Effects of Plant Growth Promoting Rhizobacteria on the Content of Abscisic Acid and Salt Resistance of Wheat Plants
Abstract
1. Introduction
2. Results
2.1. Field Experiments
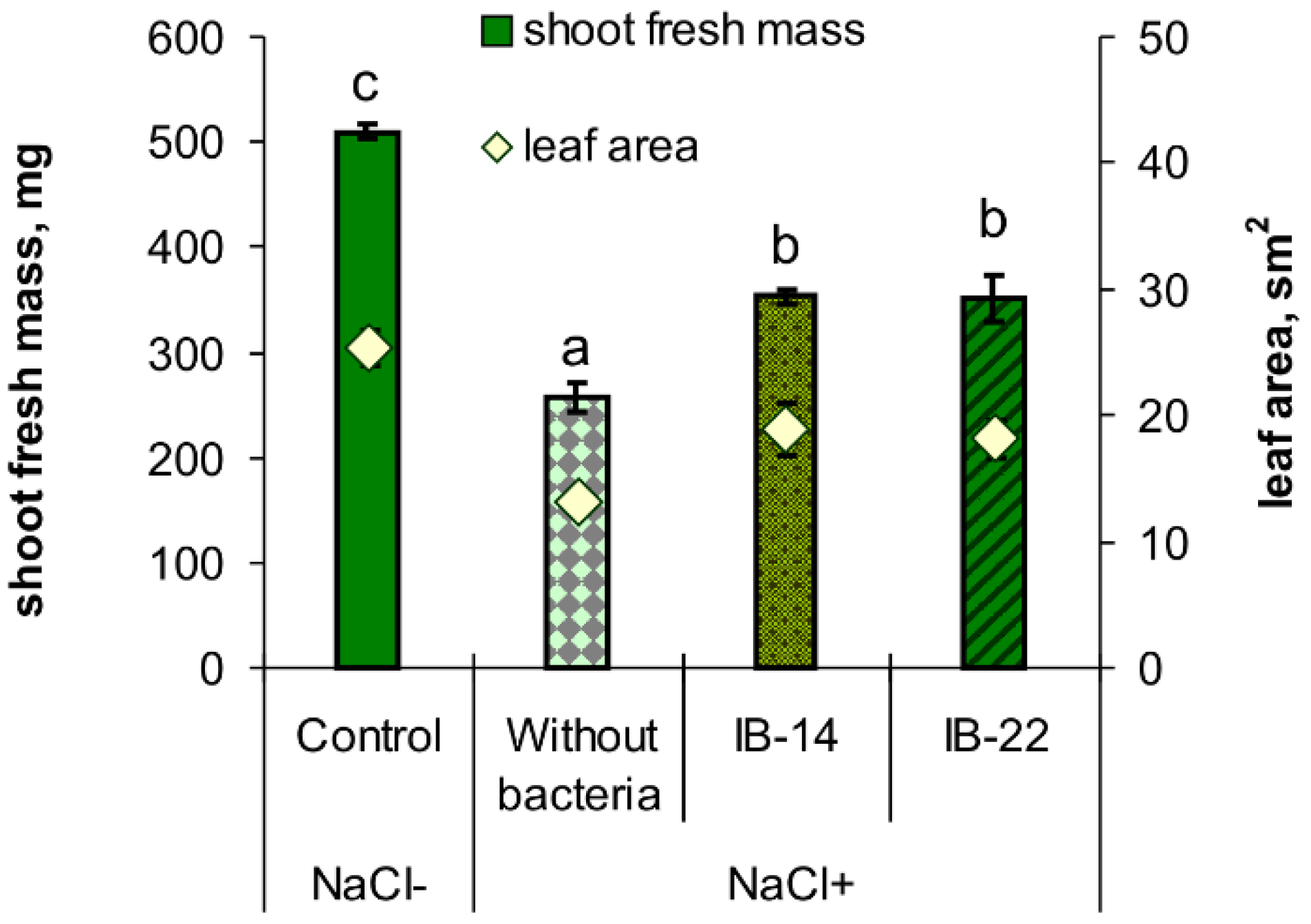
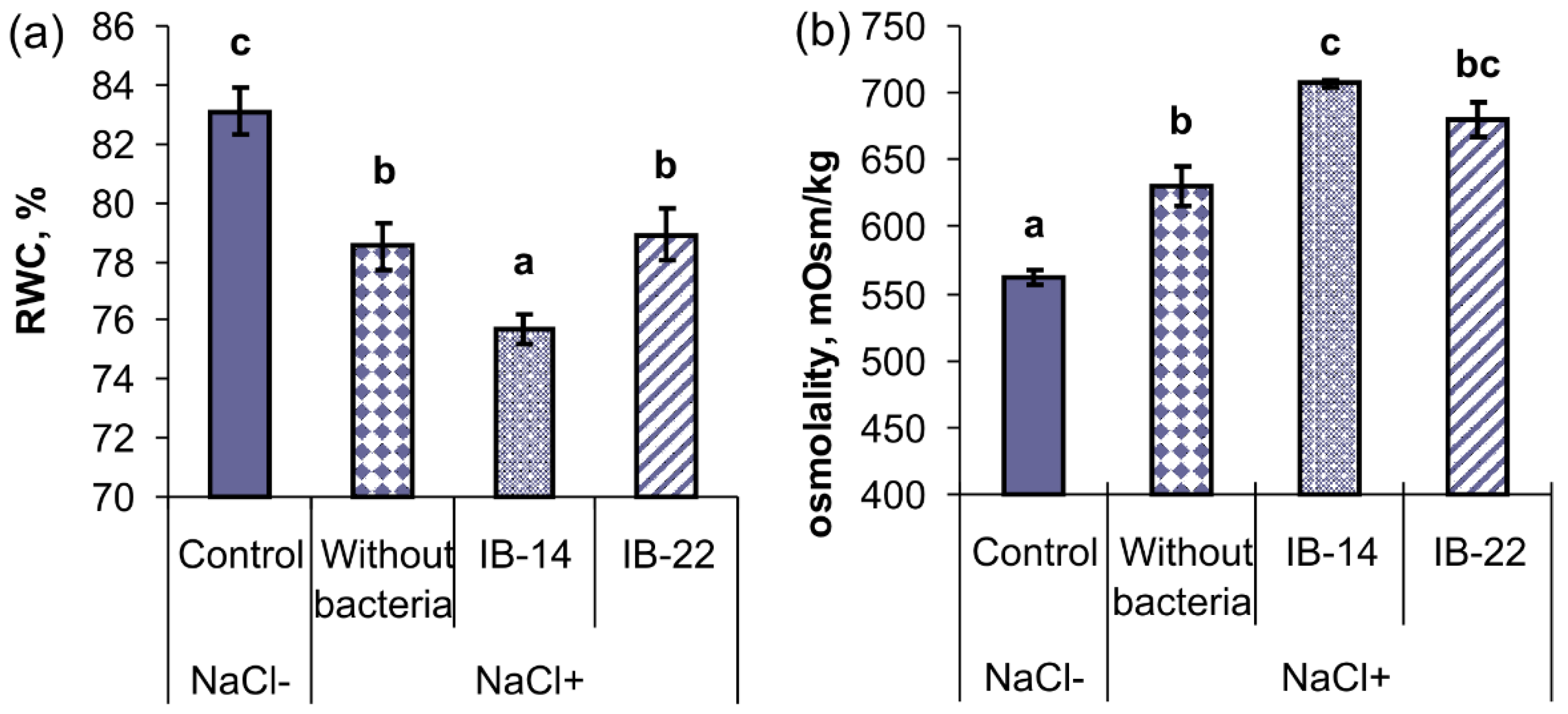
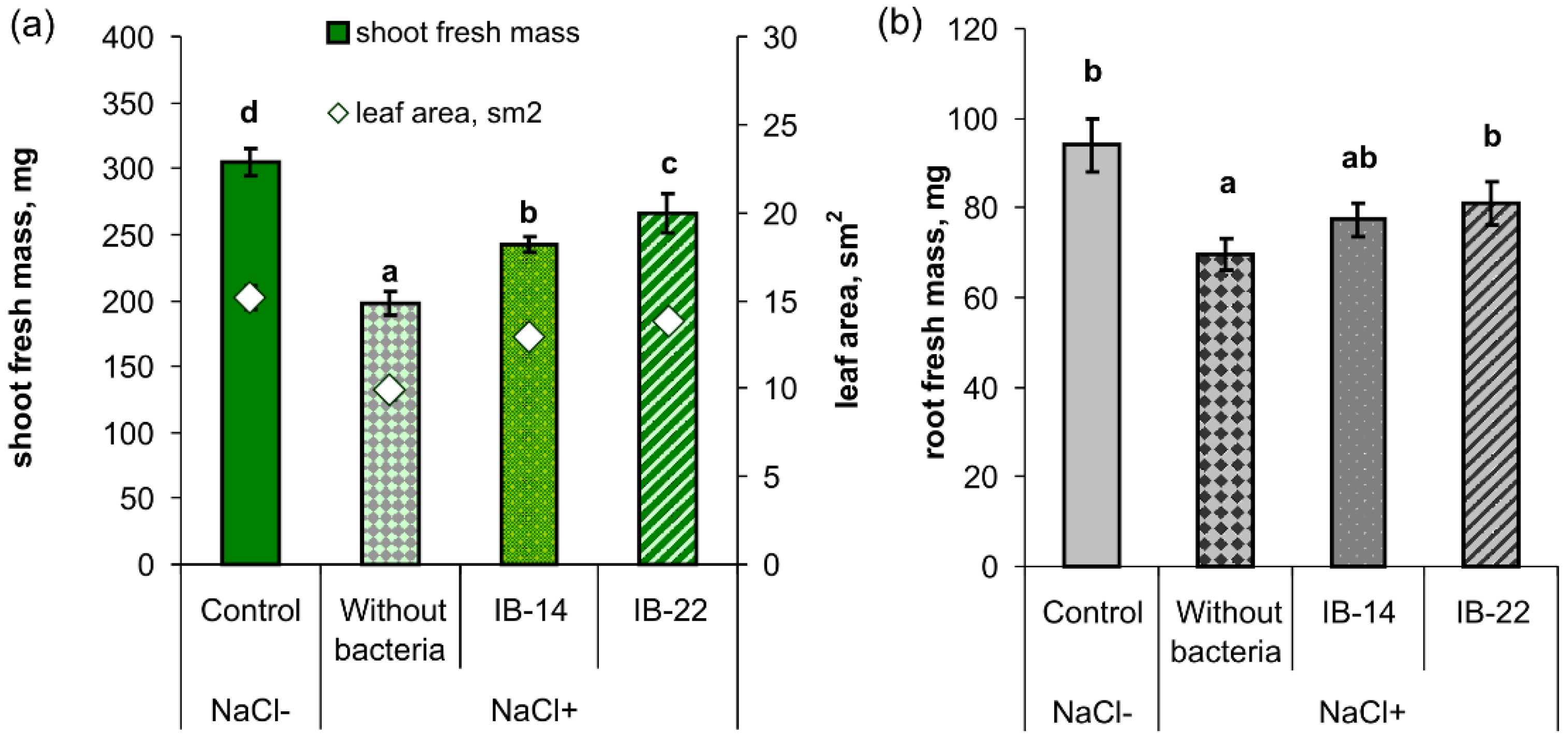
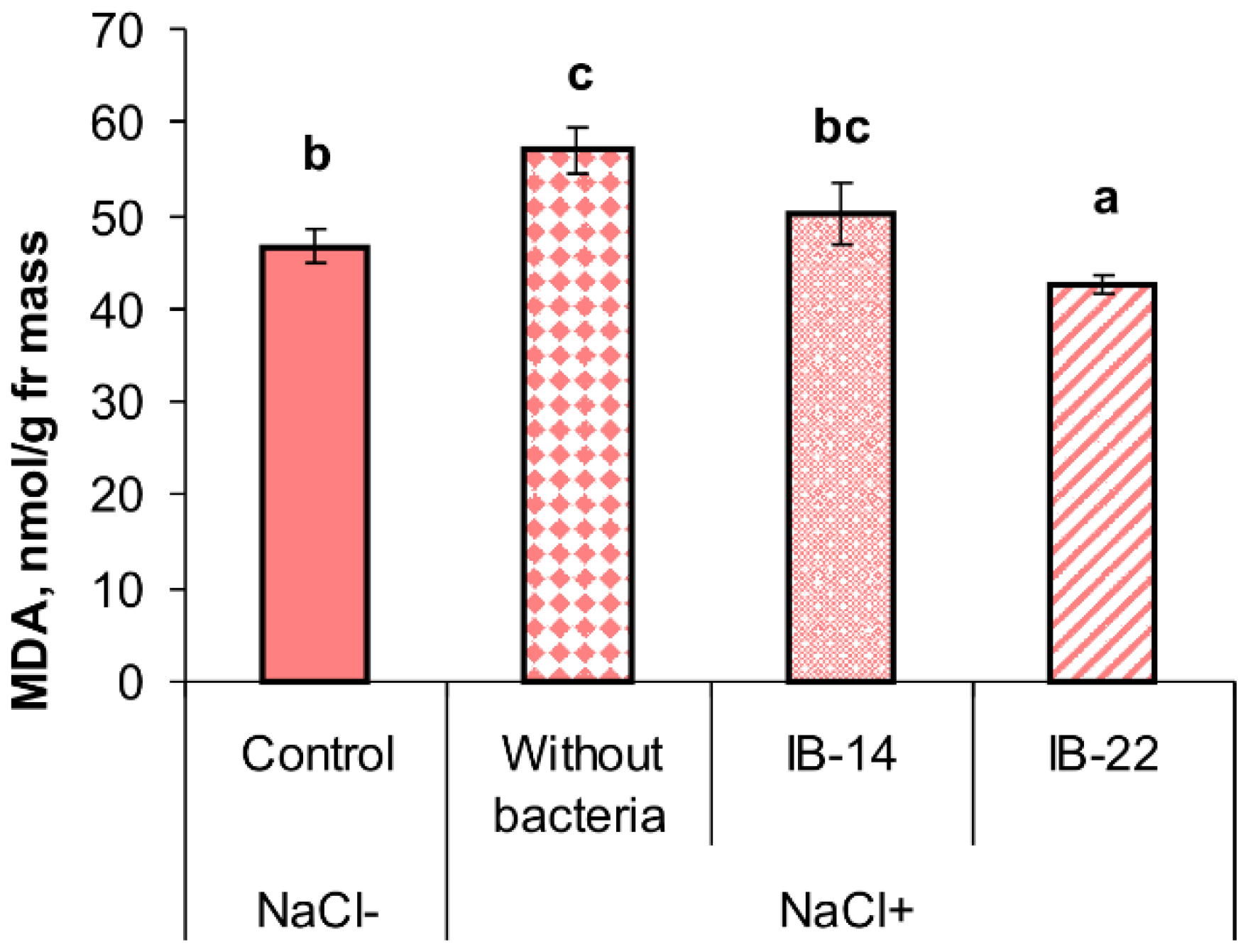
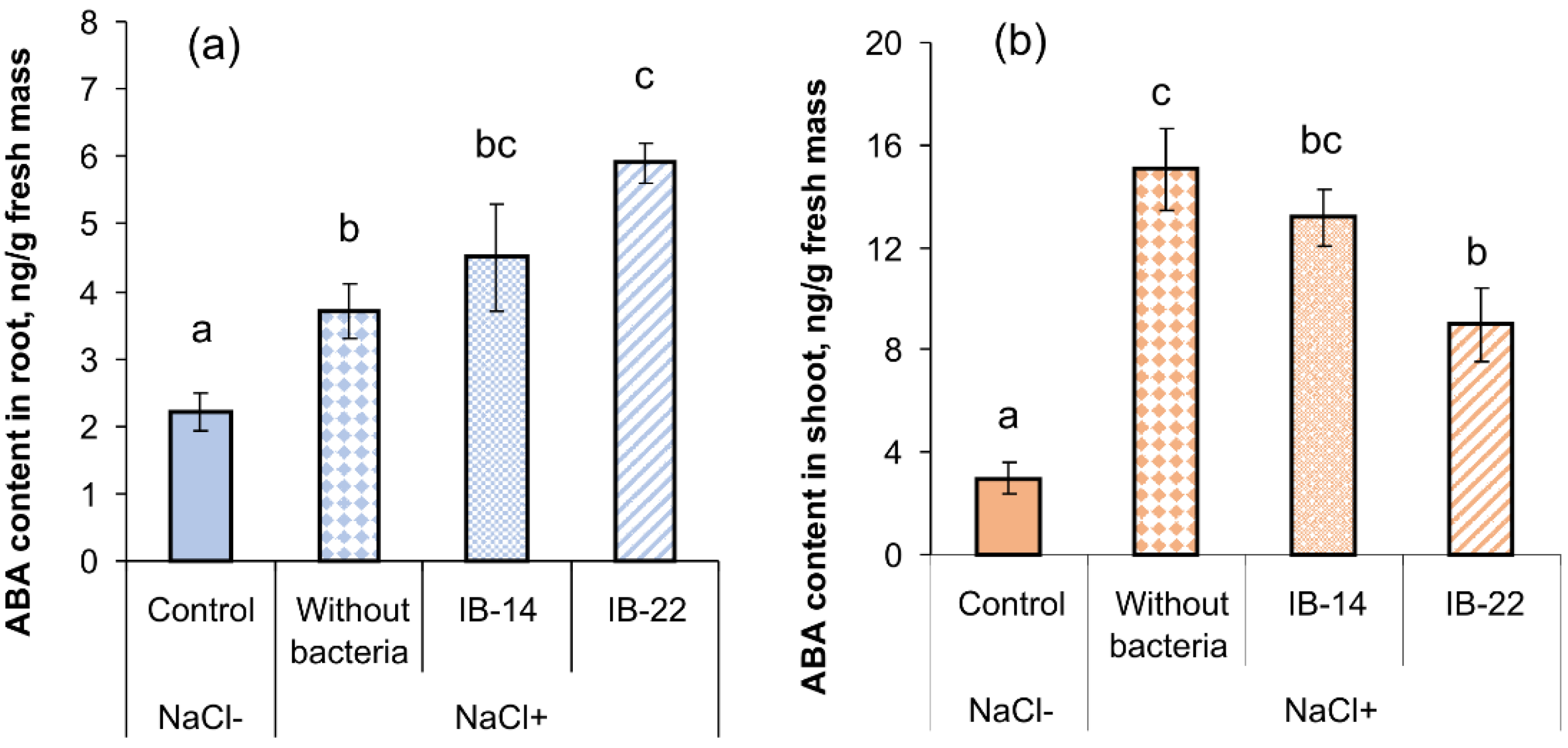
2.2. Laboratory Experiments
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant. Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth-promoting rhizobacteria. J. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- He, A.-L.; Niu, S.-Q.; Zhao, Q.; Li, Y.-S.; Gou, J.-Y.; Gao, H.-J.; Suo, S.-Z.; Zhang, J.-L. Induced salt tolerance of perennial ryegrass by a novel bacterium strain from the rhizosphere of a desert shrub Haloxylon ammodendron. Int. J. Mol. Sci. 2018, 19, 469. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant. Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Ansari, M.; Shekari, F.; Mohammadi, M.H.; Juhos, K.; Végvári, G.; Biró, B. Salt-tolerant plant growth-promoting bacteria enhanced salinity tolerance of salt-tolerant alfalfa (Medicago sativa L.) cultivars at high salinity. Acta Physiol. Plant 2019, 41, 195. [Google Scholar] [CrossRef]
- Salomon, M.V.; Bottini, R.; De Souza Filho, G.A.; Cohen, A.C.; Moreno, D.; Gil, M.; Piccoli, P. Bacteria isolated from roots and rhizosphere of Vitis vinifera retard water losses, induce abscisic acid accumulation and synthesis of defense-related terpenes in in vitro cultured grapevine. Physiol. Plant 2014, 151, 359–374. [Google Scholar] [CrossRef]
- Veselov, D.S.; Sharipova, G.V.; Veselov, S.U.; Kudoyarova, G.R. The effects of NaCl treatment on water relations, growth and ABA content in barley cultivars differing in drought tolerance. J. Plant. Growth Regul. 2008, 27, 380–386. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.A.; Pérez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Chen, K.; Wang, S.; Mur, L.A.J.; Guo, S. Exploring the roles of aquaporins in plant–microbe interactions. Cells 2018, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016, 7, 876. [Google Scholar] [CrossRef]
- Hartung, W.; Sauter, A.; Hose, E. Abscisic acid in the xylem: Where does it come from, where does it go to? J. Exp. Bot. 2002, 53, 27–32. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Yasmin Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S.; Liu, L.J. Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell Environ. 2003, 26, 1621–1631. [Google Scholar] [CrossRef]
- Hose, E.; Steudle, E.; Hartung, W. Abscisic acid and hydraulic conductivity of maize roots: A study using cell- and root-pressure probes. Planta 2000, 211, 874–882. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Veselova, S.; Hartung, W.; Farhutdinov, R.; Veselov, D.; Sharipova, G. Involvement of root ABA and hydraulic conductivity in the control of water relations in wheat plants exposed to increased evaporation demand. Planta 2011, 233, 87–94. [Google Scholar] [CrossRef]
- Sharipova, G.; Veselov, D.; Fricke, W.; Dodd, I.; Katsuhara, M.; Furuichi, T.; Ivanov, I.; Veselov, S. Exogenous application of abscisic acid (ABA) increases root and cell hydraulic conductivity and abundance of some aquaporin isoforms in the ABA deficient barley mutant Az34. Ann. Bot. 2016, 118, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.J.; Kudoyarova, G.; Hartung, W. Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant’s response to drought. J. Plant Growth Regul. 2005, 24, 285–295. [Google Scholar] [CrossRef]
- LaRosa, P.C.; Hasegawa, P.M.; Rhodes, D.; Clithero, J.M.; Watad, A.A.; Bressan, R.A. Abscisic acid stimulated osmotic adjustment and its involvement in adaptation of tobacco cells to nacl. Plant Physiol. 1987, 85, 174–181. [Google Scholar] [CrossRef]
- Sharp, R.E.; Wu, Y.; Voetberg, G.S.; Saab, I.N.; LeNoble, M.E. Confirmation that abscisic acid accumulation is required for maize primary root elongation at low water potentials. J. Exp. Bot. 1994, 45, 1743–1751. [Google Scholar] [CrossRef]
- Veselov, D.S.; Sharipova, G.V.; Veselov, S.Y.; Dodd, I.C.; Ivanov, I.; Kudoyarova, G.R. Rapid changes in root HvPIP2;2 aquaporins abundance and ABA concentration are required to enhance root hydraulic conductivity and maintain leaf water potential in response to increased evaporative demand. Funct. Plant Biol. 2018, 45, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hartung, W.; Sauter, A.; Turner, N.C.; Fillery, I.; Heilmeier, H. Abscisic acid in soils: What is its function and which factors and mechanisms influence its concentration? Plant Soil 1996, 184, 105–110. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef]
- Schubert, S.; Serra, R.; Plies-Balzer, E.; Mengel, K. Effect of drought stress on growth, sugar concentrations and amino acid accumulation in n2-fixing alfalfa (Medicago sativa). J. Plant Physiol. 1995, 146, 541–546. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Li, Y.; Wang, X.; Nan, W.; Hu, Y.; Zhang, H.; Zhao, C.; Wang, F.; Li, P.; et al. Endophytic microbes Bacillus sp. LZR216-regulated root development is dependent on polar auxin transport in Arabidopsis seedlings. Plant Cell Rep. 2015, 34, 1075–1087. [Google Scholar] [CrossRef]
- Boursiac, Y.; Leran, S.; Corratge-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA transport and transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Melentiev, A.I.; Martynenko, E.V.; Arkhipova, T.N.; Shendel, G.V.; Kuz’mina, L.Y.; Dodd, I.C.; Veselov, S.Y. Cytokinin producing bacteria stimulate amino acid deposition by wheat roots. Plant Physiol. Biochem. 2014, 83, 285–291. [Google Scholar] [CrossRef]
- Kuzmina, L.Y.; Arkhipova, T.N.; Aktuganov, G.E.; Galimzianova, N.F.; Chetverikov, S.P.; Melentiev, A.I. Bacteria belonging to genera Advenella, Bacillus and Pseudomonas as a promising biopreparations base for horticulture. Biomics 2018, 10, 16–19. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Galimsyanova, N.F.; Kuzmina, L.; Vysotskaya, L.B.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Kudoyarova, G.R. Effect of seed bacterization with plant growth-promoting bacteria on wheat productivity and phosphorus mobility in the rhizosphere. Plant Soil Environ. 2019, 65, 313–319. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Veselov, D.S.; Sharipova, G.V.; Akhiyarova, G.R.; Dodd, I.C.; Veselov, S.Y. Water relations and growth of original barley plants and its ABA-deficient mutants at increased air temperature. Russ. J. Plant Physiol. 2014, 61, 188–193. [Google Scholar] [CrossRef]
- Veselov, S.U.; Kudoyarova, G.R.; Egutkin, N.L.; Gyuli-Zade, V.G.; Mustafina, A.R.; Kof, E.K. Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole 3-acetic acid. Physiol. Plant. 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Evseeva, N.V.; Tkachenko, O.V.; Burygin, G.L.; Vysotskaya, L.B.; Akhtyamova, Z.A.; Kudoyarova, G.R. Effect of rhizobacteria on phytohormone status of potato microclones under osmotic stress in vitro. Biomolecules 2020, 10, 1231. [Google Scholar] [CrossRef]
- Bezrukova, M.; Kildibekova, A.; Shakirova, F. WGA reduces the level of oxidative stress in wheat seedlings under salinity. Plant Growth Regul. 2008, 54, 195–201. [Google Scholar] [CrossRef]


| Year of Testing | NaCl Concentration % | Crop Yield, g of Seeds m2 | ||
|---|---|---|---|---|
| Without Bacterization | Seed Bacterization with | |||
| P. mandelii IB-Ki14 | B. subtilis IB-22 | |||
| 2016 | 0 | 633 ± 12 a | 876 ± 8 b | 942 ± 10 c |
| 2018 | 0 | 400 ± 11 d | 557 ± 16 e | 581 ± 14 e |
| 10 | 93 ± 4 a | 105 ± 4 b | 121 ± 4 c | |
| 2019 | 0 | 430 ± 11 c | 478 ± 11 d | 540 ± 12 e |
| 5 | 209 ± 5 a | 254 ± 6 b | 273 ± 6 c | |
| Effect or Interaction | Root | Shoot | ||
|---|---|---|---|---|
| Fresh Mass | Dry Mass | Fresh Mass | Dry Mass | |
| Salt treatment | p ≤ 0.05 | p ≤ 0.01 | p ≤ 0.000001 | p ≤ 0.000001 |
| Inoculation | NS | NS | p ≤ 0.00001 | p ≤ 0.00001 |
| Inoculation × Salt treatment | p ≤ 0.05 | p ≤ 0.05 | NS | NS |
| NaCl Concentration, mM | Bacterization | Dry Mass, mg | |
|---|---|---|---|
| Root | Shoot | ||
| 0 | Without bacterization | 14.2 ± 0.8 b | 40.0 ± 1.9 d |
| 100 | Without bacterization | 9.3 ± 0.7 a | 24.9 ± 0.6 a |
| 100 | P. mandelii IB-Ki14 | 10.4 ± 0.9 a | 30.7 ± 0.5 b |
| 100 | B. subtilis IB-22 | 10.8 ± 0.7 a | 34.3 ± 1.0 c |
| NaCl Concentration, mM | Treatment | Transpiration | Stomatal Conductance | Hydraulic Conductivity |
|---|---|---|---|---|
| 0 | Without bacteria | 177 ± 10 c | 101 ± 9 b | 170 ± 21 b |
| 100 | Without bacteria | 108 ± 13 a | 77 ± 8 a | 77 ± 8 a |
| 100 | P. mandelii IB-Ki14 | 134 ± 4 b | 65 ± 6 a | 64 ± 5 a |
| 100 | B. subtilis IB-22 | 162 ± 5 c | 60 ± 5 a | 136 ± 15 b |
| Effect or Interaction | Root | Shoot |
|---|---|---|
| Salt treatment | p ≤ 0.0001 | p ≤ 0.0001 |
| Inoculation | p ≤ 0.05 | p ≤ 0.05 |
| Inoculation × salt treatment | p ≤ 0.05 | p ≤ 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of Plant Growth Promoting Rhizobacteria on the Content of Abscisic Acid and Salt Resistance of Wheat Plants. Plants 2020, 9, 1429. https://doi.org/10.3390/plants9111429
Arkhipova T, Martynenko E, Sharipova G, Kuzmina L, Ivanov I, Garipova M, Kudoyarova G. Effects of Plant Growth Promoting Rhizobacteria on the Content of Abscisic Acid and Salt Resistance of Wheat Plants. Plants. 2020; 9(11):1429. https://doi.org/10.3390/plants9111429
Chicago/Turabian StyleArkhipova, Tatiana, Elena Martynenko, Guzel Sharipova, Ludmila Kuzmina, Igor Ivanov, Margarita Garipova, and Guzel Kudoyarova. 2020. "Effects of Plant Growth Promoting Rhizobacteria on the Content of Abscisic Acid and Salt Resistance of Wheat Plants" Plants 9, no. 11: 1429. https://doi.org/10.3390/plants9111429
APA StyleArkhipova, T., Martynenko, E., Sharipova, G., Kuzmina, L., Ivanov, I., Garipova, M., & Kudoyarova, G. (2020). Effects of Plant Growth Promoting Rhizobacteria on the Content of Abscisic Acid and Salt Resistance of Wheat Plants. Plants, 9(11), 1429. https://doi.org/10.3390/plants9111429





