Differential Interpretation of Mountain Temperatures by Endospermic Seeds of Three Endemic Species Impacts the Timing of In Situ Germination
Abstract
1. Introduction
2. Results
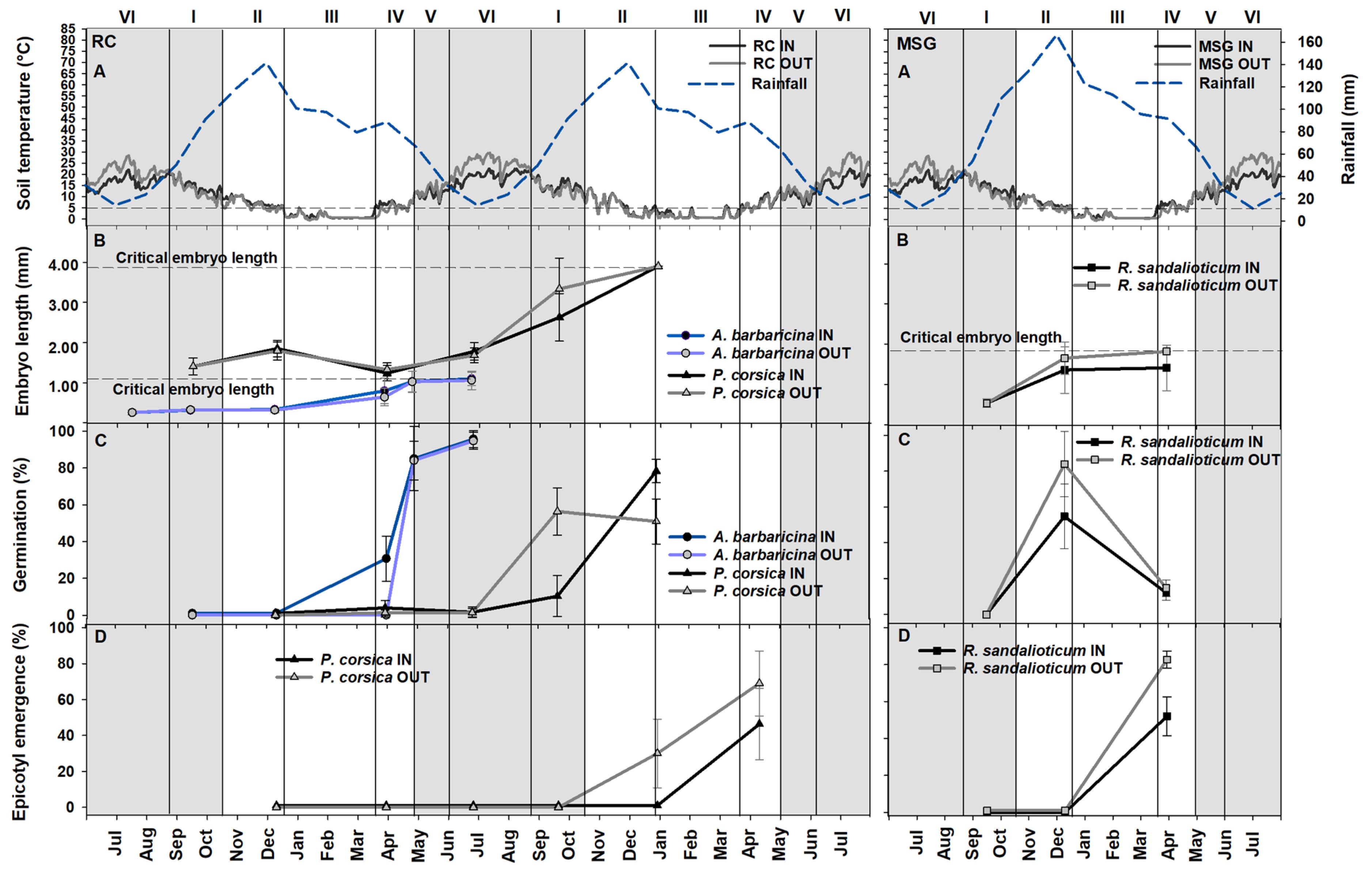
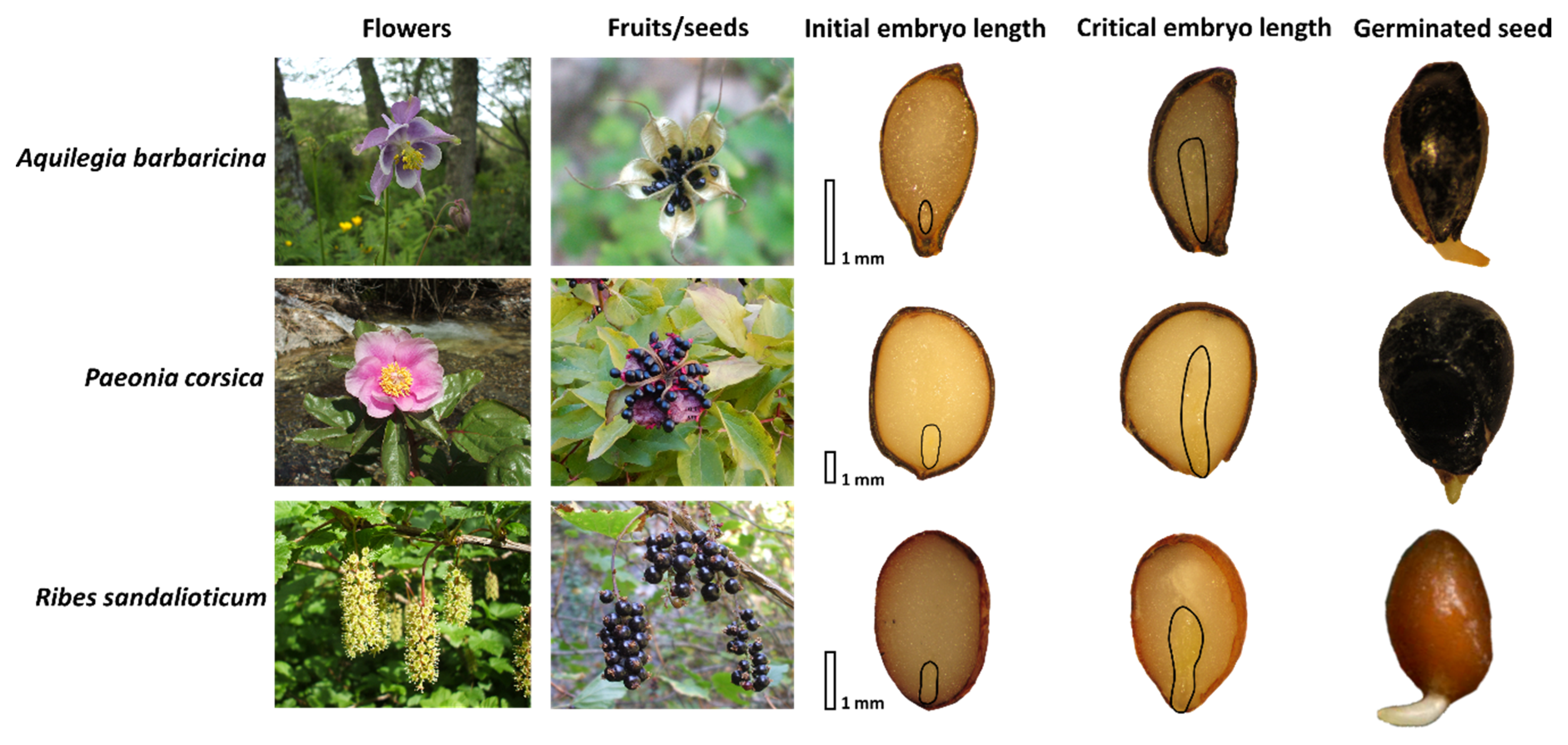
2.1. Embryo Growth and Germination Tests in Natural Conditions
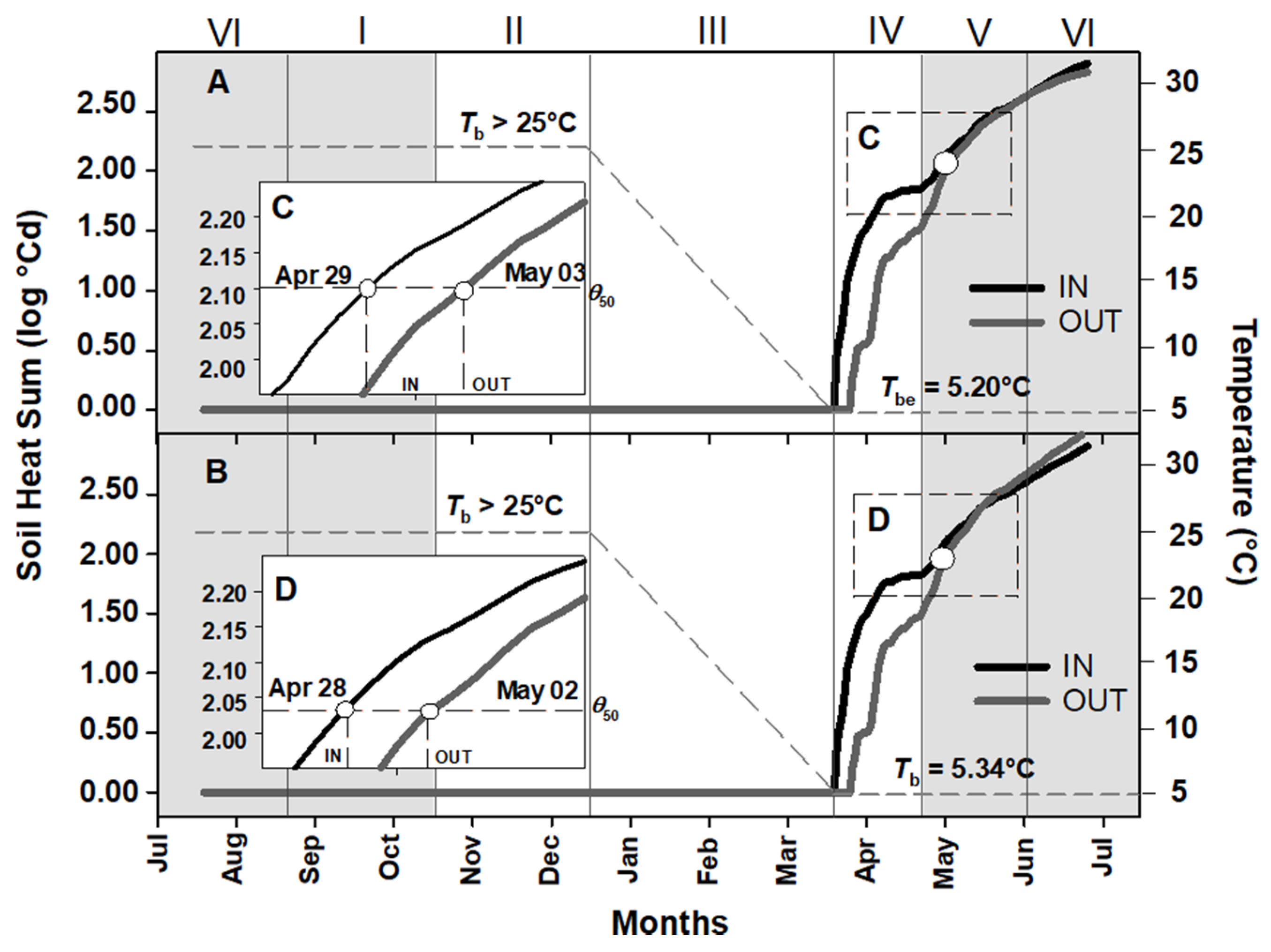
2.2. Soil Heat Sum for Embryo Growth and Seed Germination of Aquilegia barbaricina
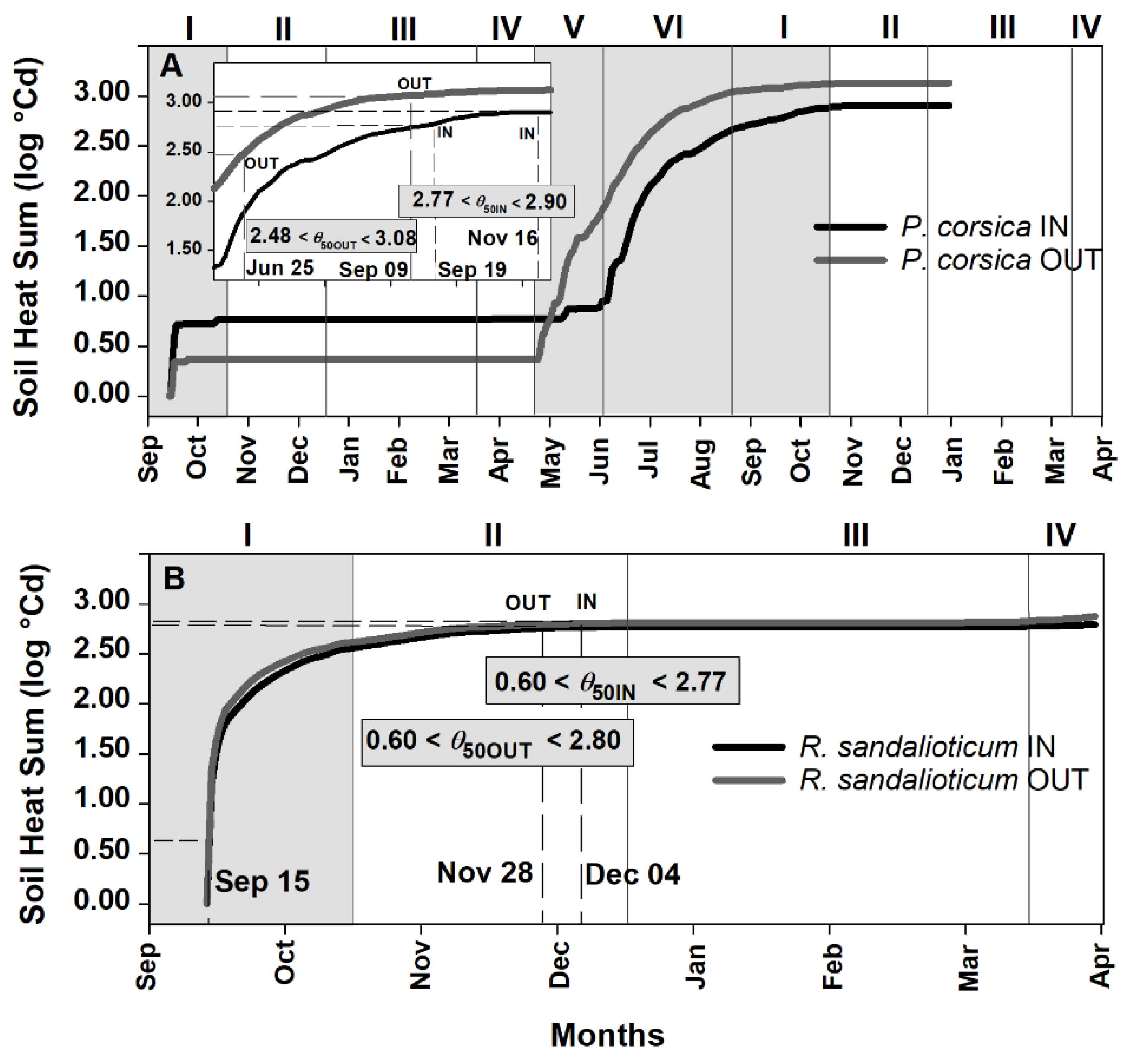
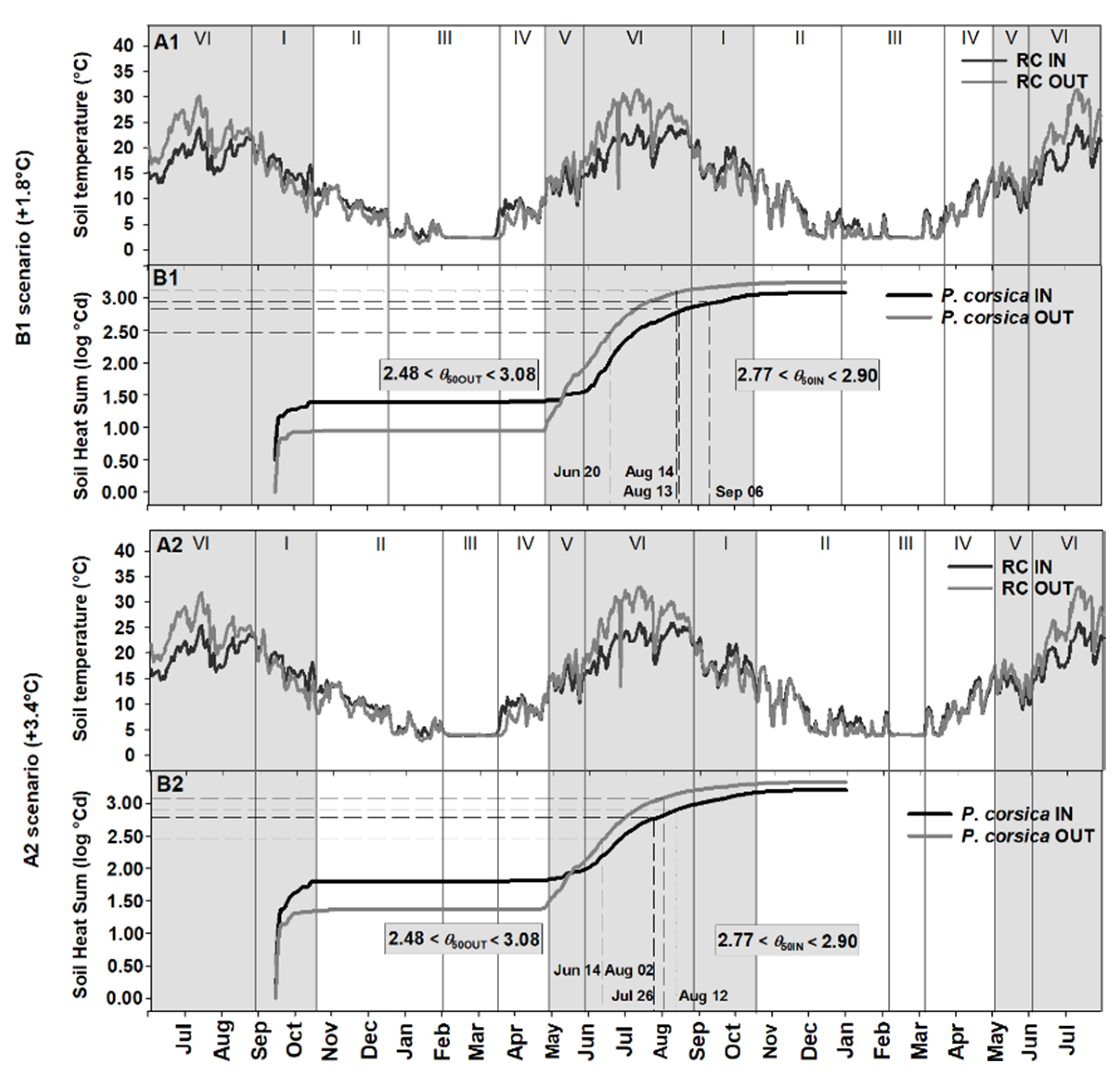
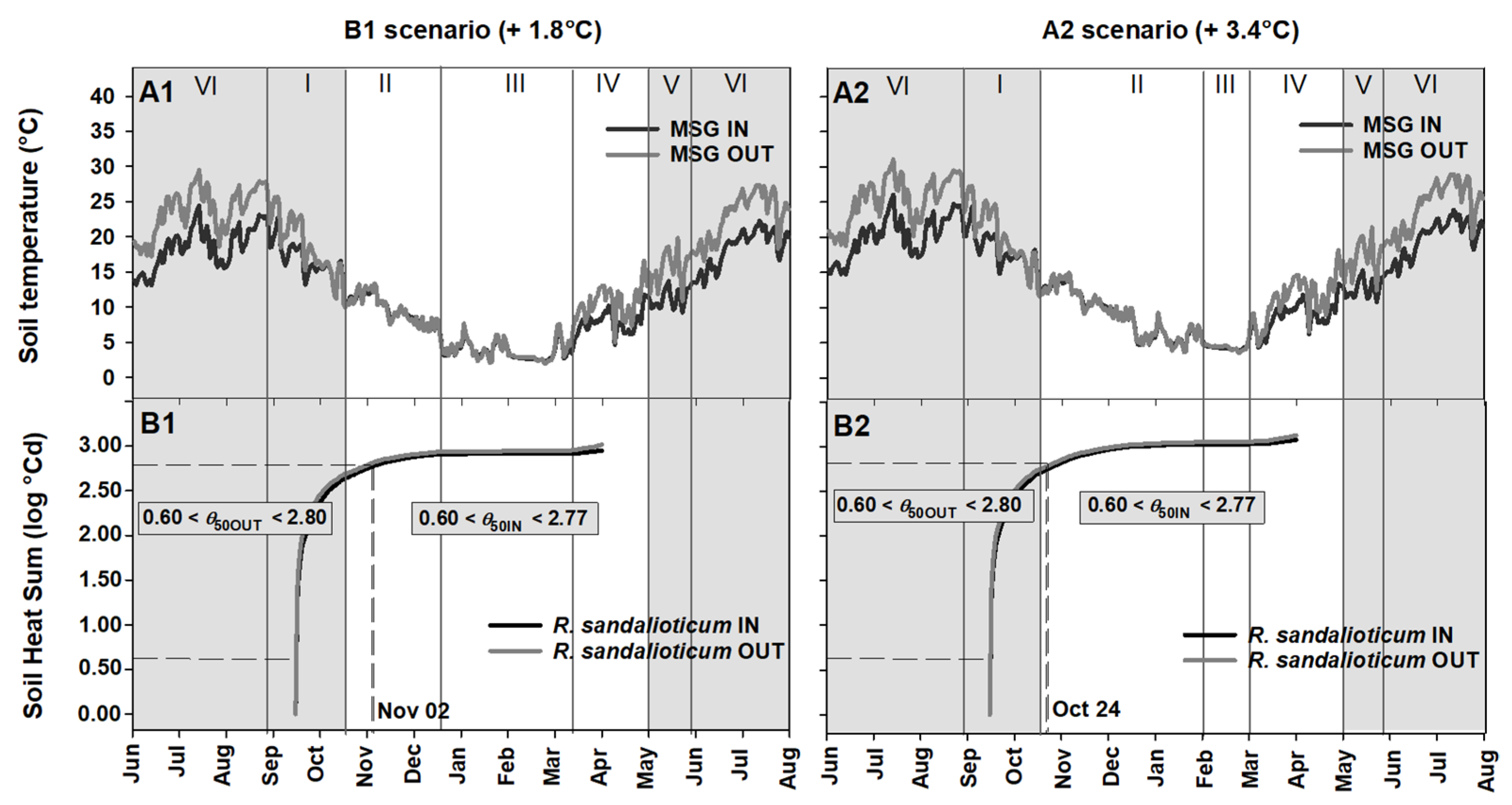
2.3. Soil Heat Sum Estimates for Seed Germination of Paeonia corsica and Ribes sandalioticum
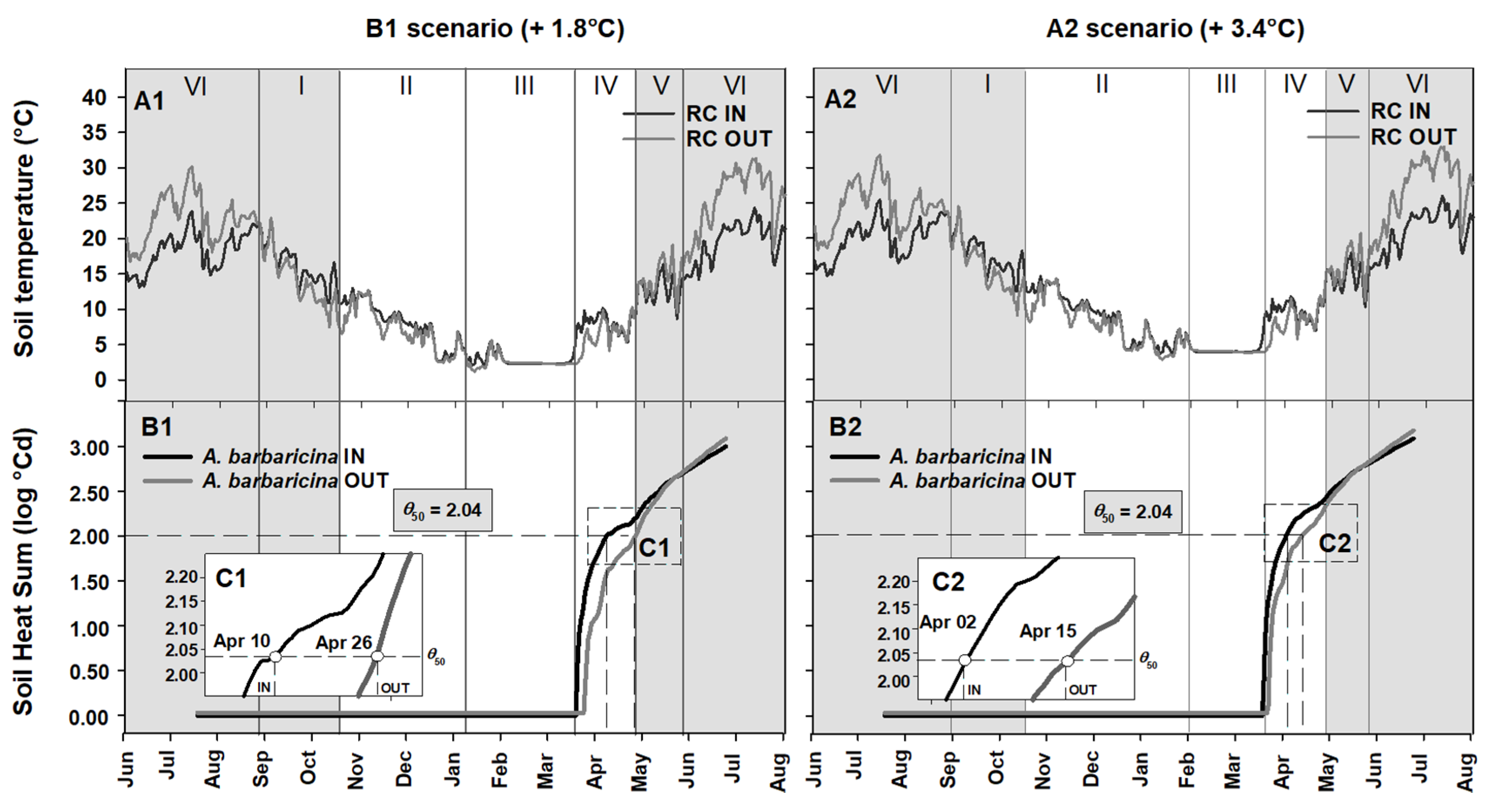
2.4. Seed Germination Phenology under Different Climate Scenarios
3. Discussion
3.1. Ecological Correlates of Embryo Growth, Seed Germination, Epicotyl Emergence and Seedling Establishment in Natural Conditions
3.2. Soil Heat Sum for In Situ Seed Germination
3.3. Phenology of Seed Germination under Global Warming
4. Materials and Methods
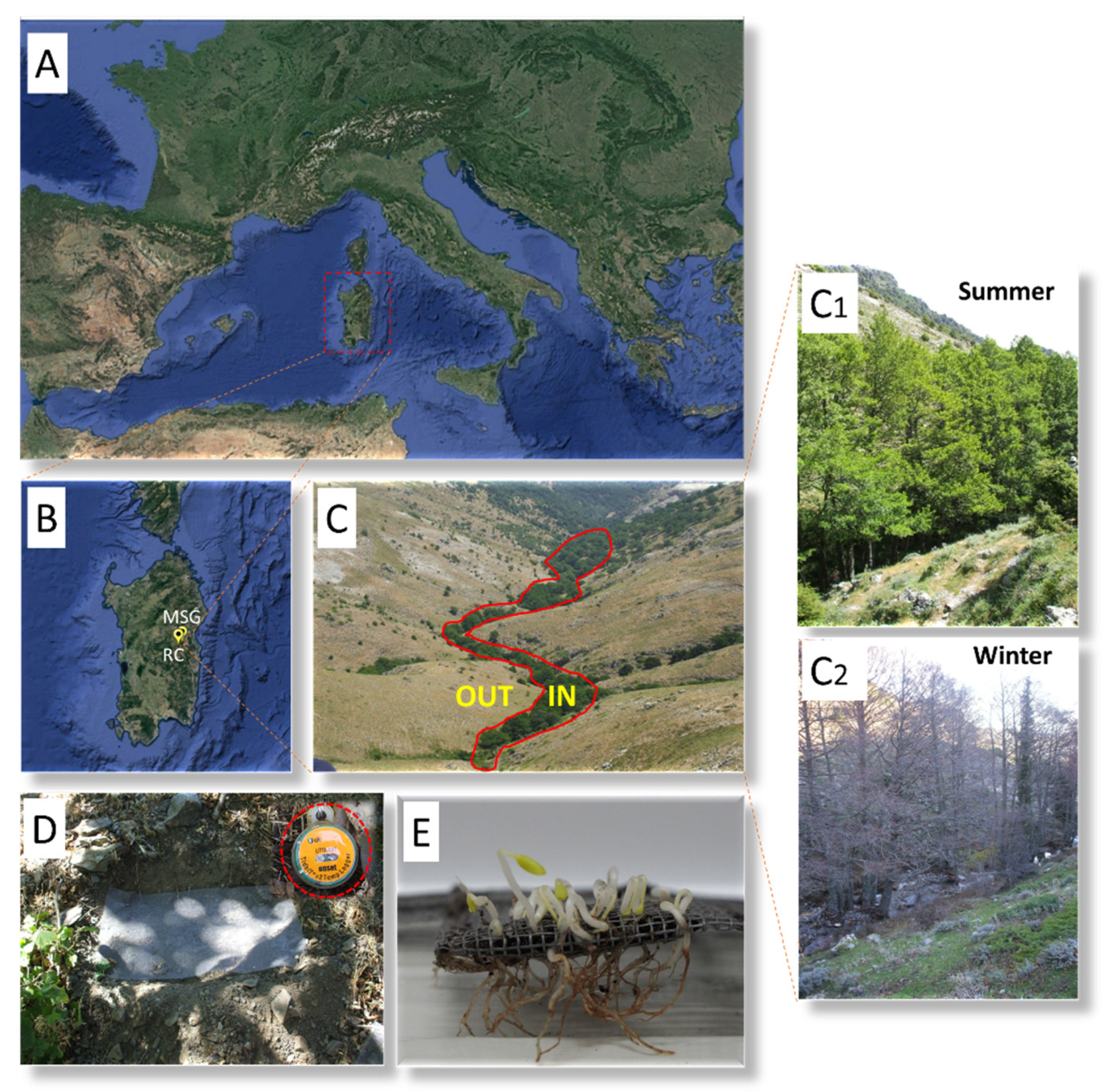
4.1. Study Species
4.2. Seed Lot Details
4.3. Seed Germination and Embryo Growth in Natural Conditions
4.4. Soil Heat Sum Approach
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lionello, P.; Malanotte-Rizzoli, P.; Boscolo, R.; Alpert, P.; Artale, V.; Li, L.; Luterbacher, J.; May, W.; Trigo, R.; Tsimplis, M.; et al. The Mediterranean climate: An overview of the main characteristics and issues. Dev. Earth Environ. Sci. 2006, 4, 1–26. [Google Scholar]
- Luna, B.; Pérez, B.; Torres, I.; Moreno, J.M. Effects of incubation temperature on seed germination of mediterranean plants with different geographical distribution ranges. Folia Geobot. 2012, 47, 17–27. [Google Scholar] [CrossRef]
- Céspedes, B.; Torres, I.; Urbieta, I.R.; Moreno, J.M. Effects of changes in the timing and duration of the wet season on the germination of the soil seed bank of a seeder-dominated Mediterranean shrubland. Plant Ecol. 2012, 213, 919–931. [Google Scholar] [CrossRef]
- Chamorro, D.; Luna, B.; Ourcival, J.-M.; Kavgacı, A.; Sirca, C.; Mouillot, F.; Arianoutsou, M.; Moreno, J.M. Germination sensitivity to water stress in four shrubby species across the Mediterranean Basin. Plant Biol. 2017, 19, 23–31. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Cochrane, J.A. Thermal requirements underpinning germination allude to risk of species decline from climate warming. Plants 2020, 9, 796. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Vandelook, F.; van Assche, J.A. Temperature requirements for seed germination and seedling development determine timing of seedling emergence of three monocotyledonous temperate forest spring geophytes. Ann. Bot. 2008, 102, 865–875. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Chien, C.T.; Chen, S.Y.; Tsai, C.C.; Baskin, J.M.; Baskin, C.C.; Kuo-Huang, L.L. Deep simple epicotyl morphophysiological dormancy in seeds of two Viburnum species, with special reference to shoot growth and development inside the seed. Ann. Bot. 2011, 108, 13–22. [Google Scholar] [CrossRef]
- Garcia-Huidobro, J.; Monteith, J.L.; Squire, G.R. Time, temperature and germination of pearl millet (Pennisetum typhoides S. & H.): I. Constant temperature. J. Exp. Bot. 1982, 33, 288–296. [Google Scholar] [CrossRef]
- Covell, S.; Ellis, R.H.; Roberts, E.H.; Summerfield, R.J. The influence of temperature on seed germination rate in grain legumes: I. A comparison of chickpea, lentil, soyabean and cowpea at constant temperatures. J. Exp. Bot. 1986, 37, 705–715. [Google Scholar] [CrossRef]
- Ellis, R.H.; Covell, S.; Roberts, E.H.; Summerfield, R.J. The influence of temperature on seed germination rate in grain legumes: II. Intraspecific variation in chickpea (Cicer arietinum L.) at constant temperatures. J. Exp. Bot. 1986, 37, 1503–1515. [Google Scholar] [CrossRef]
- Ellis, R.H.; Simon, G.; Covell, S. The influence of temperature on seed germination rate in grain legumes: III. A comparison of five faba bean genotypes at constant temperatures using a new screening method. J. Exp. Bot. 1987, 38, 1033–1043. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Manger, K.R. Quantal response of fruit and seed germination rate in Quercus robur L. and Castanea sativa Mill, to constant temperatures and photon dose. J. Exp. Bot. 1990, 41, 1549–1557. [Google Scholar] [CrossRef]
- Trudgill, D.L.; Squire, G.R.; Thompson, K. A thermal time basis for comparing the germination requirements of some British herbaceous plants. New Phytol. 2000, 145, 107–114. [Google Scholar] [CrossRef]
- Hardegree, S.P. Predicting germination response to temperature. I. Cardinal-temperature models and subpopulation-specific regression. Ann. Bot. 2006, 97, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Steadman, K.J.; Pritchard, H.W. Germination of Aesculus hippocastanum seeds following cold-induced dormancy loss can be described in relation to a temperature-dependent reduction in base temperature (Tb) and thermal time. New Phytol. 2004, 161, 415–425. [Google Scholar] [CrossRef]
- Porceddu, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Dissecting seed dormancy and germination in Aquilegia barbaricina, through thermal kinetics of embryo growth. Plant Biol. 2017, 19, 983–993. [Google Scholar] [CrossRef]
- Hardegree, S. Germination and emergence of primed grass seeds under field and simulated-field temperature regimes. Ann. Bot. 2000, 85, 379–390. [Google Scholar] [CrossRef]
- Steadman, K.J.; Bignell, G.P.; Ellery, A.J. Field assessment of thermal after-ripening time for dormancy release prediction in Lolium rigidum seeds. Weed Res. 2003, 43, 458–465. [Google Scholar] [CrossRef]
- Chantre, G.R.; Batlla, D.; Sabbatini, M.R.; Orioli, G. Germination parameterization and development of an after-ripening thermal-time model for primary dormancy release of Lithospermum arvense seeds. Ann. Bot. 2009, 103, 1291–1301. [Google Scholar] [CrossRef]
- Orrù, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Thermal thresholds as predictors of seed dormancy release and germination timing: Altitude-related risks from climate warming for the wild grapevine Vitis vinifera subsp. sylvestris. Ann. Bot. 2012, 110, 1651–1660. [Google Scholar] [CrossRef]
- Cuena-Lombraña, A.; Porceddu, M.; Dettori, C.A.; Bacchetta, G. Predicting the consequences of global warming on Gentiana lutea germination at the edge of its distributional and ecological range. PeerJ 2020, 8, e8894. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pascual, E.; Seal, C.E.; Pritchard, H.W. Simulating the germination response to diurnally alternating temperatures under climate change scenarios: Comparative studies on Carex diandra seeds. Ann. Bot. 2015, 115, 201–209. [Google Scholar] [CrossRef]
- Porceddu, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Thermal niche for in situ seed germination by Mediterranean mountain streams: Model prediction and validation for Rhamnus persicifolia seeds. Ann. Bot. 2013, 112, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Salanueva, C.A.; Seal, C.E.; Pritchard, H.W.; Orozco-Segovia, A.; Canales-Martínez, M.; Flores-Ortiz, C.M. Cardinal temperatures and thermal time in Polaskia Backeb (Cactaceae) species: Effect of projected soil temperature increase and nurse interaction on germination timing. J. Arid Environ. 2015, 115, 73–80. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the 4th Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reiginger, A., Eds.; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Penuelas, J.; Boada, M. A global change-induced biome shift in the Montseny mountains (NE Spain). Glob. Chang. Biol. 2003, 9, 131–140. [Google Scholar] [CrossRef]
- Bravo, D.N.; Araújo, M.B.; Lasanta, T.; Moreno, J.I.L. Climate change in Mediterranean mountains during the 21st century. Ambio 2008, 37, 280–285. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; García-Camacho, R.; García-Fernández, A.; Iriondo, J.M.; Lara-Romero, C.; Morente-López, J. How does climate change affect regeneration of Mediterranean high-mountain plants? An integration and synthesis of current knowledge. Plant Biol. 2018, 20, 50–62. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann. Missouri Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Bacchetta, G.; Fenu, G.; Guarino, R.; Mandis, G.; Mattana, E.; Nieddu, G.; Scudu, C. Floristic traits and biogeographic characterization of the Gennargentu Massif (Sardinia). Candollea 2013, 68, 209–220. [Google Scholar] [CrossRef]
- Fenu, G.; Mattana, E.; Congiu, A.; Bacchetta, G. The endemic vascular flora of Supramontes (Sardinia), a priority plant conservation area. Candollea 2010, 65, 347–358. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate change impacts in Alpine environments. Geogr. Compass 2010, 4, 1133–1153. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Cogoni, D.; Bacchetta, G. The reliability of conservation status assessments at regional level: Past, present and future perspectives on Gentiana lutea L. ssp. lutea in Sardinia. J. Nat. Conserv. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Breshears, D.D.; Nyhan, J.W.; Heil, C.E.; Wilcox, B.P. Effects of woody plants on microclimate in a semiarid woodland: Soil temperature and evaporation in canopy and intercanopy patches. Int. J. Plant Sci. 1998, 159, 1010–1017. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Gómez, J.M.; Zamora, R.; Boettinger, J.L. Canopy vs. soil effects of shrubs facilitating tree seedlings in Mediterranean montane ecosystems. J. Veg. Sci. 2005, 16, 191–198. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. A revision of Martin’s seed classification system, with particular reference to his dwarf-seed type. Seed Sci. Res. 2007, 17, 11–20. [Google Scholar] [CrossRef]
- Mattana, E.; Pritchard, H.W.; Porceddu, M.; Stuppy, W.H.; Bacchetta, G. Interchangeable effects of gibberellic acid and temperature on embryo growth, seed germination and epicotyl emergence in Ribes multiflorum ssp. sandalioticum (Grossulariaceae). Plant Biol. 2012, 14, 77–87. [Google Scholar] [CrossRef]
- Porceddu, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Sequential temperature control of multi-phasic dormancy release and germination of Paeonia corsica seeds. J. Plant Ecol. 2016, 9, 464–473. [Google Scholar] [CrossRef]
- Greenlee, J.T.; Callaway, R.M. Abiotic stress and the relative importance of interference and facilitation in Montane bunchgrass communities in Western Montana. Am. Nat. 1996, 148, 386–396. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Ezcurra, E. Shade as a cause of the association between the cactus Neobuxbaumia tetetzo and the nurse plant Mimosa luisana in the Tehuacan Valley, Mexico. J. Ecol. 1991, 79, 961–971. [Google Scholar] [CrossRef]
- Forcella, F.; Benech Arnold, R.L.; Sanchez, R.; Ghersa, C.M. Modeling seedling emergence. Field Crops Res. 2000, 67, 123–139. [Google Scholar] [CrossRef]
- Mondoni, A.; Rossi, G.; Orsenigo, S.; Probert, R.J. Climate warming could shift the timing of seed germination in alpine plants. Ann. Bot. 2012, 110, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Luo, X.P.; Yuan, Z.; Bai, M.J.; Hu, X.W. Seed dormancy release of Halenia elliptica in response to stratification temperature, duration and soil moisture content. BMC Plant Biol. 2020, 20, 352. [Google Scholar] [CrossRef]
- Porceddu, M.; Fenu, G.; Bacchetta, G. New findings on seed ecology of Ribes sardoum: Can it provide a new opportunity to prevent the extinction of a threatened plant species? Syst. Biodivers. 2017, 15, 480–488. [Google Scholar] [CrossRef]
- Fernández-Pascual, E.; Mattana, E.; Pritchard, H.W. Seeds of future past: Climate change and the thermal memory of plant reproductive traits. Biol. Rev. 2019, 94, 439–456. [Google Scholar] [CrossRef]
- Garrido, J.L.; Fenu, G.; Mattana, E.; Bacchetta, G. Spatial genetic structure of Aquilegia taxa endemic to the island of Sardinia. Ann. Bot. 2012, 109, 953–964. [Google Scholar] [CrossRef]
- Porceddu, M.; Picciau, R.; Fenu, G.; Bacchetta, G. Paeonia corsica Sieber ex Tausch. Inf. Bot. Ital. 2015, 47, 245–289. [Google Scholar]
- Fenu, G.; Porceddu, M.; Mattana, E.; Congiu, A.; Bacchetta, G. Ribes multiflorum Kit. ex Roem. & Schult. subsp. sandalioticum Arrigoni. Inf. Bot. Ital. 2011, 43, 381–458. [Google Scholar]
- Porceddu, M.; Santo, A.; Orrù, M.; Meloni, F.; Ucchesu, M.; Picciau, R.; Sarigu, M.; Cuena Lombrana, A.; Podda, L.; Sau, S.; et al. Seed conservation actions for the preservation of plant diversity: The case of the Sardinian Germplasm Bank (BG-SAR). Plant Sociol. 2017, 54, 111–117. [Google Scholar] [CrossRef]
- Vandelook, F.; Bolle, N.; van Assche, J.A. Multiple environmental signals required for embryo growth and germination of seeds of Selinum carvifolia (L.) L. and Angelica sylvestris L. (Apiaceae). Seed Sci. Res. 2007, 17, 283–291. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
| Species | A. barbaricina | P. corsica | R. sandalioticum |
|---|---|---|---|
| Population | Rio Correboi (Villagrande Strisaili, NU) | Rio Correboi (Villagrande Strisaili, NU) | Monte Novo San Giovanni (Orgosolo, NU) |
| Maximum Germination in Laboratory (%) | 81 ± 12 | 63± 10 | 88 ± 3 |
| Tb (°C) Dormant Seeds | > 25 | 15 * | 10 * |
| Tb (°C) Non-dormant Seeds | 5.34 ± 1.38 | 10 * | 5 * |
| Initial Embryo Length (mm) | 0.29 ± 0.06 | 1.40 ± 0.20 | 0.52 ± 0.08 |
| Critical Embryo Length (mm) | 1.17 ± 0.23 | 3.90 ± 0.70 | 1.80 ± 0.39 |
| Tbe (°C) | 5.20 ± 0.60 | ND | ND |
| θe50 (log °Cd) | 2.10 | ND | ND |
| θg50 (log °Cd) | 2.04 | ND | ND |
| Source | [19] | [44] | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porceddu, M.; Pritchard, H.W.; Mattana, E.; Bacchetta, G. Differential Interpretation of Mountain Temperatures by Endospermic Seeds of Three Endemic Species Impacts the Timing of In Situ Germination. Plants 2020, 9, 1382. https://doi.org/10.3390/plants9101382
Porceddu M, Pritchard HW, Mattana E, Bacchetta G. Differential Interpretation of Mountain Temperatures by Endospermic Seeds of Three Endemic Species Impacts the Timing of In Situ Germination. Plants. 2020; 9(10):1382. https://doi.org/10.3390/plants9101382
Chicago/Turabian StylePorceddu, Marco, Hugh W. Pritchard, Efisio Mattana, and Gianluigi Bacchetta. 2020. "Differential Interpretation of Mountain Temperatures by Endospermic Seeds of Three Endemic Species Impacts the Timing of In Situ Germination" Plants 9, no. 10: 1382. https://doi.org/10.3390/plants9101382
APA StylePorceddu, M., Pritchard, H. W., Mattana, E., & Bacchetta, G. (2020). Differential Interpretation of Mountain Temperatures by Endospermic Seeds of Three Endemic Species Impacts the Timing of In Situ Germination. Plants, 9(10), 1382. https://doi.org/10.3390/plants9101382







