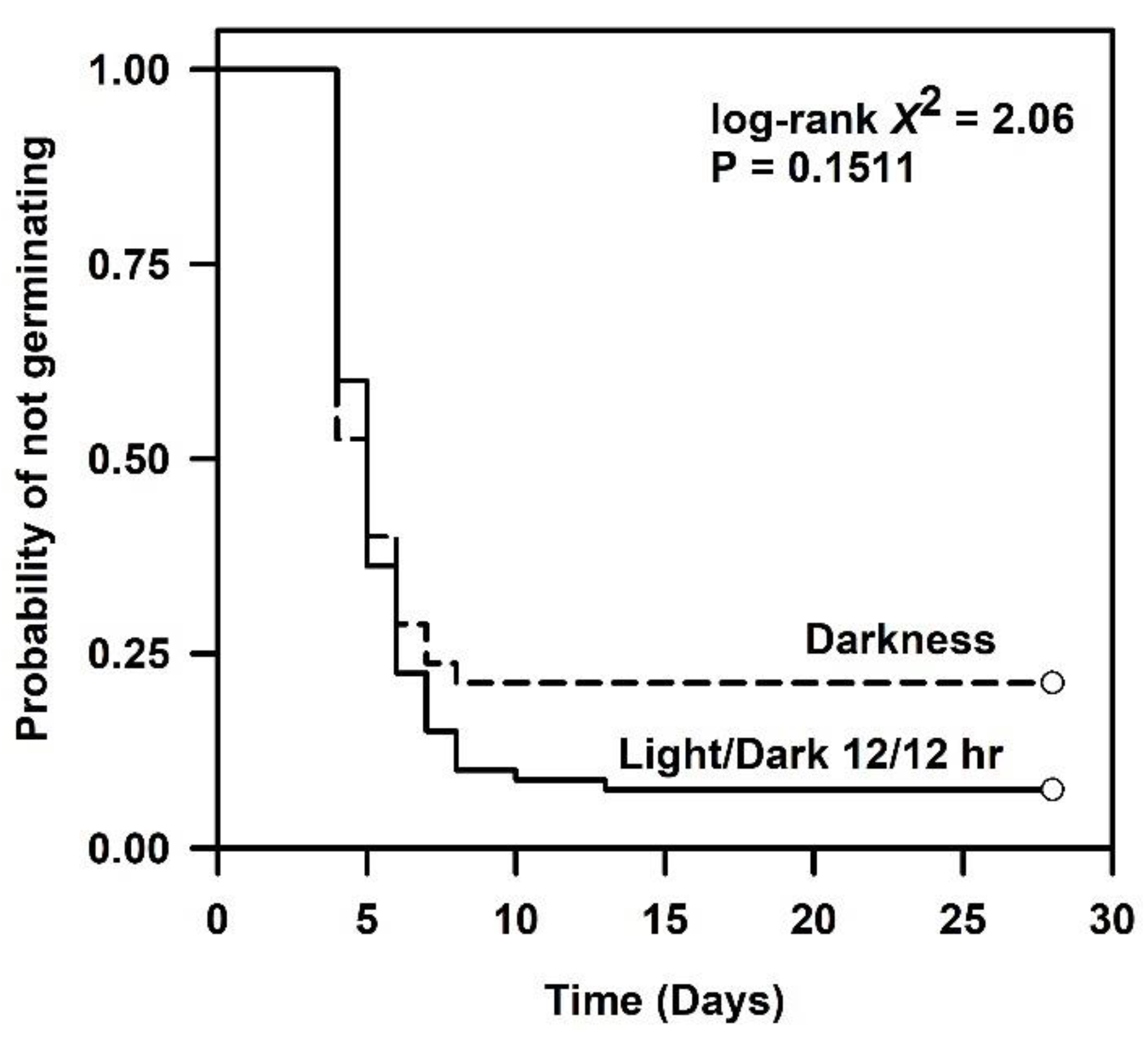
2.2. Germination Ability in a Diurnal Photoperiod or Darkness
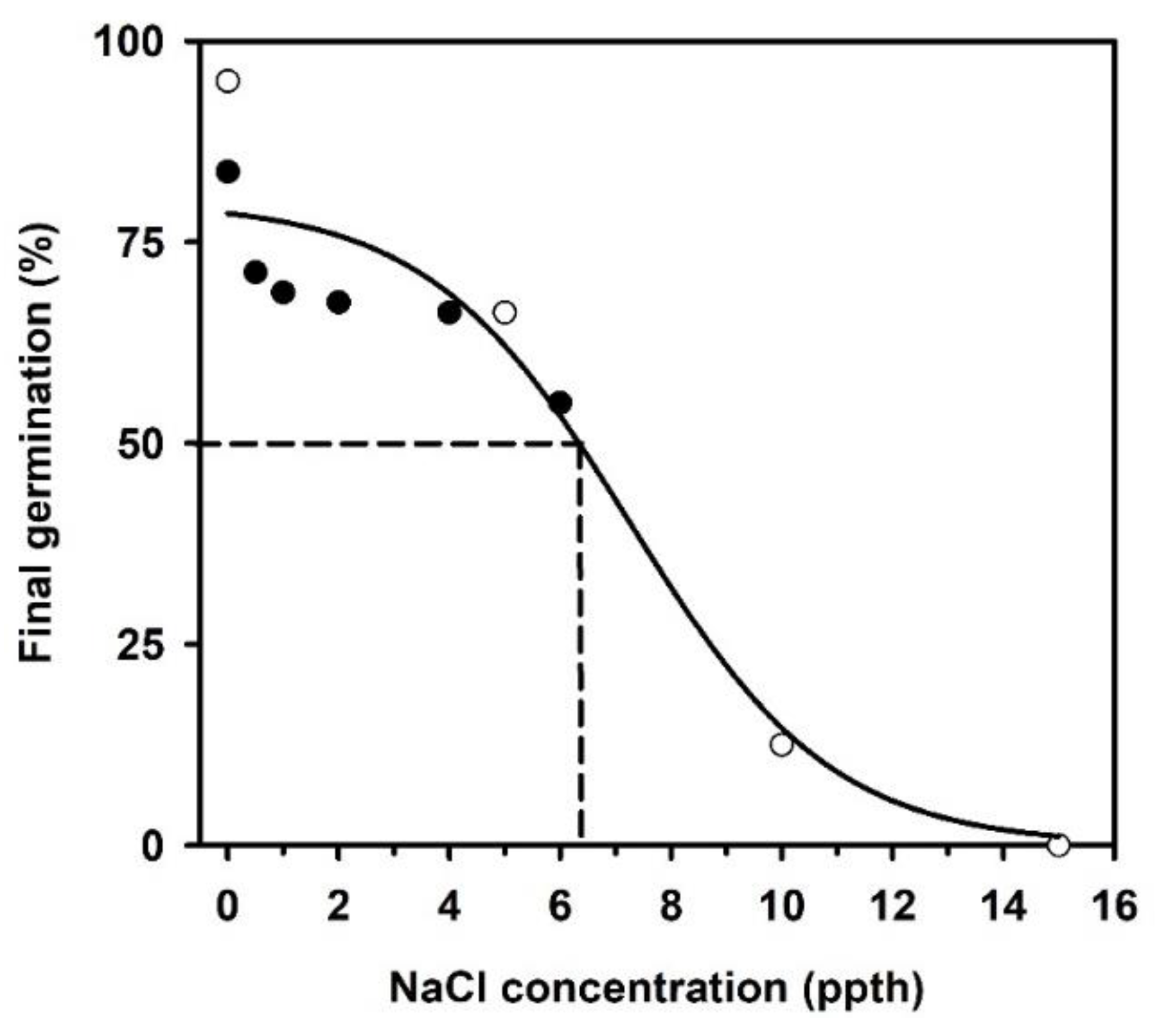
2.3. Germination Following a Salinity Concentration Challenge
2.4. Germination Niche Breadths
3. Discussion
3.1. Seed Functional Traits
3.2. Multi-Dimensional Germination Niche Breadth
4. Materials and Methods
4.1. Fruit Collection, Seed Handling, and General Methods
4.2. Assessing Germination Capacity under Constant or Simulated Seasonal Temperatures
4.3. Evaluating Germination Ability with a Diurnal Photoperiod or Darkness
4.4. Measuring Germination Following a Salinity Challenge
4.5. Estimating Germination Niche Breadths
4.6. Data Analyses
5. Conclusions and Implications for Conservation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, ditribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Joppa, L.N.; Roberts, D.L.; Pimm, S.L. How many species of flowering plants are there? Proc. R. Soc. B 2010, 278, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Lughadha, E.N.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Endangered Species Act of 1973. In 16 U.S.C. 1531–1544; United States of America: Washington, DC, USA, 1973.
- Business Case and Plan Botanic Gardens Conservation International 2015–2020. Available online: https://www.bgci.org/resources/bgci-tools-and-resources/bgci-business-case-and-plan/ (accessed on 10 July 2020).
- The IUCN Red List of Threatened Species. Version 2020-2. Available online: https://www.iucnredlist.org (accessed on 9 July 2020).
- Strategic Plan for Biodiversity 2011–2020, Including Aichi Biodiversity Targets. Available online: https://www.cbd.int/sp/ (accessed on 10 July 2020).
- Boersma, P.D.; Kareiva, P.; Fagan, W.F.; Clark, A.; Hoekstra, J.M. How good are endangered species recovery plans? BioScience 2011, 51, 643–649. [Google Scholar] [CrossRef]
- Clark, J.A.; Hoekstra, J.M.; Boersma, P.D.; Kareiva, P. Improving U.S. Endangeres Species Act recovery plans: Key findings and recommendations of the SCB recovery plan project. Conserv. Biol. 2002, 16, 1510–1519. [Google Scholar] [CrossRef]
- Wyse, S.V.; Dickie, J.B. Predicting the global incidence of seed desiccation sensitivity. J. Ecol. 2017, 105, 1082–1093. [Google Scholar] [CrossRef]
- Falk, D.A.I.; Millar, C.I.; Olwell, M. (Eds.) Restoring Diversity: Strategies for Reintroduction of Endangered Plants; Island Press: Washington, DC, USA, 1996. [Google Scholar]
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J.; et al. International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 2019, 27, S1–S46. [Google Scholar] [CrossRef]
- Raven, P.H. Ex Situ Plant Conservation: Supporting Species Survival in the Wild; Guerrant, E.O., Havens, K., Maunder, M., Eds.; Island Press: Washington, DC, USA, 2004; p. 504. [Google Scholar]
- Thompson, K. The functional ecology of soil seed banks. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: New York, NY, USA, 2000; pp. 215–235. [Google Scholar]
- Jimenez-Alfaro, B.; Silveira, F.A.O.; Fidelis, A.; Poschold, P.; Commander, L. Seed germination traits can contribute better to plant community ecology. J. Veg. Sci. 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Saatkamp, A.; Cochrane, A.; Commander, L.; Guja, L.K.; Jimenez-Alfaro, B.; Larson, J.; Nicotra, A.; Poschold, P.; Silveira, F.A.O.; Cross, A.T.; et al. A research agenda for seed-trait functional ecology. New Phytol. 2019, 221, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Grubb, P.J. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Sexton, J.P.; Montiel, J.; Shay, J.E.; Stephens, M.R.; Slatyer, R.A. Evolution of ecological niche breadth. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 183–206. [Google Scholar] [CrossRef]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments; Princeton University Press: Princeton, NJ, USA, 1968; p. 134. [Google Scholar]
- Rao, P.B.; Singh, S.P. Response breadths on environmental gradients of germination and seedling growth in two dominant forest tree species of central Himalaya. Ann. Bot. 1985, 56, 783–794. [Google Scholar]
- Bandara, R.G.; Finch, J.; Walck, J.L.; Hidayati, S.N.; Havens, K. Germination niche breadth and potential response to climate change differ among three North American perennials. Folia Geobot. 2019, 54, 5–17. [Google Scholar] [CrossRef]
- Finch, J.; Walck, J.L.; Hidayati, S.N.; Kramer, A.T.; Lason, V.; Havens, K. Germination niche breadth varies inconsistently among three Asclepias congeners along a latitudinal gradient. Plant Biol. 2019, 21, 425–438. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Ecology, Biogepgraphy, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Batlla, D.; Benech-Arnold, R.L. Weed seed germination and the light environment: Implications for weed management. Weed Biol. Manag. 2014, 14, 77–87. [Google Scholar] [CrossRef]
- Bradford, K.J. Applications of hydrothermal time to quantifying and modelling seed germination and dormancy. Weed Sci. 2002, 50, 248–260. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Footitt, S. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. J. Exp. Bot. 2017, 68, 843–856. [Google Scholar] [CrossRef]
- Nanda, A.K.; El Habti, A.; Hocart, C.H.; Masle, J. ERECTA receptor-kinases play a key role in the appropriate timing of seed germination under changing salinity. J. Exp. Bot. 2019, 70, 6417–6435. [Google Scholar] [CrossRef]
- Saatkamp, A.; Affre, L.; Dumas, P.J.; Gasmi, A.; Gachet, S.; Arene, F. Soil depth detection by seeds and diurnally fluctuating temperatures: Different dynamics in 10 annual plants. Plant Soil 2011, 349, 331–340. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Broadhurst, L.; Coates, D. Plant conservation in Australia: Current directions and future challenges. Plant Divers. 2017, 39, 348–356. [Google Scholar] [CrossRef]
- Endangered and Threatened Wildlife and Plants: Endangered Species Status for Chamaecrista lineata var. keyensis (Big Pine Partridge Pea), Chamaesyce deltoidea ssp. serphyllum (Wedge Spruge), and Linum arenicola (Sand Flax), Threatened Status for Argythamnia blodgettii (Blodgett’s Silverbush). Available online: https://www.federalregister.gov/documents/2016/09/29/2016-23546/endangered-and-threatened-wildlife-and-plants-endangered-species-status-for-chamaecrista-lineata-var (accessed on 3 March 2017).
- Chumana, L.A.O. Germination Response of Linum arenicola Seeds to Various Abiotic Factors: Implications for Conservation. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2019. [Google Scholar]
- Maschinski, J.; Ross, M.S.; Liu, H.; O’Brien, J.; von Wettberg, E.J.; Haskins, K.E. Sinking ships: Conservation options for endemic taxa threatened by sea level rise. Clim. Chang. 2011, 107, 147–167. [Google Scholar] [CrossRef]
- Linum arenicola: Sand Flax. Available online: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.152712/Linum_arenicola (accessed on 2 August 2020).
- South Florida Multi-Species Recovery Plan—Ecological Communities: Pine Rocklands. Available online: https://www.fws.gov/verobeach/MSRPPDFs/Pinerock.pdf (accessed on 12 July 2020).
- Snyder, J.R.; Herndon, A.; Robertson, W.B. South Florida Rockland. In Ecosystems of Florida; Myers, R.L., Ewel, J.J., Eds.; University Press of Central Florida: Orlando, FL, USA, 1990; pp. 230–277. [Google Scholar]
- Gordon, D.R. Effects of invasive, non-indigenous plant species on ecosystem processes: Lessons from Florida. Ecol. Appl. 1998, 8, 975–989. [Google Scholar] [CrossRef]
- Prinos, S.T. Map of the Approximate Inland Extent of Saltwater at the Base of the Biscayne Aquifer in the Model Land Area of Miami-Dade County, Florida, 2016. Available online: https://pubs.er.usgs.gov/publication/sim3380 (accessed on 16 May 2020).
- Hefty, L.N. Model Lands Hydrology. Available online: https://www.nrc.gov/docs/ML1907/ML19077A088.pdf (accessed on 17 June 2020).
- Ross, M.S.; O’Brien, J.; Sternberg, L. Sea-level rise and the reduction in pine forests in the Florida Keys. Ecol. Appl. 1994, 4, 144–156. [Google Scholar] [CrossRef]
- Bradley, K.; Saha, S. Post-Hurricane Responses of Rare Plant Species and Vegetation of Pine Rocklands in the Lower Florida Keys. Available online: https://www.regionalconservation.org/ircs/aboutus/Reports_all.asp (accessed on 23 June 2020).
- Kurt, O.; Bozkurt, D. Effect of temperature and photoperiod on seedling emergence of Flax (Linum usitatissimum L.). J. Agron. 2006, 5, 541–545. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Breaking seed dormancy during dry storage: A useful tool or major problem for successful restoration via direct seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Bazin, J.; Batlla, D.; Dussert, S.; El-Maarouf-Bouteau, H.; Bailly, C. Role of relative humidity, temperature, and water status in dormancy alleviation of sunflower seeds during dry after-ripening. J. Exp. Bot. 2011, 62, 627–640. [Google Scholar] [CrossRef]
- Comparative Climatic Data. Available online: https://www1.ncdc.noaa.gov/pub/data/ccd-data/relhum18.dat (accessed on 7 July 2020).
- Pérez, H.E.; Kane, M.E. Different plant provenance same seed tolerance to abiotic stress: Implications for ex situ germplasm conservation of a widely distributed coastal dune grass (Uniola paniculata L.). Plant Growth Regul. 2017, 82, 123–137. [Google Scholar]
- DataTools: 1981–2010 Normals. Available online: https://www.ncdc.noaa.gov/cdo-web/datatools/normals (accessed on 9 July 2020).
- Abiy, A.Z.; Melesse, A.M.; Abtew, W.; Whitman, D. Rainfall trend and variability in Southeast Florida: Implications for freshwater availability in the Everglades. PLoS ONE 2019, 14, e0212008. [Google Scholar] [CrossRef]
- Hopkins, W.G. Introduction to Plant Physiology; John Wiley and Sons: New York, NY, USA, 1995; p. 464. [Google Scholar]
- Kettner, K.; Pérez, H.E. Dose-response of germinating Rudbeckia mollis (Asteraceae) seeds exposed to various thermal scenarios. Seed Sci. Res. 2012, 22, 191–197. [Google Scholar] [CrossRef]
- Pérez, H.E.; Kettner, K. Characterizing Ipomopsis rubra (Polemoniaceae) germination under various thermal scenarios with non-parametric and semi-parametric statistical methods. Planta 2013, 238, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Côme, D.; Corbineau, F. Germination—influences of temperature. In The Encyclopedia of Seeds: Science, Technology and Uses; Black, M., Bewley, J.D., Halmer, P., Eds.; CABI: Wallingford, UK, 2006; pp. 271–274. [Google Scholar]
- Callaway, R.M. Positive interactions among plants. Bot. Rev. 1995, 61, 306–349. [Google Scholar] [CrossRef]
- Munguía-Rosas, M.A.; Sosa, V.J. Nurse plants vs. nurse objects: Effects of woody plants and rocky cavities on the recruitment of the Pilosocereus leucocephalus columnar cactus. Ann. Bot. 2008, 101, 175–185. [Google Scholar]
- Peters, E.M.; Martorell, C.; Ezcurra, E. Nurse rocks are more important than nurse plants in determining the distribution and establishment of globose cacti (Mammillaria) in the Tehuacán Valley, Mexico. J. Arid Environ. 2008, 72, 593–601. [Google Scholar] [CrossRef]
- Liu, S.; Bradford, K.J.; Huang, Z.; Venable, D.L. Hydrothermal sensitivities of seed populations underlie fluctuations of dormancy states in an annual plant community. Ecology 2020, 101, e02958. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Berriri, S.; Basso, D.P.; Ly-Vu, B.; Dang, T.T.; Lalanne, D.; da Silva, E.A.A. The seed-specific heat shock factor A9 regulates the depth of dormancy in Medicago truncatula seeds via ABA signalling. Plant Cell Environ. 2020, 43, 2508–2522. [Google Scholar] [CrossRef]
- Huo, H.; Bradford, K. Molecular and hormonal regulation of thermoinhibition of seed germination. In Advances in Plant Dormancy; Anderson, J., Ed.; Springer: Cham, Switzerland, 2015; pp. 3–33. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Sea Level Rise Task Force: Report on Flooding and Salt Water Intrusion. Available online: https://www.miamidade.gov/green/library/sea-level-rise-flooding-saltwater-intrusion.pdf (accessed on 20 July 2020).
- Wendelberger, K.S. Evaluating Plant Community Response to Sea Level Rise and Anthropogenic Drying: Can Life Stage and Competitive Ability Be Used as Indicators in Guiding Conservation Activities? Ph.D. Thesis, Florida International University, Miami, FL, USA, 2016. [Google Scholar]
- Moghaddam, M.; Babaei, K.; Pooya, E.S. Germination and growth response of flax (Linum usitatissimum) to salinity stress by different salt types and concentrations. J. Plant Nutr. 2018, 41, 563–573. [Google Scholar] [CrossRef]
- Donohue, K. Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology 2002, 83, 1006–1016. [Google Scholar] [CrossRef]
- Climate Change Indicators: U.S. and Global Temperatures. Available online: https://www.epa.gov/climate-indicators/climate-change-indicators-us-and-global-temperature (accessed on 18 August 2020).
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013: The Physical Science Basis. Contribution of Workign Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136. [Google Scholar]
- Schoenfeld, D.A.; Richter, J.R. Nomograms for calculating the number of patients needed for clinical trial with survival as an endpoint. Biometrics 1982, 38, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 12, 1373–1379. [Google Scholar] [CrossRef]
- Feinsinger, P.; Spears, E.E.; Poole, R.W. A simple measure of niche breadth. Ecology 1981, 62, 27–32. [Google Scholar] [CrossRef]
- McNair, J.N.; Sunkara, A.; Frobish, D. How to analyse seed germination data using statistical time-to-event analysis: Non-parametric and semi-parametric methods. Seed Sci. Res. 2012, 22, 77–95. [Google Scholar] [CrossRef]
- Scott, S.J.; Jones, R.A.; Williams, W.A. Review of data analysis methods for seed germination. Crop Sci. 1984, 24, 1192–1199. [Google Scholar] [CrossRef]
- Allison, P.D. Survival Analysis Using SAS®: A Practical Guide; SAS Institute: Cary, NC, USA, 2010; p. 324. [Google Scholar]

| Treatment | n | Final Germination (%) | Percentile Estimates (95% CI) | Germination Rate (1/t50) | U | ||
|---|---|---|---|---|---|---|---|
| t25 | t50 | t75 | |||||
| Constant temperature (°C) | |||||||
| 2017 (fresh) | |||||||
| 6 | 40 | 0.0 | - | - | - | - | - |
| 11 | 40 | 0.0 | - | - | - | - | - |
| 17 | 40 | 0.0 | - | - | - | - | - |
| 22 | 40 | 0.0 | - | - | - | - | - |
| 25 | 40 | 0.0 | - | - | - | - | - |
| 31 | 40 | 0.0 | - | - | - | - | - |
| 36 | 40 | 0.0 | - | - | - | - | - |
| 40 | 40 | 0.0 | - | - | - | - | - |
| 2017 (post-storage) | |||||||
| 6 | 40 | 0.0 | - | - | - | - | - |
| 11 | 40 | 62.5 | 14.0 (13, 19) | 24.0 (17, -) x | - y | 0.04 | - |
| 17 | 40 | 85.0 | 8.0 (6, 9) | 9.5 (9, 10) | 10.0 (10, -) | 0.10 | 2.0 |
| 22 | 40 | 100.0 | 6.0 (5, 6) | 7.0 (6, 7) | 8.0 (7, 9) | 0.14 | 2.0 |
| 25 | 40 | 90.0 | 5.5 (5, 7) | 7.5 (6, 9) | 10.0 (8, 10) | 0.10 | 4.5 |
| 31 | 40 | 0.0 | - | - | - | - | - |
| 36 | 40 | 0.0 | - | - | - | - | - |
| 40 | 40 | 0.0 | - | - | - | - | - |
| 2018 (post-storage) | |||||||
| 10 | 80 | 0.0 | - | - | - | - | - |
| 15 | 80 | 97.5 | 6.0 (-, -) | 6.0 (6, 7) | 9.0 (7, 11) | 0.16 | 3.0 |
| 20 | 80 | 98.7 | 5.0 (-, -) | 5.0 (-, -) | 7.0 (6, 8) | 0.20 | 2.0 |
| 25 | 80 | 25.0 | - | - | - | - | - |
| 30 | 80 | 0.0 | - | - | - | - | - |
| 35 | 80 | 0.0 | - | - | - | - | - |
| Simulated seasonal temperatures z (°C) | |||||||
| Prior to transfer to 22 °C | |||||||
| 33/24 (summer) | 80 | 0.0 | - | - | - | - | - |
| 29/19 (early fall or late spring) | 80 | 15.0 | - | - | - | - | - |
| 27/15 (late fall or early spring) | 80 | 25.0 | - | - | - | - | - |
| 22/11 (winter) | 80 | 0.0 | - | - | - | - | - |
| Following transfer to 22 °C | |||||||
| 33/24 (summer) | 80 | 88.8 | 7 (6, 7) | 8 (7, 8) | 8 (8, 9) | 0.12 | 1 |
| 29/19 (early fall or late spring) | 68 | 86.8 | 3 (3, 4) | 4 (-, -) | 6 (5, 8) | 0.25 | 3 |
| 27/15 (late fall or early spring) | 60 | 85.0 | 3 (-, -) | 3 (3, 4) | 4 (4, -) | 0.33 | 1 |
| 22/11 (winter) | 80 | 90.0 | 3 (3, 4) | 4 (-, -) | 4 (-, -) | 0.25 | 1 |
| Photoperiod z | |||||||
| Darkness | 80 | 78.60 | 4 (-, -) | 5 (4, 6) | 7 (6, -) | 0.20 | 3 |
| 12 h Photoperiod | 80 | 92.50 | 4 (-, -) | 5 (4, 5) | 6 (6, 7) | 0.20 | 2 |
| Salinity z (ppth NaCl) | |||||||
| 0 | 160 | 89.37 | 5.0 (4, 5) | 7.0 (6, 7) | 8.0 (8, 11) | 0.14 | 3.0 |
| 0.5 | 80 | 71.25 | 6.0 (5, 6) | 8.0 (6, 10) | - | 0.12 | - |
| 1 | 80 | 68.75 | 6.0 (-, -) | 7.0 (7, 9) | - | 0.14 | - |
| 2 | 80 | 67.50 | 7.0 (6, 7) | 9.0 (7, 14) | - | 0.11 | - |
| 4 | 80 | 66.25 | 11.0 (9, 12) | 14.5 (13, 17) | - | 0.07 | - |
| 5 | 80 | 66.25 | 14.5 (12, 17) | 19 (18, 21) | - | 0.05 | - |
| 6 | 80 | 55.00 | 13.0 (12, 17) | 22 (17, -) | - | 0.04 | - |
| 10 | 80 | 12.50 | - | - | - | - | - |
| 15 | 80 | 0.0 | - | - | - | - | - |
| 20 | 80 | 0.0 | - | - | - | - | - |
| 25 | 80 | 0.0 | - | - | - | - | - |
| 30 | 80 | 0.0 | - | - | - | - | - |
| 35 | 80 | 0.0 | - | - | - | - | - |
| 40 | 80 | 0.0 | - | - | - | - | - |
| 45 | 80 | 0.0 | - | - | - | - | - |
| Experiment | Model Covariates | Coefficient β | SE of βi | Wald χ2 | p | Hazard Ratio, e(βi) |
|---|---|---|---|---|---|---|
| 2017 harvest (constant 11, 17, 22, 25 °C) | ||||||
| Temperature | 0.412 | 0.068 | 36.688 | <0.0001 | 1.510 | |
| Temperature × day | −0.029 | 0.007 | 17.603 | <0.0001 | 0.971 | |
| 2018 harvest (constant 15, 20, 25 °C) | ||||||
| Temperature | −0.174 | 0.019 | 86.811 | <0.001 | 0.840 | |
| Simulated seasonal temperatures (29/19 and 27/15 °C) | ||||||
| Temperature | −0.281 | 0.183 | 2.369 | 0.1238 | 0.755 | |
| Simulated seasonal temperatures followed by 22 °C (33/24, 29/19, 27/15, 22/11 → 22 °C) | ||||||
| Temperature | −0.546 | 0.060 | 82.460 | <0.0001 | 0.579 | |
| Temperature × day | 0.101 | 0.014 | 54.451 | <0.0001 | 1.106 | |
| Salinity (0, 0.5, 1, 2, 4, 5, 6, 10 ppth NaCl) | ||||||
| Salinity | −0.25 | 0.019 | 174.279 | <0.0001 | 0.778 | |
| Salinity × day | 0.045 | 0.004 | 128.117 | <0.0001 | 1.047 |
| Comparison | df | Wald χ2 | p |
|---|---|---|---|
| 2017 (constant 11, 17, 22, 25 °C) | |||
| 11 vs. 17 | 1 | 18.591 | <0.0001 |
| 11 vs. 22 | 1 | 74.133 | <0.0001 |
| 11 vs. 25 | 1 | 36.859 | <0.0001 |
| 17 vs. 22 | 1 | 29.921 | <0.0001 |
| 17 vs. 25 | 1 | 3.687 | 0.0548 |
| 22 vs. 25 | 1 | 14.147 | 0.0002 |
| 2018 (constant 15, 20, 25 °C) | |||
| 15 vs. 20 | 1 | 21.000 | <0.0001 |
| 15 vs. 25 | 1 | 91.678 | <0.0001 |
| 20 vs. 25 | 1 | 145.054 | <0.0001 |
| Simulated seasonal temperatures followed by 22 °C (33/24, 29/19, 27/15, 22/11 → 22 °C) | |||
| 22/11 vs. 27/15 | 1 | 0.180 | 0.6712 |
| 22/11 vs. 29/19 | 1 | 4.357 | 0.0368 |
| 22/11 vs. 33/24 | 1 | 34.920 | <0.0001 |
| 27/15 vs. 29/19 | 1 | 2.316 | 0.1281 |
| 27/15 vs. 33/24 | 1 | 25.780 | <0.0001 |
| 29/19 vs. 33/24 | 1 | 13.514 | 0.0002 |
| Salinity (ppth NaCl) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 4 | 5 | 6 | 10 | |
| 0 | - | 2.0 (<0.0001) | 2.1 (<0.0001) | 2.6 (<0.0001) | 3.4 (<0.0001) | 3.9 (<0.0001) | 4.8 (<0.0001) | 25.5 (<0.0001) |
| 0.5 | - | 1.0 (0.8284) | 1.3 (0.2100) | 1.7 (0.0067) | 1.9 (0.0007) | 2.4 (<0.0001) | 12.6 (<0.0001) | |
| 1 | - | 1.2 (0.3037) | 1.6 (0.0134) | 1.8 (0.0016) | 2.3 (<0.0001) | 12.1 (<0.0001) | ||
| 2 | - | 1.3 (0.1486) | 1.5 (0.0338) | 1.9 (0.0023) | 9.9 (<0.0001) | |||
| 4 | - | 1.1 (0.4982) | 1.4 (0.0944) | 7.5 (<0.0001) | ||||
| 5 | - | 1.2 (0.3041) | 6.6 (<0.0001) | |||||
| 6 | - | 5.3 (<0.0001) | ||||||
| 10 | - | |||||||
| Seed Collection Year | Environmental Gradient | 1/R | Bn | Scaled Z Bn |
|---|---|---|---|---|
| 2017 | Constant temperatures | 0.125 | - | - |
| After-ripening, constant temperatures | 0.111 | 0.433 | - | |
| 2018 | After-ripening, constant temperatures | 0.143 | 0.352 | 0.328 |
| Simulated seasonal temperatures | 0.250 | 0.471 | - | |
| Simulated seasonal temperatures, transfer to 22 °C | 0.200 | 0.800 | 0.813 | |
| Photoperiod | 0.500 | 0.993 | - | |
| Salinity | 0.067 | 0.480 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, H.E.; Chumana, L.A.O. Enhancing Conservation of a Globally Imperiled Rockland Herb (Linum arenicola) through Assessments of Seed Functional Traits and Multi-Dimensional Germination Niche Breadths. Plants 2020, 9, 1493. https://doi.org/10.3390/plants9111493
Pérez HE, Chumana LAO. Enhancing Conservation of a Globally Imperiled Rockland Herb (Linum arenicola) through Assessments of Seed Functional Traits and Multi-Dimensional Germination Niche Breadths. Plants. 2020; 9(11):1493. https://doi.org/10.3390/plants9111493
Chicago/Turabian StylePérez, Héctor Eduardo, and Luis Andres Ochoa Chumana. 2020. "Enhancing Conservation of a Globally Imperiled Rockland Herb (Linum arenicola) through Assessments of Seed Functional Traits and Multi-Dimensional Germination Niche Breadths" Plants 9, no. 11: 1493. https://doi.org/10.3390/plants9111493
APA StylePérez, H. E., & Chumana, L. A. O. (2020). Enhancing Conservation of a Globally Imperiled Rockland Herb (Linum arenicola) through Assessments of Seed Functional Traits and Multi-Dimensional Germination Niche Breadths. Plants, 9(11), 1493. https://doi.org/10.3390/plants9111493






