Foliar Application of 24-Epibrassinolide Improves Growth, Ascorbate-Glutathione Cycle, and Glyoxalase System in Brown Mustard (Brassica juncea (L.) Czern.) under Cadmium Toxicity
Abstract
1. Introduction
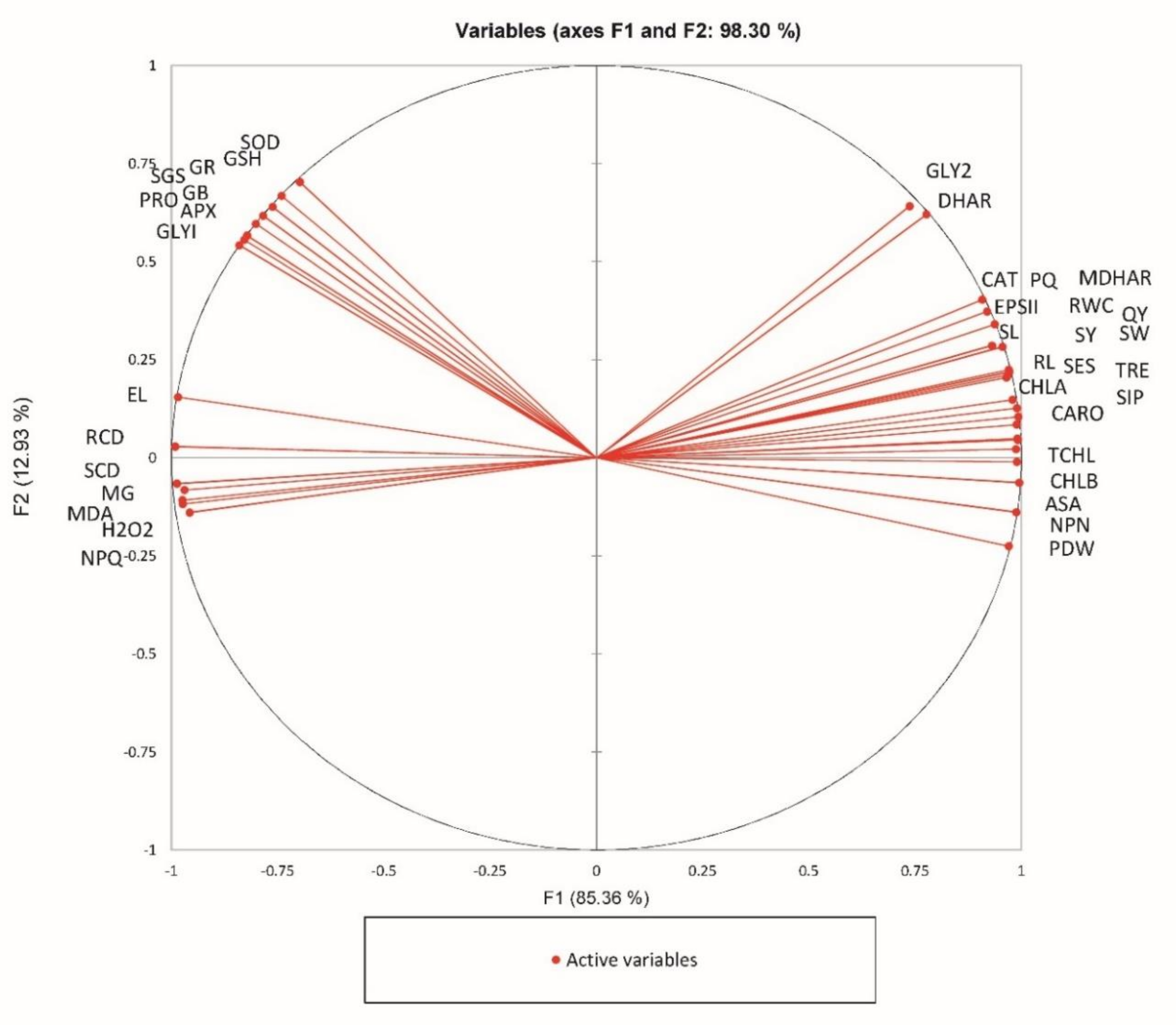
2. Results
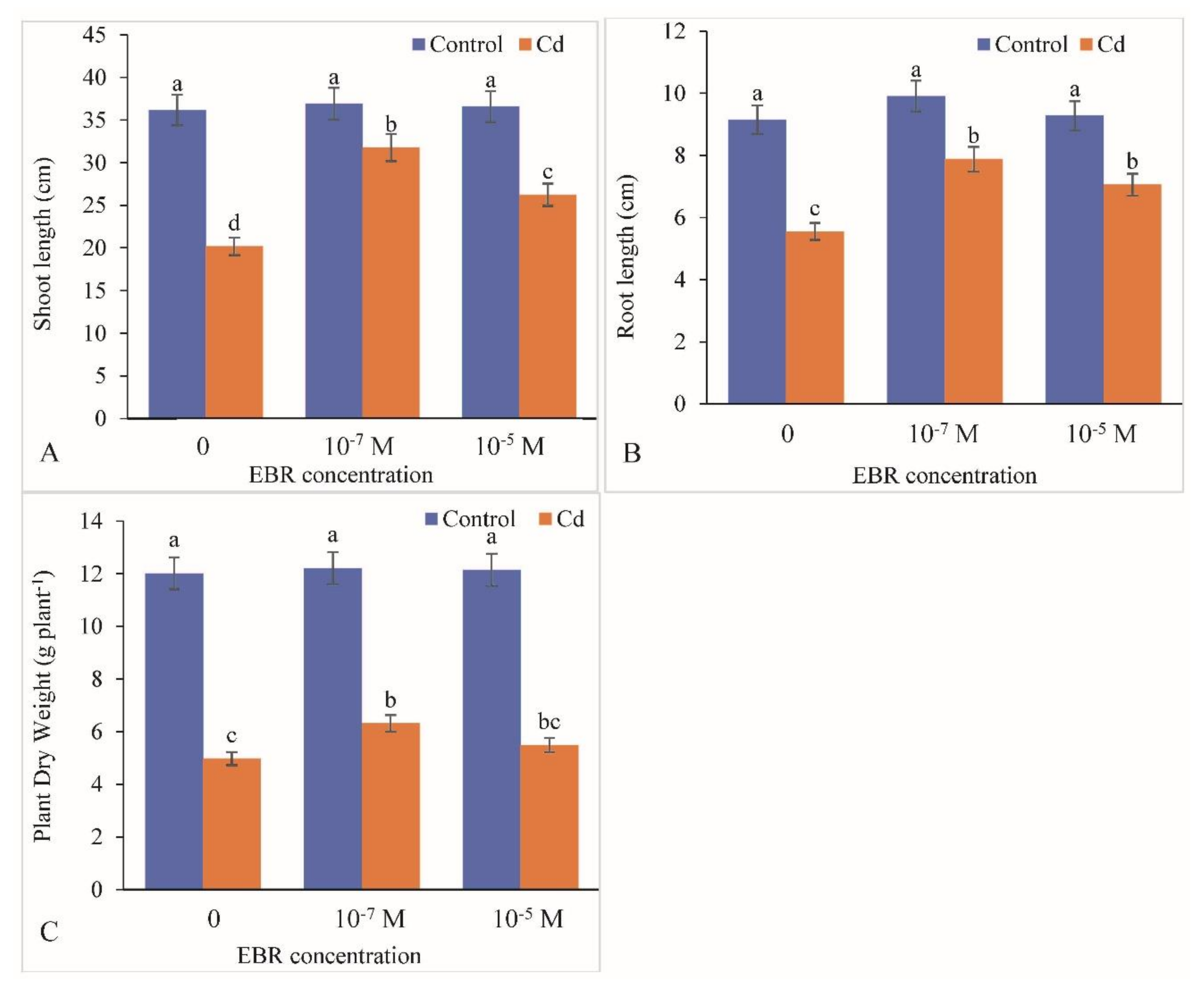
2.1. Improvement in Mustard Growth by 24-EBR Application
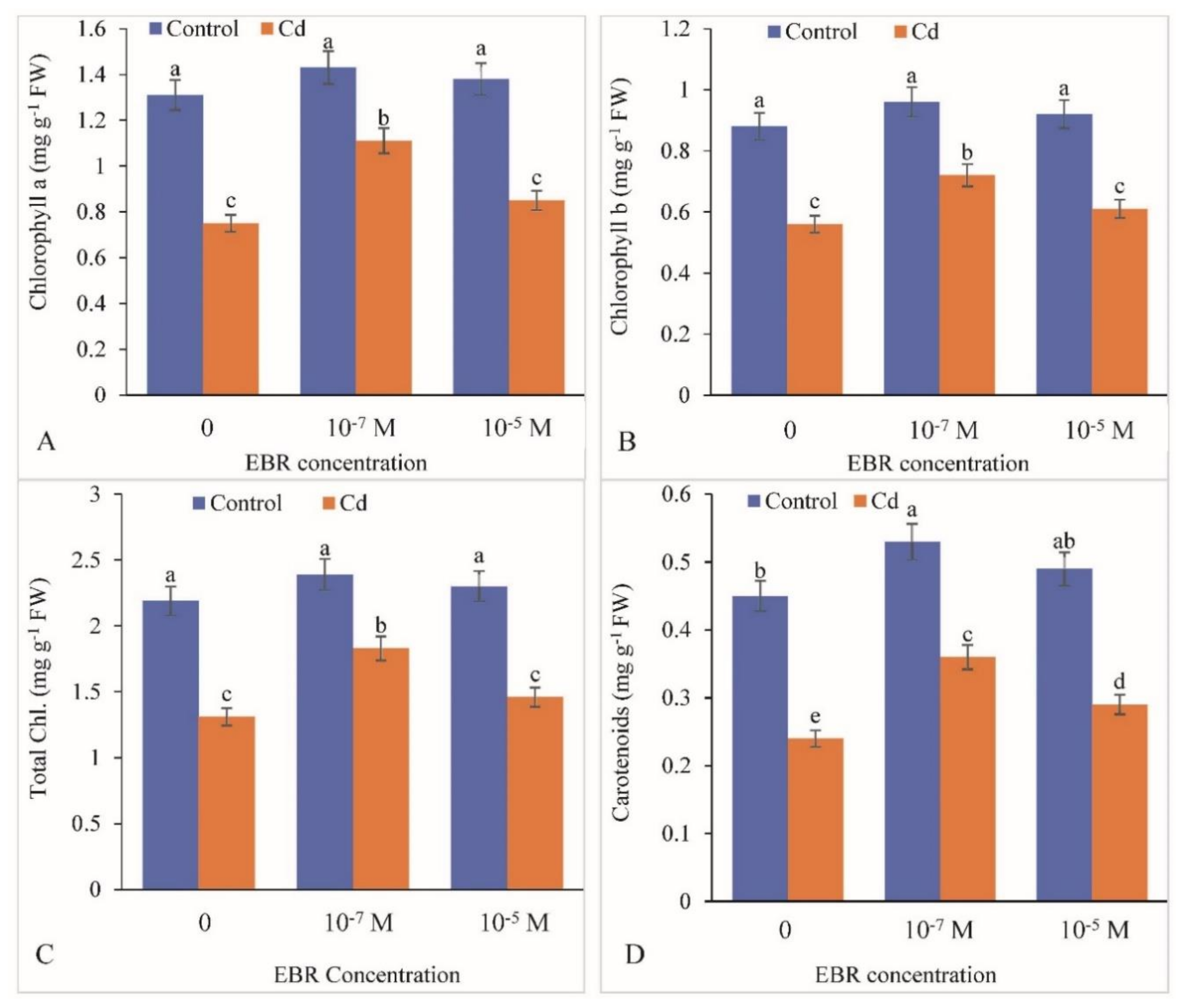
2.2. Enhancement in Chlorophyll and Carotenoid levels by 24-EBR Supplementation
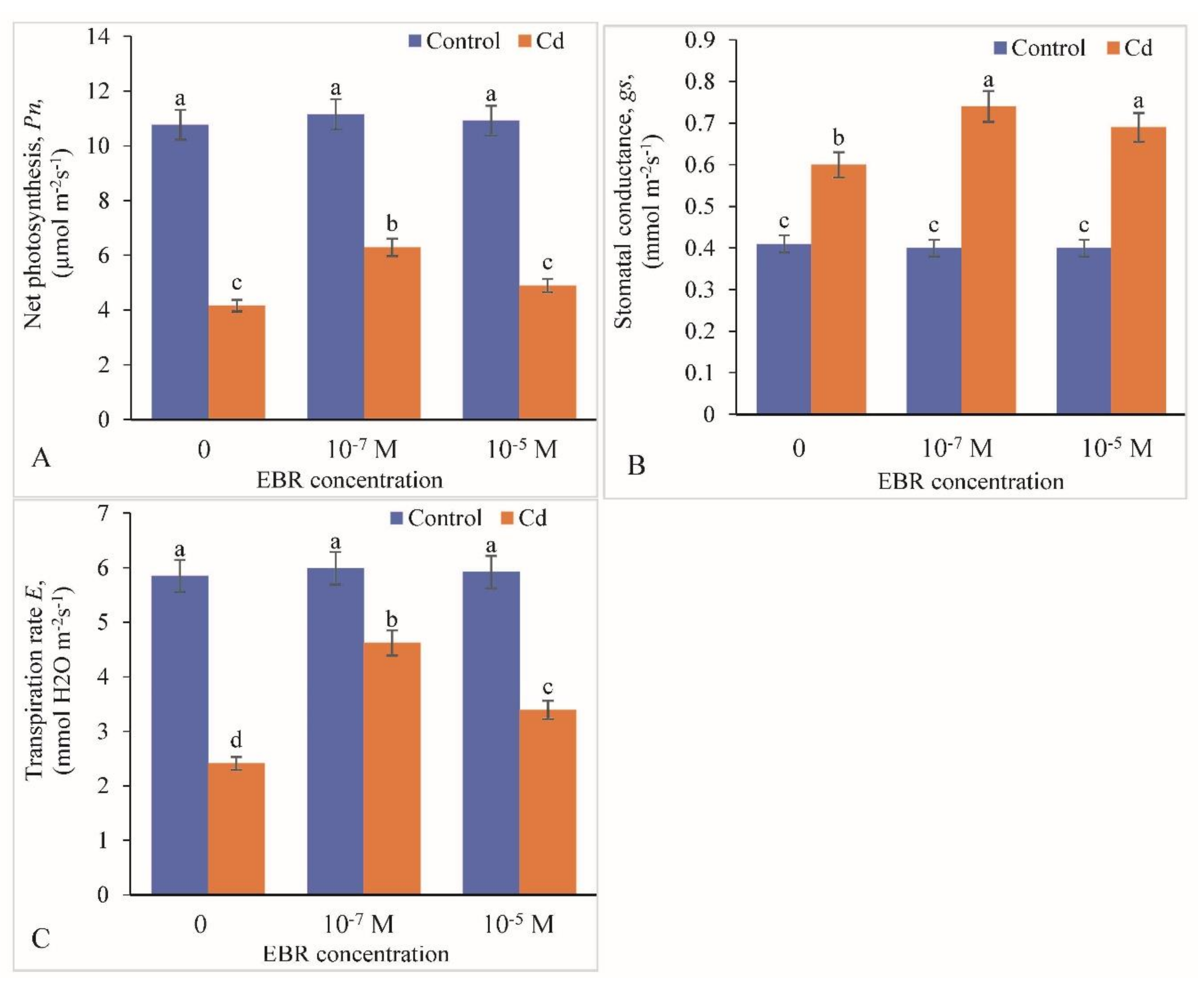
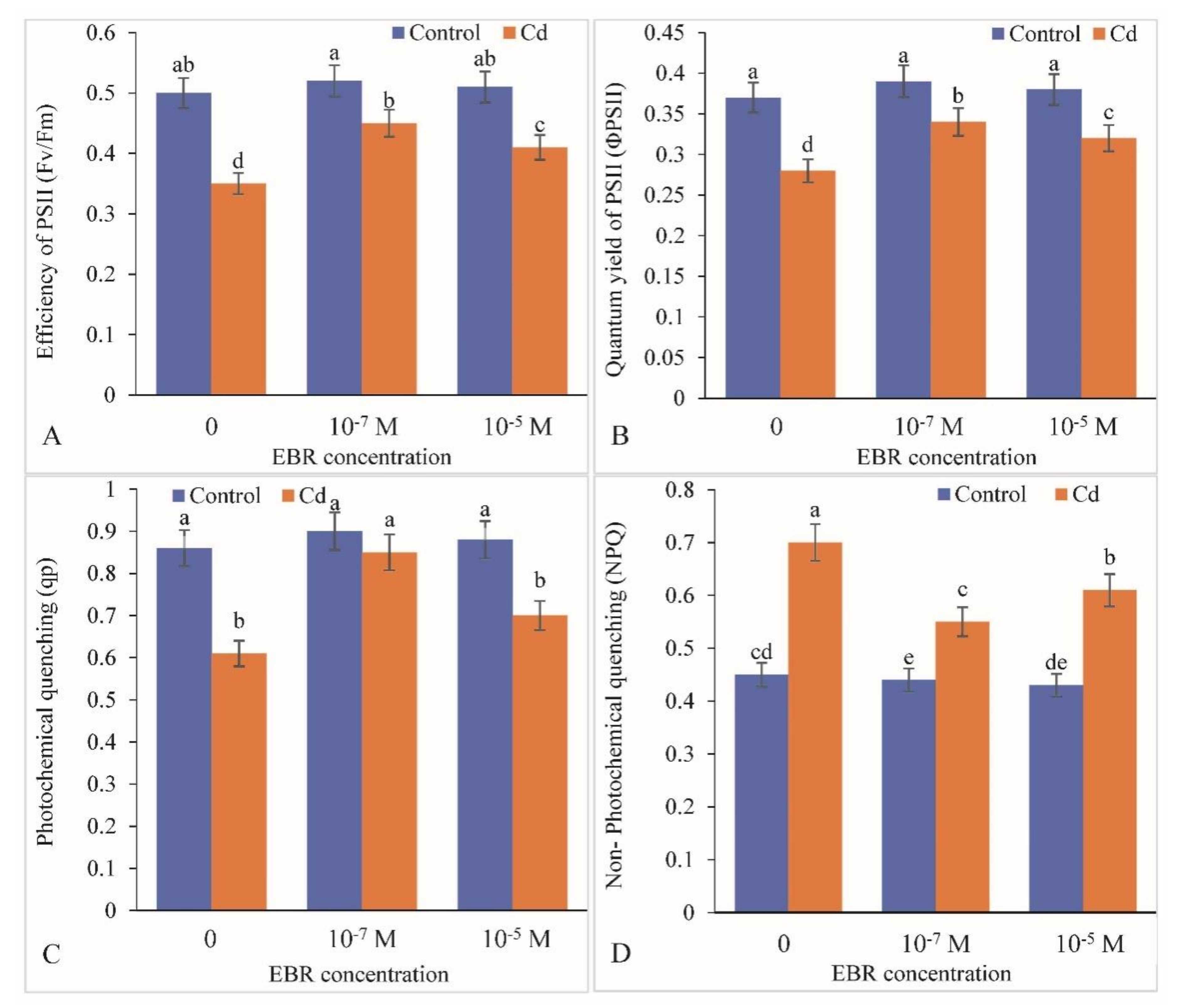
2.3. Variation in Photosynthetic Parameters (Chlorophyll Fluorescence)
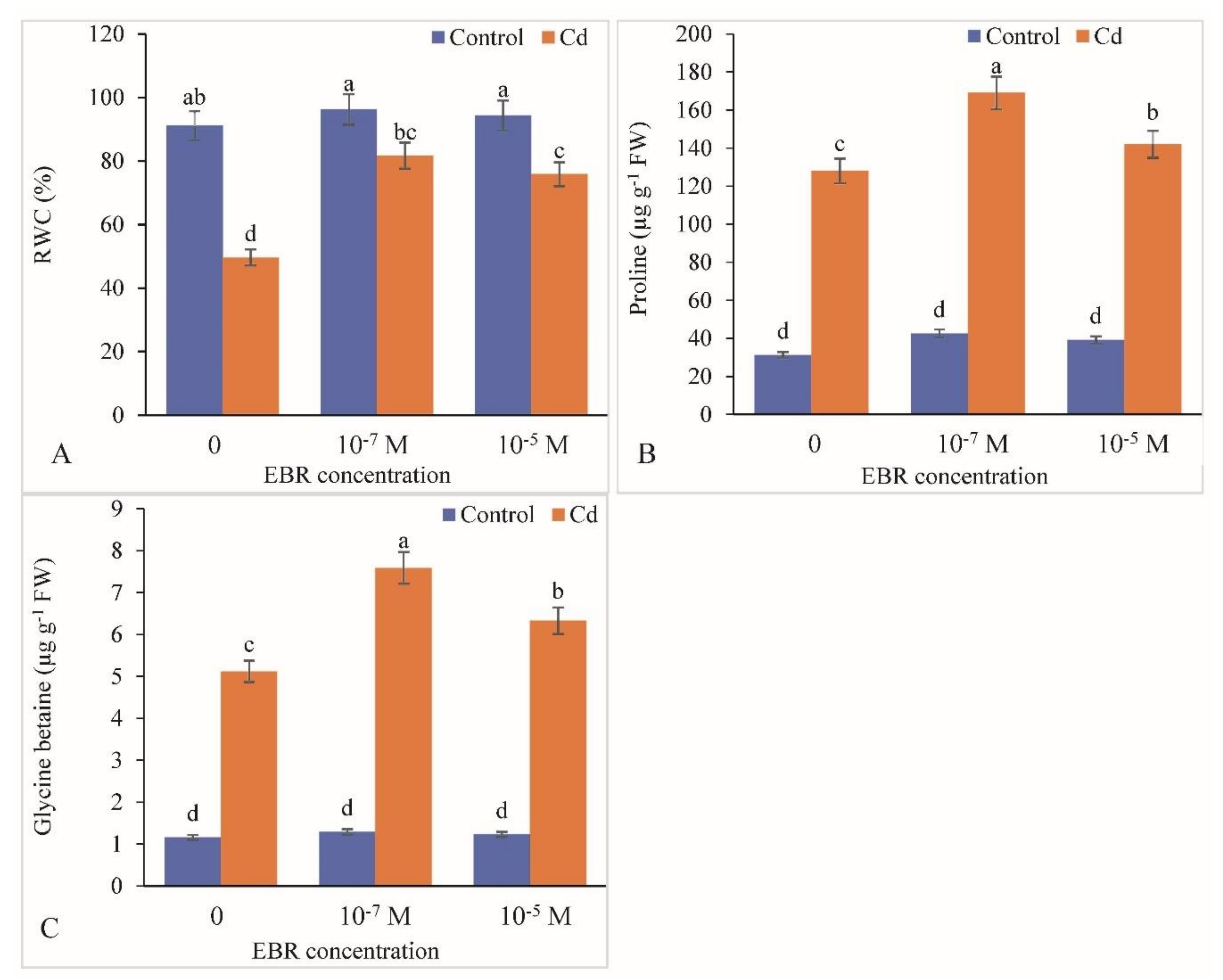
2.4. RWC, Proline and Glycine Betaine
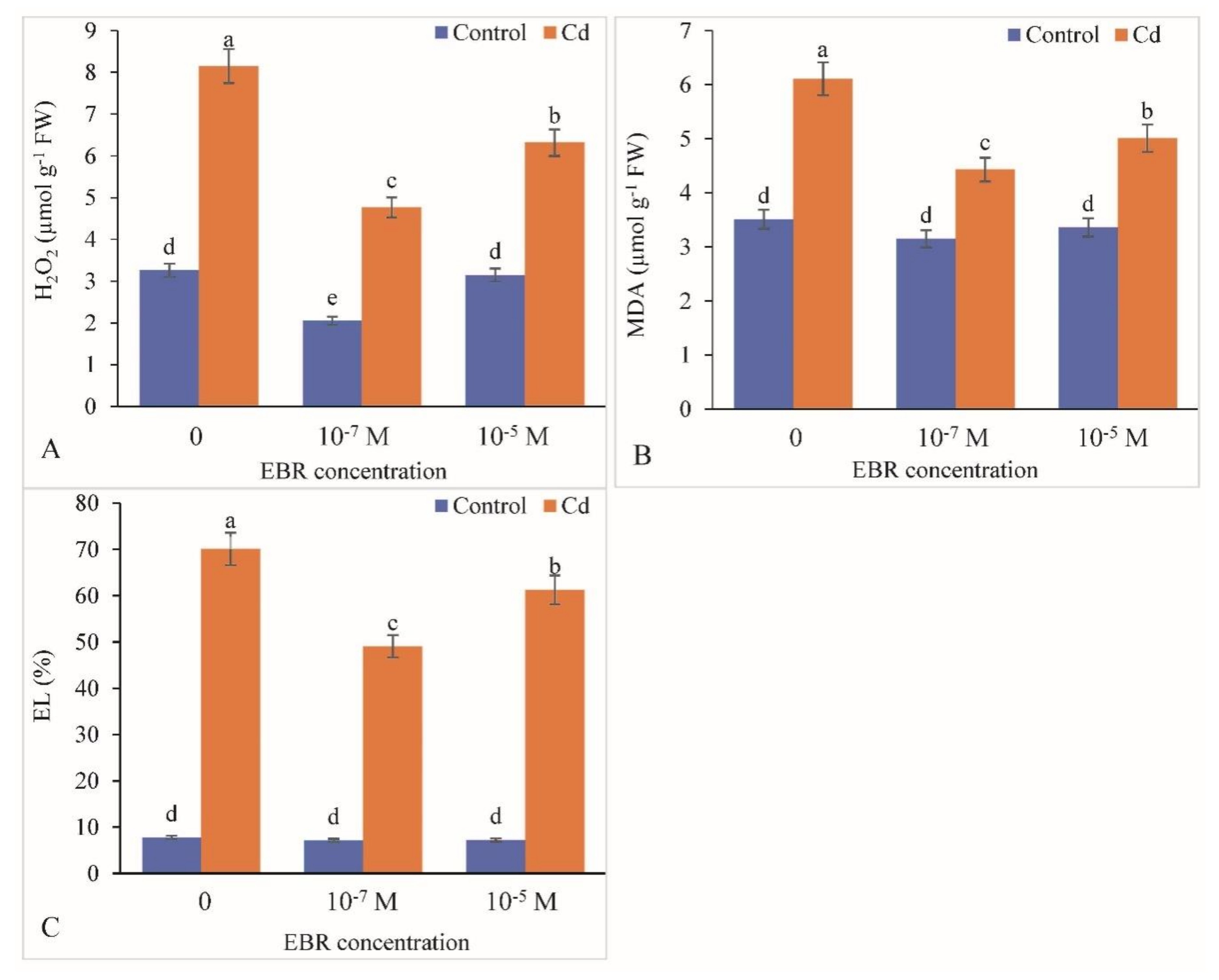
2.5. Decline in Oxidative Damage in Response to 24-EBR Treatment
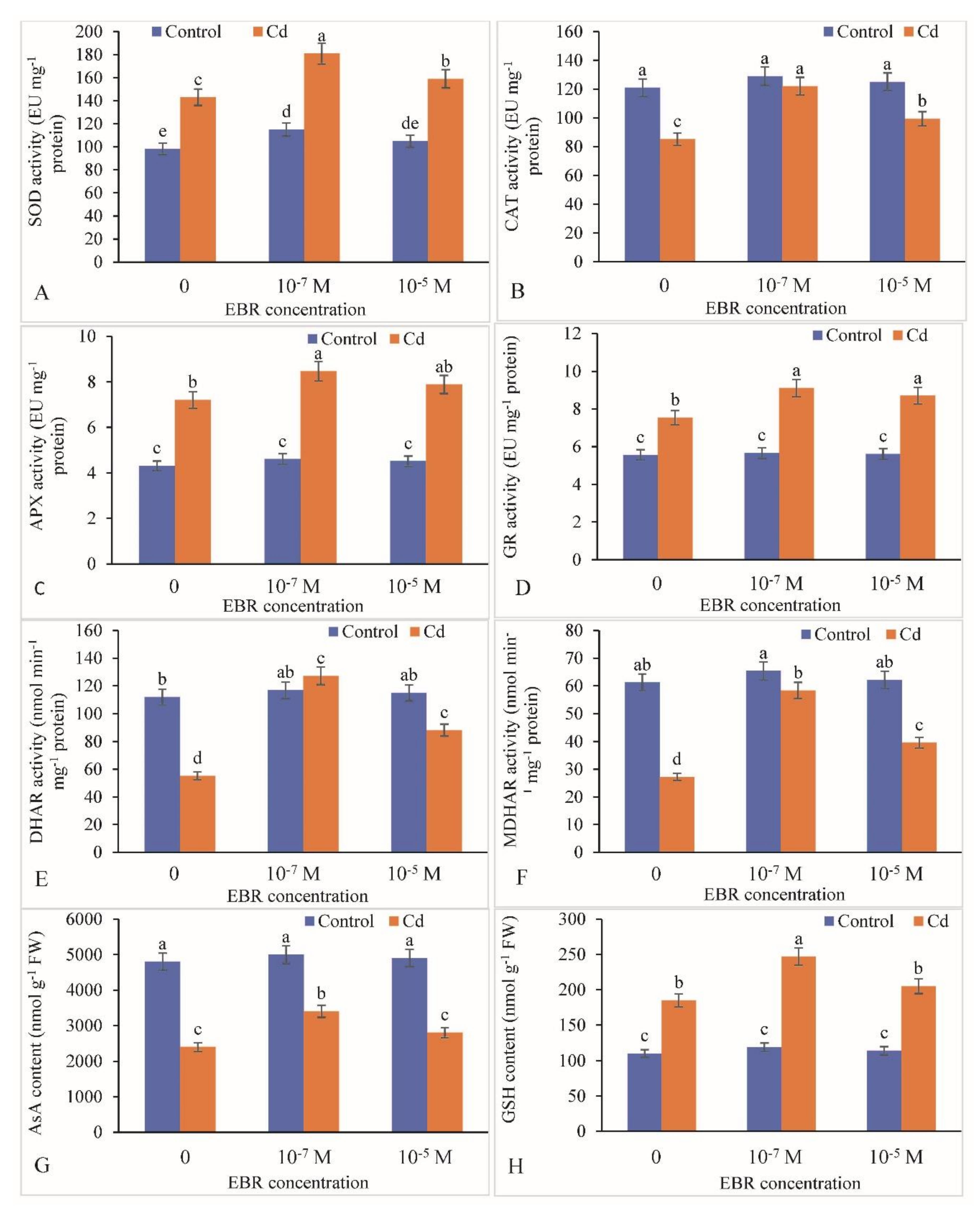
2.6. Change in Antioxidative Defense Components by the 24-EBR Treatment
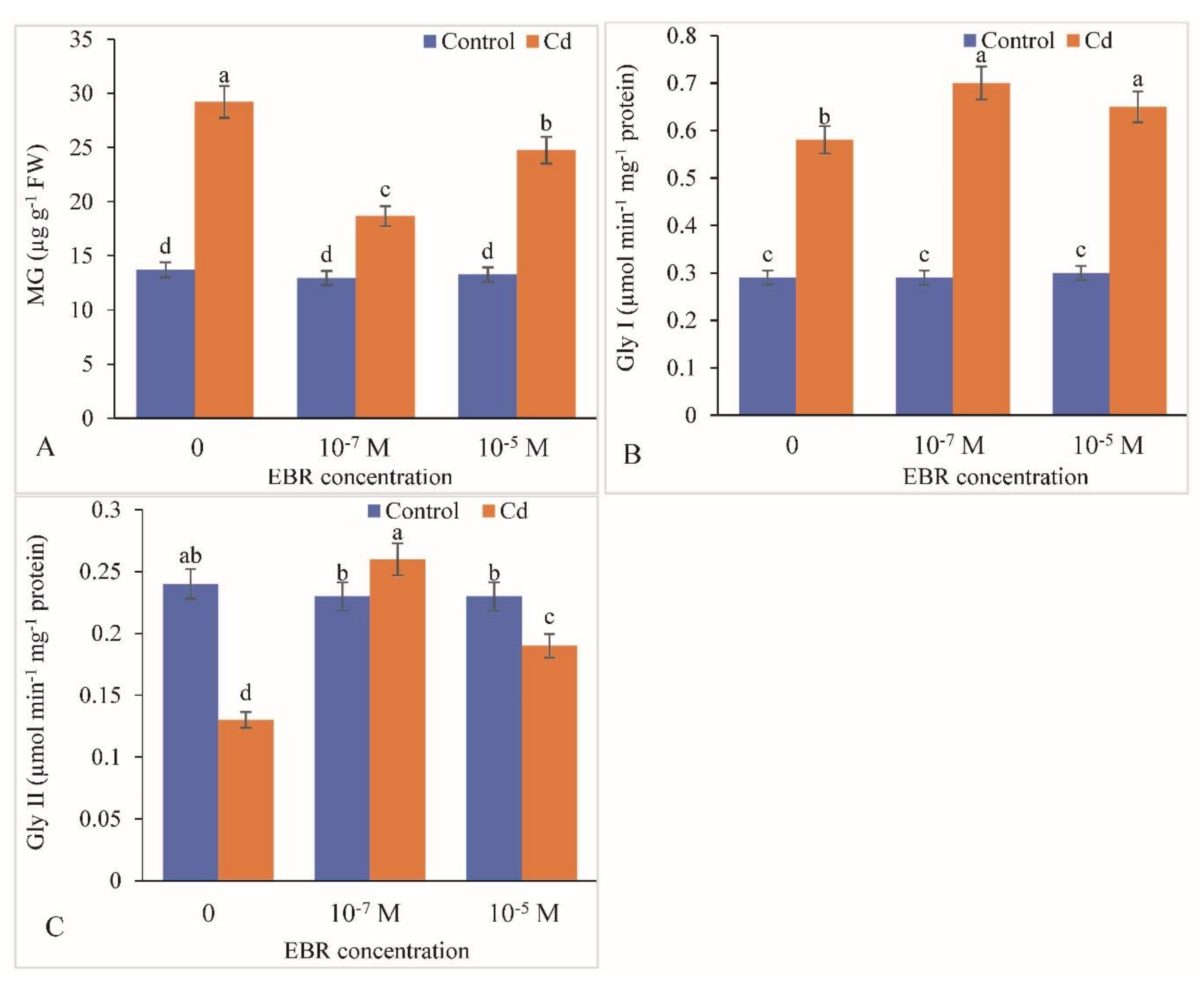
2.7. Methylglyoxalase System as Altered by 24-EBR Supplementation
2.8. Cadmium Accumulation in Roots and Shoots
2.9. Translocation Factor and Shoot and Root Tolerance Indices as Altered by 24-EBR Application
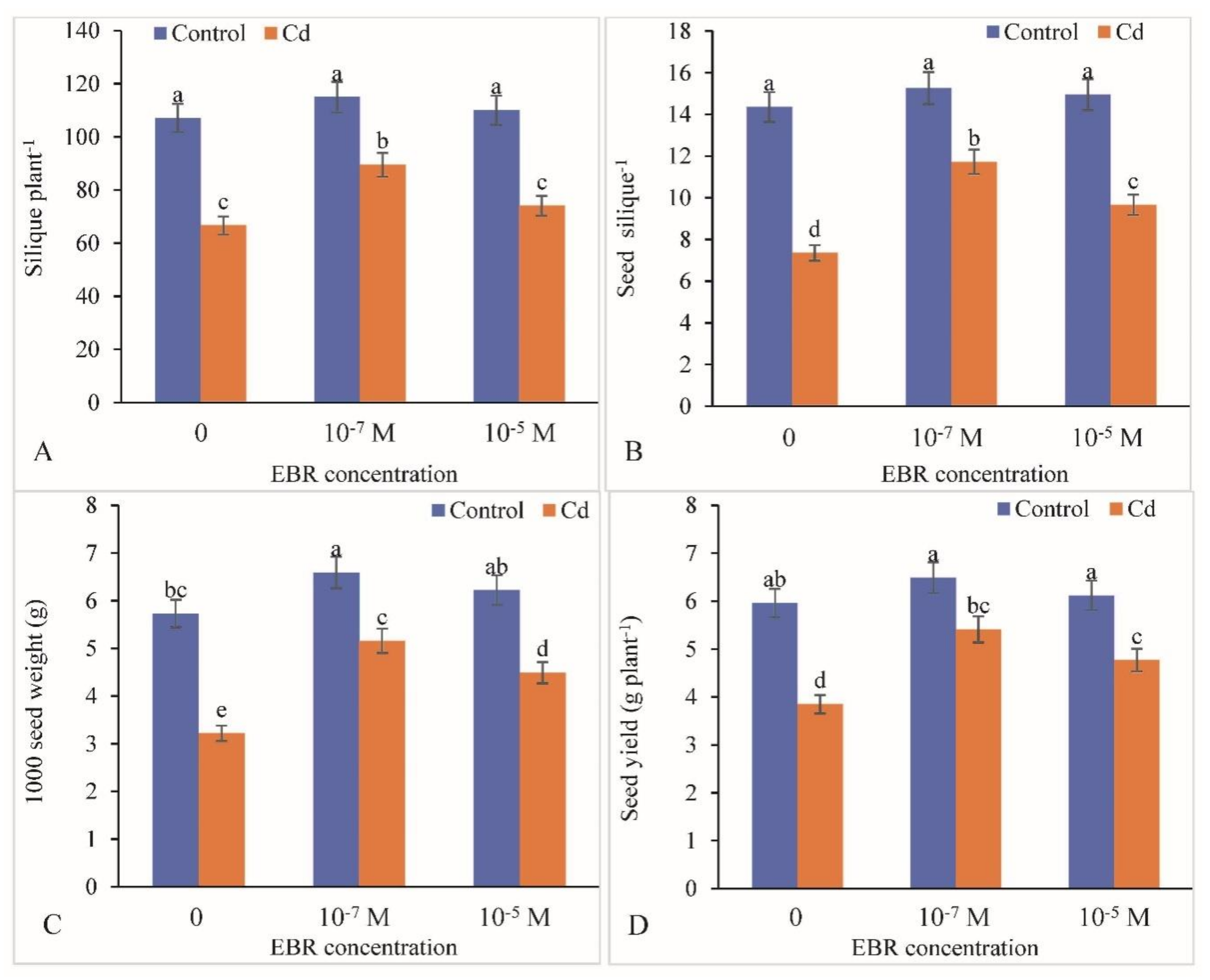
2.10. Alterations in Yield Attributes
3. Discussion
4. Materials and Methods
4.1. Determination of Growth Traits
4.2. Determination of Pigment Content
4.3. Estimation of Cd
4.4. Cadmium Translocation Factor and Tolerance Index
4.5. Gas Exchange Attributes
4.6. Estimation of Chlorophyll Fluorescence
4.7. Determination of Leaf Relative Water Content (LRWC)
4.8. Glycine Betaine and Proline Estimation
4.9. Determination of H2O2, MDA and EL
4.10. Appraisal of Activities of Antioxidative Enzymes and Components of the Ascorbate-Glutathione Cycle
4.11. Estimation of MG, Gly I (EC4.4.1.5) and GlyII (EC3.1.2.6)
4.12. Yield Attributes
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goix, S.; Lévêque, T.; Xiong, T.-T.; Schreck, E.; Baeza-Squiban, A.; Geret, F.; Uzu, G.; Austruy, A.; Dumat, C. Environmental and health impacts of fine and ultrafine metallic particles: Assessment of threat scores. Environ. Res. 2014, 133, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Maqbool, A. A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef]
- ur Rehman, M.Z.; Rizwan, M.; Hussain, A.; Saqib, M.; Ali, S.; Sohail, M.I.; Shafiq, M.; Hafeez, F. Alleviation of cadmium (Cd) toxicity and minimizing its uptake in wheat (Triticum aestivum) by using organic carbon sources in Cd-spiked soil. Environ. Pollut. 2018, 241, 557–565. [Google Scholar] [CrossRef]
- Tucker, P. Cadmium Toxicity: What Is the Biological Fate of Cadmium in the Body. 2011; pp. 10–44. Available online: https://www.atsdr.cdc.gov/csem/csem.asp?csem=6&po=9 (accessed on 6 October 2020).
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-Ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Li, Z.; Lin, W. Synergetic effects of DA-6/24-EBL and NTA on uptake, subcellular distribution and chemical form of Cd in Amaranthus hybridus L. Soil Sci. Plant Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Ma, X.; Guo, L.; He, Y.; Ren, Z.; Kuang, Z.; Zhang, X.; Zhang, Z. Analysis of potential strategies for cadmium stress tolerance revealed by transcriptome analysis of upland cotton. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Rehman, M.Z.U.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2017, 25, 25668–25680. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Rinklebe, J.; Tsang, D.C.W.; Bashir, A.; Maqbool, A.; Tack, F.M.G.; Ok, Y.S. Cadmium phytoremediation potential of Brassica crop species: A review. Sci. Total. Environ. 2018, 1175–1191. [Google Scholar] [CrossRef]
- Yang, G.; Chen, C.; Wang, Y.; Cai, L.; Kong, X.; Qian, Y.; Wang, Q. Joint toxicity of chlorpyrifos, atrazine, and cadmium at lethal concentrations to the earthworm Eisenia fetida. Environ. Sci. Pollut. Res. 2015, 22, 9307–9315. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Shah, F.U.R.; Ahmad, N.; Masood, K.R.; Peralta-Videa, J.R.; Ahmad, F.U.D. Heavy Metal Toxicity in Plants. In Plant Adaptation and Phytoremediation; Springer: Dordrecht, The Netherlands, 2010; pp. 71–97. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Halim, N.I.A.; Phang, I.C. Salicylic Acid Mitigates Pb Stress in Nicotiana Tabacum. Sci. Herit. J. 2017, 1, 16–19. [Google Scholar] [CrossRef]
- Kohli, A.; Sreenivasulu, N.; Lakshmanan, P.; Kumar, P.P. The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep. 2013, 32, 945–957. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2017, 255, 11–24. [Google Scholar] [CrossRef]
- Alam, P.; AlBalawi, T.H.; Altalayan, F.H.; Bakht, M.A.; Ahanger, M.A.; Raja, V.; Ashraf, M.; Ahmad, P. 24-Epibrassinolide (EBR) Confers Tolerance against NaCl Stress in Soybean Plants by Up-Regulating Antioxidant System, Ascorbate-Glutathione Cycle, and Glyoxalase System. Biomolecules 2019, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Jamwal, V.L.; Kohli, S.K.; Kaur, P.; Tejpal, R.; Bhalla, V.; Ohri, P.; Gandhi, S.G.; Bhardwaj, R.; Al-Huqail, A.A.; et al. Jasmonic acid application triggers detoxification of lead (Pb) toxicity in tomato through the modifications of secondary metabolites and gene expression. Chemosphere 2019, 235, 734–748. [Google Scholar] [CrossRef]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. 24-Epibrassinolide Alleviates the Injurious Effects of Cr(VI) Toxicity in Tomato Plants: Insights into Growth, Physio-Biochemical Attributes, Antioxidant Activity and Regulation of Ascorbate–Glutathione and Glyoxalase Cycles. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Nitrate reductase rather than nitric oxide synthase activity is involved in 24-epibrassinolide-induced nitric oxide synthesis to improve tolerance to iron deficiency in strawberry (Fragaria × annassa) by up-regulating the ascorbate-glutathione cycle. Plant Physiol. Biochem. 2020, 151, 486–499. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Kaya, C.; Sarıoğlu, A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Gibberellic acid-induced generation of hydrogen sulfide alleviates boron toxicity in tomato (Solanum lycopersicum L.) plants. Plant Physiol. Biochem. 2020, 153, 53–63. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Khan, M.N.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 2020, 398, 122882. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Arteca, R.N.; Foolad, M.R. The Physiological, Biochemical and Molecular Roles of Brassinosteroids and Salicylic Acid in Plant Processes and Salt Tolerance. Crit. Rev. Plant Sci. 2010, 29, 162–190. [Google Scholar] [CrossRef]
- Clouse, S.D. Brassinosteroids. Arab. Book 2011, 9, e0151. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in Plant Tolerance to Abiotic Stress. J. Plant Growth Regul. 2020, 1–14. [Google Scholar] [CrossRef]
- Janeczko, A.; Kościelniak, J.; Pilipowicz, M.; Szarek-Łukaszewska, G.; Skoczowski, A. Protection of winter rape photosystem 2 by 24-epibrassinolide under cadmium stress. Photosynthetica 2005, 43, 293–298. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Da Silva, J.A.T.; Fujita, M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In Crop Stress and its Management: Perspectives and Strategies; Springer: Dordrecht, The Netherlands, 2011; pp. 261–315. [Google Scholar]
- Ahmad, P.; Sarwat, M.; Bhat, N.A.; Wani, M.R.; Kazi, A.G.; Tran, L.-S.P. Alleviation of Cadmium Toxicity in Brassica juncea L. (Czern. & Coss.) by Calcium Application Involves Various Physiological and Biochemical Strategies. PLoS ONE 2015, 10, e0114571. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Nan, Z. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J. Hazard. Mater. 2010, 175, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Mohammad, F.; Fariduddin, Q. Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol. Mol. Biol. Plants 2020, 26, 25–39. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef]
- Rao, S.S.R.; Vardhini, B.V.; Sujatha, E.; Anuradha, S. Brassinosteroids—A new class of phytohormones. Curr. Sci. 2002, 82, 1239–1245. [Google Scholar]
- Jazi, S.; Yazdi, H.; Ranjbar, M. Effect of salicylic acid on some plant growth parameters under lead stress in Brassica napus var. Okapi. Iran J. Plant Physiol. 2011, 1, 177–185. [Google Scholar]
- Saeidnejad, A.H.; Mardani, H.; Naghibolghora, M. Protective effects of salicylic acid on physiological parameters and antioxidants response in maize seedlings under salinity stress. J. Appl. Environ. Biol. Sci. 2012, 2, 364–373. [Google Scholar]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ying, R.-R.; Qiu, R.-L.; Tang, Y.-T.; Hu, P.-J.; Qiu, H.; Chen, H.-R.; Shi, T.-H.; Morel, J.-L. Cadmium tolerance of carbon assimilation enzymes and chloroplast in Zn/Cd hyperaccumulator Picris divaricata. J. Plant Physiol. 2010, 167, 81–87. [Google Scholar] [CrossRef]
- Prasad, D.D.K.; Prasad, A.R.K. Altered δ-aminolevulinic Acid Metabolism by Lead and Mercury in Germinating Seedlings of Bajra (Pennisetum typhoideum). J. Plant Physiol. 1987, 127, 241–249. [Google Scholar] [CrossRef]
- Prasad, D.D.K.; Prasad, A.R.K. Effect of lead and mercury on chlorophyll synthesis in mung bean seedlings. Phytochemistry 1987, 26, 881–883. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Wang, Y.-S. Physiological and biochemical responses in the leaves of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) exposed to multiple heavy metals. J. Hazard. Mater. 2010, 182, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Xie, C.; Hu, D.; Pu, S.; Xiong, X.; Ma, J.; Sun, L.; Huang, Z.; Jiang, M.; Li, X. Effect of 24-epibrassinolide on reactive oxygen species and antioxidative defense systems in tall fescue plants under lead stress. Ecotoxicol. Environ. Saf. 2020, 187, 109831. [Google Scholar] [CrossRef]
- Dos Santos, L.R.; da Silva, B.R.S.; Pedron, T.; Batista, B.L.; da Silva Lobato, A.K. 24-Epibrassinolide Improves Root Anatomy and Antioxidant Enzymes in Soybean Plants Subjected to Zinc Stress. J. Soil Sci. Plant Nutr. 2020, 20, 105–124. [Google Scholar] [CrossRef]
- Verma, A.; Malik, C.P.; Gupta, V.K. In Vitro Effects of Brassinosteroids on the Growth and Antioxidant Enzyme Activities in Groundnut. ISRN Agron. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef]
- Bukhari, S.A.H.; Wang, R.; Wang, W.; Ahmed, I.M.; Zheng, W.; Cao, F. Genotype-dependent effect of exogenous 24-epibrassinolide on chromium-induced changes in ultrastructure and physicochemical traits in tobacco seedlings. Environ. Sci. Pollut. Res. 2016, 23, 18229–18238. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, G.; Guo, S.; Ren, Y.; Xu, Y.; Ling, K.-S. Screening the USDA Watermelon Germplasm Collection for Drought Tolerance at the Seedling Stage. HortScience 2011, 46, 1245–1248. [Google Scholar] [CrossRef]
- Arshad, M.; Ali, S.; Noman, A.; Ali, Q.; Rizwan, M.; Farid, M.; Irshad, M.K. Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch. Agron. Soil Sci. 2016, 62, 533–546. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ashraf, U.; Khan, I.; Wang, L. Alteration in Growth, Leaf Gas Exchange, and Photosynthetic Pigments of Maize Plants Under Combined Cadmium and Arsenic Stress. Water Air Soil Pollut. 2017, 228, 13. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elkelish, A.; Soliman, M.; Elansary, H.O.; Zaid, A.; Wani, S.H. Serratia marcescens BM1 Enhances Cadmium Stress Tolerance and Phytoremediation Potential of Soybean Through Modulation of Osmolytes, Leaf Gas Exchange, Antioxidant Machinery, and Stress-Responsive Genes Expression. Antioxidants 2020, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-J.; Huang, L.-F.; Zhou, Y.H.; Mao, W.-H.; Shi, K.; Wu, J.-X.; Asami, T.; Chen, Z.; Yu, J.Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 2009, 230, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Pervez, M.; Balal, R.; Mattson, N.; Rashid, A.; Ahmad, R.; Ayyub, C.; Abbas, T. Brassinosteroid (24-epibrassinolide) enhances growth and alleviates the deleterious effects induced by salt stress in pea (’Pisum sativum’ L.). Aust. J. Crop Sci. 2011, 5, 500–510. [Google Scholar]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Li, J.; Bian, Z. Effects of Brassinosteroids on Photosynthetic Performance and Nitrogen Metabolism in Pepper Seedlings under Chilling Stress. Agronomy 2019, 9, 839. [Google Scholar] [CrossRef]
- Azhar, N.; Ashraf, M.Y.; Hussain, M.; Hussain, F. Phytoextraction of lead (Pb) by EDTA application through sunflower (Helianthus annuus L.) cultivation: Seedling growth studies. Pak. J Bot. 2006, 38, 1551–1560. [Google Scholar]
- Brunet, J.; Repellin, A.; Varrault, G.; Terryn, N.; Zuily-Fodil, Y. Lead accumulation in the roots of grass pea (Lathyrus sativus L.): A novel plant for phytoremediation systems? Comptes Rendus Biol. 2008, 331, 859–864. [Google Scholar] [CrossRef]
- Jan, S.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Siddique, K.H.; Ahmad, P. Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol. 2018, 18, 1–18. [Google Scholar] [CrossRef]
- Anuradha, S.; Rao, S.E.R. Amelioration of lead toxicity in radish (Raphanus sativus L) plants by brassinolide. J. Appl. Biol. Sci. 2011, 5, 43–48. [Google Scholar]
- Irfan, M.; Ahmad, A.; Hayat, S. Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J. Biol. Sci. 2014, 21, 125–131. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Gan, Y.; Yu, J.; Xie, J. Brassinosteroid alleviates chilling-induced oxidative stress in pepper by enhancing antioxidation systems and maintenance of photosystem II. Acta Physiol. Plant. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Pandey, N.; Singh, G.K. Studies on antioxidative enzymes induced by cadmium in pea plants (Pisum sativum). J. Environ. Biol. 2012, 33, 201. [Google Scholar]
- Chmielowska-Bąk, J.; Gzyl, J.; Rucińska-Sobkowiak, R.; Arasimowicz-Jelonek, M.; Deckert, J. The new insights into cadmium sensing. Front. Plant Sci. 2014, 5, 245. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Arora, N.; Sharma, P.; Arora, H.K. Effects of 28-homobrassinolide on Seedling Growth, Lipid Peroxidation and Antioxidative Enzyme Activities under Nickel Stress in Seedlings of Zea mays L. Asian J. Plant Sci. 2007, 6, 765–772. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Bhardwaj, R.; Gupta, B.D.; Dutt, P.; Gupta, R.K.; Kanwar, M.; Dutt, P. Changes induced by Cu2+ and Cr6+ metal stress in polyamines, auxins, abscisic acid titers and antioxidative enzymes activities of radish seedlings. Braz. J. Plant Physiol. 2010, 22, 263–270. [Google Scholar] [CrossRef][Green Version]
- Ramakrishna, B.; Rao, S.S.R. Foliar application of brassinosteroids alleviates adverse effects of zinc toxicity in radish (Raphanus sativus L.) plants. Protoplasma 2015, 252, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A. Blockade of heavy metals accumulation in Chlorella vulgaris cells by 24-epibrassinolide. Plant Physiol. Biochem. 2000, 38, 797–801. [Google Scholar] [CrossRef]
- Deng, X.-G.; Zhu, T.; Peng, X.-J.; Xi, D.-H.; Guo, H.; Yin, Y.; Zhang, D.-W.; Lin, H.-H. Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci. Rep. 2016, 6, 20579. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Liu, Y.J.; Xu, X.L.; Jin, M.; An, L.Z.; Zhang, H. Effect of 24-epibrassinolide on drought stress-induced changes in Chorispora bungeana. Biol. Plant. 2012, 56, 192–196. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Al Mahmud, J.; Hossain, S.; Alam, K.; Oku, H.; Fujita, M. Actions of Biological Trace Elements in Plant Abiotic Stress Tolerance. In Essential Plant Nutrients; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 213–274. [Google Scholar]
- Hossain, M.A.; Hossain, M.Z.; Fujita, M. Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 2009, 3, 53–64. [Google Scholar]
- Santos, L.R.; Batista, B.L.; Lobato, A.K.S. Brassinosteroids mitigate cadmium toxicity in cowpea plants. Photosynthetica 2018, 56, 591–605. [Google Scholar] [CrossRef]
- Wani, A.S.; Tahir, I.; Ahmad, S.S.; Dar, R.A.; Nisar, S. Efficacy of 24-epibrassinolide in improving the nitrogen metabolism and antioxidant system in chickpea cultivars under cadmium and/or NaCl stress. Sci. Hortic. 2017, 225, 48–55. [Google Scholar] [CrossRef]
- Ahmad, P.; Allah, E.F.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Siddique, K.H.M. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Waisi, H.K.; Petković, A.Z.; Nikolić, B.R.; Janković, B.; Raičević, V.; Lalević, B.; Giba, Z.S. Influence of 24-epibrassinolide on seedling growth and distribution of mineral elements in two maize hybrids. Chem. Ind. 2017, 71, 201–209. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Yu, J.-Q.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Benefits of brassinosteroid crosstalk. Trends Plant Sci. 2012, 17, 594–605. [Google Scholar] [CrossRef]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and Response of Plants to Heavy Metals Action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, D.; Li, W. Leaf gas exchange characteristics and chlorophyll fluorescence of three wetland plants in response to long-term soil flooding. Photosynthetica 2007, 45, 222–228. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.R. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Rao, K.V.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought Tolerance in Two Mosses: Correlated with Enzymatic Defence Against Lipid Peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Colowick, S., Kaplan, N., Eds.; Elsevier: Tampa, FL, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foster, J.G.; Hess, J.L. Responses of Superoxide Dismutase and Glutathione Reductase Activities in Cotton Leaf Tissue Exposed to an Atmosphere Enriched in Oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef]
- Miyake, C.; Asada, K. Thylakoid-Bound Ascorbate Peroxidase in Spinach Chloroplasts and Photoreduction of Its Primary Oxidation Product Monodehydroascorbate Radicals in Thylakoids. Plant Cell Physiol. 1992, 33, 541–553. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Yu, C.-W.; Murphy, T.M.; Lin, C.-H. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-l-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef]
| EBR (Molar) | Shoot Cd (mg kg−1 DW) | Root Cd (mg kg−1 DW) | Translocation Factor | Shoot Tolerance Index (STI%) | Root Tolerance Index (RTI%) | ||
|---|---|---|---|---|---|---|---|
| Control | Cd | Control | Cd | ||||
| 0 | ND | 22.42 ± 0.595 a | ND | 35.31 ± 0.917 a | 0.634 ± 0.029 a | 55.81 ± 5.05 c | 60.65 ± 5.62 c |
| 10−7 | ND | 9.32 ± 0.327 c | ND | 18.45 ± 0.461 c | 0.505 ± 0.013 c | 86.02 ± 7.33 a | 79.51 ± 7.10 a |
| 10−5 | ND | 14.76 ± 0.326 b | ND | 24.74 ± 0.529 b | 0.596 ± 0.021 b | 71.67 ± 6.85 b | 76.07 ± 6.95 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, P.; Kaur Kohli, S.; Al Balawi, T.; Altalayan, F.H.; Alam, P.; Ashraf, M.; Bhardwaj, R.; Ahmad, P. Foliar Application of 24-Epibrassinolide Improves Growth, Ascorbate-Glutathione Cycle, and Glyoxalase System in Brown Mustard (Brassica juncea (L.) Czern.) under Cadmium Toxicity. Plants 2020, 9, 1487. https://doi.org/10.3390/plants9111487
Alam P, Kaur Kohli S, Al Balawi T, Altalayan FH, Alam P, Ashraf M, Bhardwaj R, Ahmad P. Foliar Application of 24-Epibrassinolide Improves Growth, Ascorbate-Glutathione Cycle, and Glyoxalase System in Brown Mustard (Brassica juncea (L.) Czern.) under Cadmium Toxicity. Plants. 2020; 9(11):1487. https://doi.org/10.3390/plants9111487
Chicago/Turabian StyleAlam, Pravej, Sukhmeen Kaur Kohli, Thamer Al Balawi, Fahad H. Altalayan, Prawez Alam, Muhammad Ashraf, Renu Bhardwaj, and Parvaiz Ahmad. 2020. "Foliar Application of 24-Epibrassinolide Improves Growth, Ascorbate-Glutathione Cycle, and Glyoxalase System in Brown Mustard (Brassica juncea (L.) Czern.) under Cadmium Toxicity" Plants 9, no. 11: 1487. https://doi.org/10.3390/plants9111487
APA StyleAlam, P., Kaur Kohli, S., Al Balawi, T., Altalayan, F. H., Alam, P., Ashraf, M., Bhardwaj, R., & Ahmad, P. (2020). Foliar Application of 24-Epibrassinolide Improves Growth, Ascorbate-Glutathione Cycle, and Glyoxalase System in Brown Mustard (Brassica juncea (L.) Czern.) under Cadmium Toxicity. Plants, 9(11), 1487. https://doi.org/10.3390/plants9111487








